Remdesivir: Is the Chance to Improve the COVID-19 Course Negligible?
Miro Morović* and Vedrana Terkeš
Department of Infectious Diseases, Zadar General Hospital, Croatia
Received Date: 18/01/2024; Published Date: 28/05/2024
*Corresponding author: Prof. Miro Morovic, MD, PhD, Department of Infectious Diseases, Zadar General Hospital, Boze Pericica 5, 23000 Zadar, Croatia
ORCID: https://orcid.org/0000-0002-9745-074X
Background
Two big randomized trials, ACTT-1 [1] and PINETREE [2], showed clinical benefits of remdesivir in COVID-19 patients, but two other trials, Solidarity [3] and DisCoVeRy [4], showed little or no effect of remdesivir. However, there is a general agreement that early treatment with remdesivir reduces viral loads and improves recovery for certain patients with COVID-19 [5]. In a recent Cochrane Database review, it has been concluded that remdesivir probably has little or no effect on all-cause mortality or in-hospital mortality of individuals with moderate to severe COVID-19 and that future studies are needed, especially for different population subgroups [6]. We earlier reported in a small observational study in 137 patients with different disease severities, a beneficial effect of remdesivir, administrated in the early stage of COVID-19, at least in moderately ill patients with a high risk of progression, before the transition to a more severe stage [7].
Methods
The study population consisted of 956 patients diagnosed and hospitalized with COVID-19. All the patients were grouped according to the National Institute of Health (NIH) guidelines disease severity [8]. 451 (47.2%) patients belonged to the severe disease group, 223 (23.3%) to the critical disease group. The mean age was 69.5±14.07 years, most of them 643 (67.7%) were male. Most of the comorbidities were arterial hypertension (63.5%) and diabetes (26.4%). Bilateral pneumonia was registered in 879 (91.9%) of patients. Only 96 (10%) of patients were vaccinated once. 304 (31.8%) of patients died. 228 (23.8%) of patients were treated with remdesivir, almost all within the first 10 days from the beginning of the symptoms, others received SOC (Standard of Care) measures. No serious adverse effects were reported in remdesivir treated patients.
Results
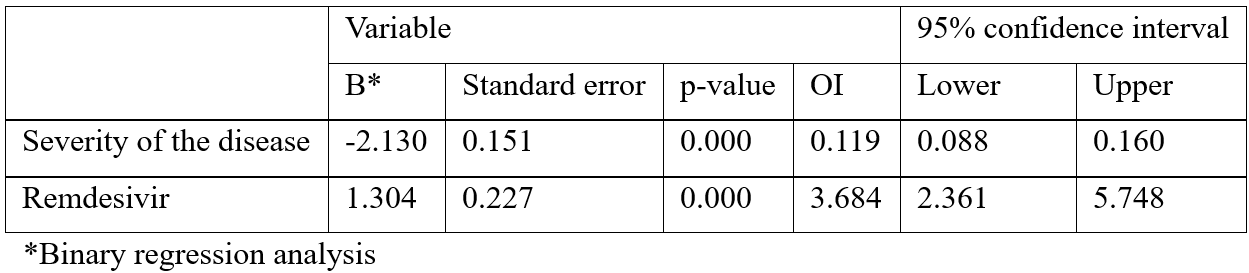
The first analysis includes the results of the binary regression analysis of remdesivir treatment and of disease severity in predicting the disease outcomes, death or survival, and showed that both predictors explained 41.8% of survival variance in the criteria; both parameters showed to be statistically significant of the disease outcome. The results showed that the more severe the clinical condition, the more likely the outcome to be fatal (for every degree of severity the probability of death rose by 12%, p=.000). On the other hand, remdesivir treated patients had a 3.7 times bigger chance to survive opposite to the non-treated ones (p=.000) (Table 1).
Table 1: Prediction of disease survival depending on severity of the disease and remdesivir treatment.
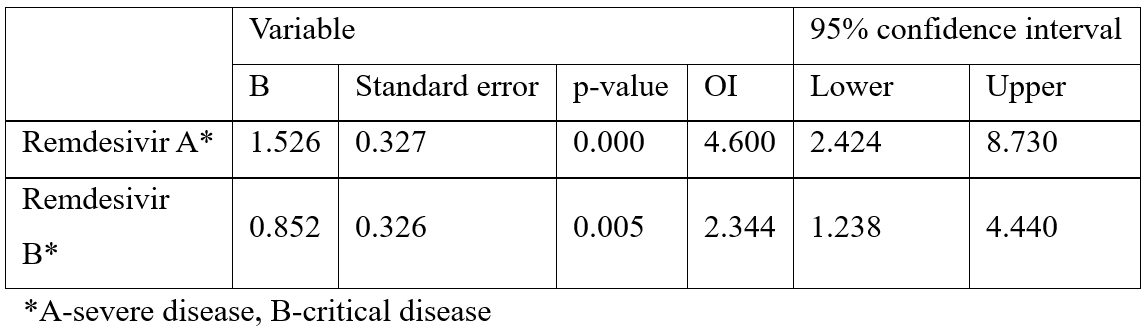
The same binary regression analysis, according to various disease severity groups, showed also that the chance of survival in the severe patients treated with remdesivir was 4.6 times bigger than in the non-treated ones (p=.000), and moreover, the chance of survival in critical patients also was 2.3 times bigger in treated than in non-treated patients (p=.005) (Table 2).
Table 2: Prediction of disease outcome (survival/death) in severe (A) and critical (B) patients depending on remdesivir treatment.
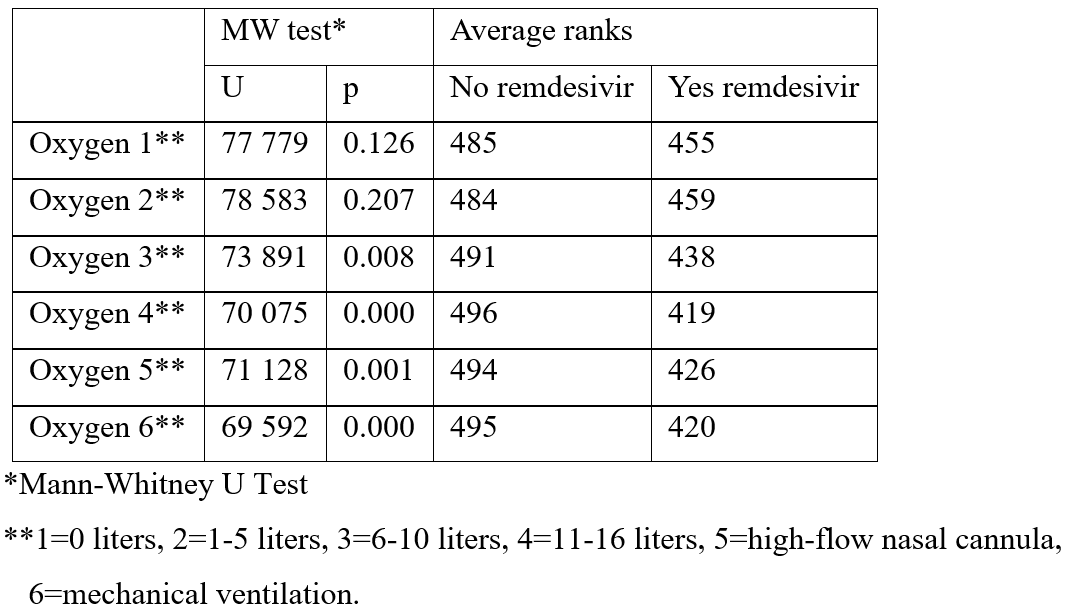
The second analysis dealt with the remdesivir influence on supportive oxygen treatment in COVID-19 patients during hospitalization. The value of supportive oxygen was evaluated according to the 6-degree scale where number 1 means 0 liters of oxygen, 2 means 1-5 liters, 3 means 6-10 liters, 4 means 11-16 liters, 5 means the treatment with high flow over nasal cannula and 6 means mechanical ventilation. The results of Mann-Whitney U-test showed a statistically significant difference in the direction of significant lower level of oxygen needed in the treated patients from points 3 to 6, in contrast to the non-treated ones who required much more oxygen (Table 3).
Table 3: Difference in level of supportive care oxygen requirements in different time points during remdesivir treatment.
Discussion
From the beginning of the COVID-19 pandemic a countless number of clinical investigations tried to find the most efficient treatment. Remdesivir has remained one of the drugs with an unclear benefit for patients [1-5]. The results of our investigations contribute to the opinion that remdesivir has more benefit than harm. First our results showed that remdesivir could be of benefit even in severe and critical patients, rising their chance to survive. Second, remdesivir showed to be of significant benefit in the supplement oxygen treatment by reducing the oxygen need in more severe patients during the hospitalization period. Until presently. only sparse reports deal with this remdesivir effect [9,10].
Conclusion
Regardless of name, type or magnitude of the clinical studies our conclusion is that the chance to improve the COVID-19 course with remdesivir is far from negligible. Moreover, it could be of significant benefit in certain categories of COVID-19 patients and during some disease phases also.
Acknowledgement: Sincere thanks go to Mrs. Jasminka Bajlo and Mr. Predrag Jelicic for their great help in creating the manuscript.
References
- Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. ACTT-1 Study Group Members. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med, 2020; 383(19): 1813-1826. doi: 10.1056/NEJMoa2007764.
- Gottlieb RL, Vaca CE, Paredes R, Mera J, Webb BJ, Perez G, et al. GS-US-540-9012 (PINETREE) Investigators. Early Remdesivir to Prevent Progression to Severe Covid-19 in Outpatients. N Engl J Med, 2022; 386(4): 305-315. doi: 10.1056/NEJMoa2116846.
- WHO Solidarity Trial Consortium. Remdesivir and three other drugs for hospitalised patients with COVID-19: final results of the WHO Solidarity randomised trial and updated meta-analyses. Lancet, 2022; 399(10339): 1941-1953. doi: 10.1016/S0140-6736(22)00519-0.
- Ader F, Bouscambert-Duchamp M, Hites M, Peiffer-Smadja N, Poissy J, Belhadi D, et al. DisCoVeRy Study Group. Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): a phase 3. randomised. controlled. open-label trial. Lancet Infect Dis, 2022; 22(2): 209-221. doi: 10.1016/S1473-3099(21)00485-0.
- Qaseem A, Yost J, Etxeandia-Ikobaltzeta I, Abraham GM, Jokela JA, Forciea MA, et al. Should Remdesivir Be Used for the Treatment of Patients With COVID-19? Rapid. Living Practice Points From the American College of Physicians (Version 2). Ann Intern Med, 2021; 174(5): 673-679. doi: 10.7326/M20-8101.
- Grundeis F, Ansems K, Dahms K, Thieme V, Metzendorf MI, Skoetz N, et al. Remdesivir for the treatment of COVID-19. Cochrane Database Syst Rev, 2023; 1(1): CD014962. doi: 10.1002/14651858.CD014962.
- Terkes V, Lisica K, Marusic M, Verunica N, Tolic A, Morovic M. Remdesivir Treatment in Moderately Ill COVID-19 Patients: A Retrospective Single Center Study. J Clin Med, 2022; 11(17): 5066. doi: 10.3390/jcm11175066.
- COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health, 2019.
- Lee TC, Murthy S, Del Corpo O, Senécal J, Butler-Laporte G, Sohani ZN, et al. Remdesivir for the treatment of COVID-19: a systematic review and meta-analysis. Clin Microbiol Infect, 2022; 28(9): 1203-1210. doi: 10.1016/j.cmi.2022.04.018.
- Libra A, Ciancio N, Sambataro G, Sciacca E, Muscato G, Marino A, et al. Use of Remdesivir in Patients Hospitalized for COVID-19 Pneumonia: Effect on the Hypoxic and Inflammatory State. Viruses, 2023; 15(10): 2101. doi: 10.3390/v15102101.

