The Role of Early -Life Nutrition in Preventing Micronutrient Deficiencies Focus on Vitamin D, Omega3 and Iron During the First 1000 Days& Role of Gum Versus Cow Milk in Toddlers’ Nutrition
Said Eldeib1,*, Dinesh banur1, Jayaraj Damodaran1, Mohammad Othman Mohamed Eldesoky2, Rafia Owera3 and Hadi Haddad4
1Department of paediatrics, ADSCC/Yas clinic Hospital, UAE
2Department of paediatrics, Specialized Rehabilitation Hospitall, AD, UAE
3Department of paediatrics, Prime Medical Centre, Dubai, UAE
4Department of paediatrics, Lebanese American university mc, Lebanon
Received Date: 28/03/2025; Published Date: 01/05/2025
*Corresponding author: Dr. Said Eldeib, Department of paediatrics ADSCC/ Yas clinic Hospital, Abu Dhabi, UAE
Abstract
Optimal nutrition in the first 1000 days of life has a lifelong positive impact on child development. Specific intrauterine and perinatal factors, pathological conditions, and dietary restrictions can represent potential risk factors for micronutrient deficiencies in the first 1000 days of life, which can have negative systemic consequences , the micronutrient status of children should be investigated in order to tailor strategies specific to the individual’s metabolic needs, with a particular focus on deficiencies which can impair or delay the physical and cognitive development of children, namely, vitamin B12, vitamin D and folic acid, as well as oligo-elements such as iron, zinc, calcium, sodium, magnesium, and phosphorus, and essential fatty acids such as omega-3. Nutrition is essential for human growth, particularly in newborns and children. An optimal growth needs a correct diet, to ensure an adequate intake of macronutrients and micronutrients. Macronutrients are the compounds that humans consume in largest quantities, mainly classified in carbohydrates, proteins and fats. Micronutrients are instead introduced in small quantities, but they are required for an adequate growth in the paediatric age, especially zinc, iron, vitamin D and folic acid and omega 3.
Keywords: Micronutrient; Vit D; Omega 3; Toddler’s nutrition; Growth; Infant nutrition; Development
Abbreviations: GUM - Growing Up Milk; VIT D - Vitamin D; PUFA - Polyunsaturated Fatty Acid; EPA - Eicosapentaenoic Acid; DHA - Docosahexaenoic; ALA - Alpha-Linolenic Acid
Introduction
The first 1000 days (conception, pregnancy, and the first two years of life) represent a period of enormous vulnerability to nutritional deficiency as well opportunity for promoting better outcomes in children’s lives. The foetuses, infants, and children experience unique physiological changes and have specific nutritional needs [1]. In this context, an optimal nutrition during this time acts as the first line of prevention against developmental shortfalls [2-6]. The traditional concept of optimal nutrition during the first 1000 days of life included only macronutrient and energy balance. This concept was recently extended to include an adequate supplementation of micronutrients, given the evidence that diet influences gene expression through epigenetic mechanisms during the first 1000 days of life. Adequate micronutrient intake is essential for neural, visual, and skeletal system development because of its role in early foetal organ development and cell differentiation [7-26]. Micronutrient can only be provided by the diet and act as coenzymes in the production of hormones and essential substances for proper growth. Hence, their role in phases of rapid growth as the first 1000 days is essential. There is no definitive evidence on the benefits of supplementation of other micronutrients without demonstrated deficiency, both for lactating mothers and both for breastfed and weaning infants. At the same time, a supplementation of micronutrients become necessary during the first 1000 days of life in infants or children adopting restricted or unbalanced diets and affected by particular diseases [16,27,28]
Importance of Micronutrients During the First 1000 Days
Micronutrients at significant risk of deficiency during the first 1000 days are reported in Table 1. Among those, deficiencies of omega-3 fatty acids, vitamins C, B9, B12, and D, and minerals such as iodine and iron seem the most involved in clinical syndromes [1]. It is important to note that unbalanced diets can lead to micronutrient deficiencies even when following an omnivorous diet due to maternal and child undernutrition and food insecurity especially in low-income and middle-income countries. In this paragraph, we have focused on dietary regimens that involve the conscious choice to exclude food groups or the therapeutic necessity to do so.
Vitamin D promotes the absorption of calcium and phosphorus and bone remodeling. Studies also show its anti-inflammatory, anti-tumoral, and cardiovascular protection effects [22]. In vitamin D deficiency, the body absorbs less calcium and phosphate, causing bone disorders associated with bone weakness (rickets in children or osteomalacia in adults). Vitamin D deficiency during pregnancy can cause deficiency in the foetus; sometimes, the deficiency is severe enough to cause osteomalacia in women. Muscle spasms (tetany) caused by a low calcium level in the blood in people may be newborns’ first signs of rickets when severe vitamin D deficiency occurs. If the spasms are severe, they can lead to seizures. In younger children with rickets, the entire skull may be soft. Infants may have difficulty sitting up, crawling, and learning to walk. Closing of fontanelles may take longer. In children older aged one year or more, bone growth may be impaired, resulting in an abnormal spine curvature (scoliosis) and varus or valgus knees. Vitamin D supplementation is advised in all infants for the first year with 400 UI/die, whether breast or formula-fed [20-22,26].
Iron deficiency develops in stages. First, the demand for iron exceeds the amount consumed in the diet, causing the progressive depletion of iron reserves in the bone marrow. When reserves are reduced, the absorption of dietary iron increases to compensate for this deficiency. The deficiency impairs red blood cell synthesis in later stages, ultimately causing anaemia. Severe and prolonged iron deficiency can also cause a functional alteration of cellular enzymes that contain iron. Most iron deficiency symptoms are due to anaemia. In addition, patients may suffer from pica, a compulsive desire to eat non-food substances (e.g., ice, dirt, paint, starch, ash). Other severe deficiency symptoms include skin and mucosal damage, such as glossitis and cheilosis [7,19,20,32].
Omega-3 s is polyunsaturated fatty acid (PUFA) that includes eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), as well as the essential precursor alpha-linolenic acid (ALA). Omega-3 deficiency is associated with skin problems, mood disorders, and attention deficits and difficulty to concentrate. In the foetus, they act in neuronal and eye development [30,33,34].
Scientific evaluation of growing-up milk (GUM) versus fresh cow milk
GUM was developed in response to this imbalanced nutritional intake of young children. It is specially formulated to meet young children’s needs, containing large amounts of nutrients which they often do not get in sufficient quantities from their diet. Growing-up milk can be described as a “nutrient-optimised” form of cow’s milk, as it replicates low-fat cow’s milk in terms of the nutrients it contains. A number of studies on humans have investigated the extent to which growing-up milk compensates for the nutrient deficiencies described above. Data on the consumption of growing-up milk and its role in nutrition for children between the ages of 1 and 3 is limited, but the studies that have been conducted show that consuming growing-up milk has a positive influence on young children’s nutrient supply. Can growing-up milk improve the supply of critical nutrients? The table below gives an overview of growing-up milk studies and the observed improvement in nutrient intake and supply
Table 1: Overview of growing-up milk studies.
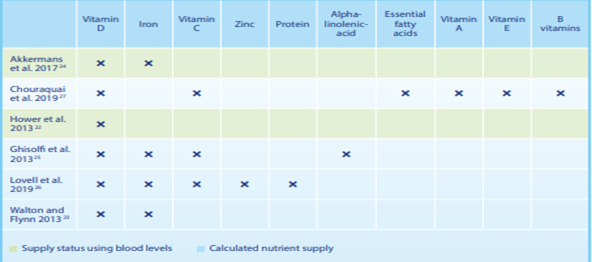
Overview of studies on the effects of growing-up milk
These products are usually referred to as growing-up milk (GUM). However, the term “Young Child Formula” (YCF) is also used by some studies and organisations. The terms are interchangeable. Influence on the supply of micronutrients and macronutrients A prospective, randomised, double-blind, controlled intervention study tested whether growing-up milk with high vitamin D content contributes to improved vitamin D supply in the winter months, and whether enriching growing-up milk with vitamin D can be regarded as safe the whole year round. Over a period of 10 months, children were given either a growing-up milk enriched with vitamin D (2.85 µg per 100 ml = 115 IU) or semi-skimmed cow’s milk with a natural vitamin D content (0.03 µg per 100 ml). The vitamin D supply at the start of the study was more or less the same in both groups (21.5 vs. 18.4 ng/ml 25(OH)D in serum). By the end of the five-month winter period, the children in the growing-up milk group had been supplied with significantly more vitamin D. Their vitamin D levels were in the ideal range (24.8 ng/ml 25(OH)D). In contrast, the levels in the children from the control group were in the subclinical deficiency range (13.6 ng/ ml 25(OH)D,. During the summer, when we produce more of our own vitamin D in our skin, the 25(OH)D serum concentration in the growing-up milk group increased only marginally to 27.6 ng/ml, which means that growing-up milk does not lead to an excessive supply of vitamin D in summer. This first prospective, double-blind intervention study with growing-up milk in Europe showed that consuming growing-up milk with approx. 2.9 µg per 100 ml of vitamin D instead of low-fat cow’s milk is a simple and safe way to prevent the drop in 25(OH)D serum concentration during the winter without increasing the 25(OH)D serum concentration to excessively high levels during the summer [36].
The cross-sectional study in 2013 used a 3-day diet record to investigate the diet and nutrient supply of 241 children who drank growing-up milk, and 206 children who did not drink growing-up milk but drank cow’s milk or consumed other dairy products instead. Even though the protein and sodium intake in the growing-up milk group was lower than in the group that did not drink growing-up milk, these values were still above the quantities recommended by the EFSA. In all age groups, the children in the growing-up milk group were significantly better supplied with essential fatty acids, vitamins C, A, D and E and all B vitamins. This also applied to iron intake, which was significantly higher in the growing-up milk group than in the group which were not given any growing-up milk (Figure 1). The intake of DHA and ARA was similar. To reach the quantities recommended by the EFSA, at least 360 ml growing-up milk was required. The authors conclude that growing-up milk can help children get their recommended amounts of nutrients. However, the quantities of protein, sodium and vitamin A were above the EFSA recommendations, and the quantities of DHA, ARA and vitamin D were below them (Figure 1 and 2). 37
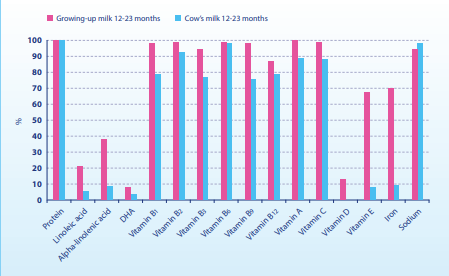
Figure 1: Adherence to EFSA recommendations when consuming growing-up milk vs. cow’s milk or dairy products at the age 12–23 months [37].
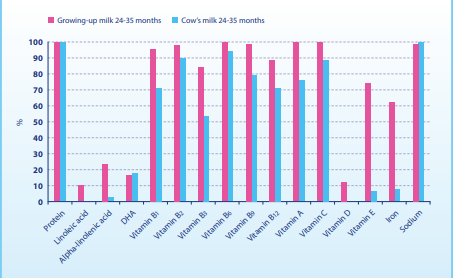
Figure 2: Adherence to EFSA recommendations when consuming growing-up milk vs. cow’s milk or dairy products at the age 24–35 months [37].
Conclusion
Early life malnutrition in terms of both macro and micronutrients can cause metabolic derangements which could impair or delay the physical and cognitive development of the individual. Deficiencies should be promptly identified to tailor interventional strategies specifically to the metabolic needs of the individual [38,39].
Nutritional biomarkers are useful for assessing nutrient intake, but they have limitations in terms of accuracy. Just combining anamnesis, clinical evaluation, growth, and dietary assessment, we can effectively identify and address potential micronutrient deficiencies in children during the first 1000 days of life. Overall, most micronutrient deficiencies can be prevented during the first 1000 days of life through nutrition guidance, food fortification, or supplementation. And the role of GUM over the fresh cow milk in terms of preventing micronutrients deficiencies specially Vit D, Iron, Omega 3 can't be neglected and should be considered. Timely supplementation can provide a lifelong advantageous impact on child development. contributing to inadequate intake of micronutrients [40].
In conclusion, nutrition in the first 1000 days of life through correct, specific, and precise guidance could help prevent mainly nutrition deficiencies over time.
Funding: This research received no external funding.
Institutional Review Board Statement: Not applicable.
Informed Consent Statement: Not applicable.
Data Availability Statement: Not applicable.
Acknowledgments: The authors would like to acknowledge the use of Bio render for the conception of the figures.
Conflicts of Interest: No conflict of interest
References
- Baltuska-Turkan K, Korczak R, Hartell B, et al. Nutritional gaps and supplementation in the first 1000 days. Nutrients, 2019; 11(12): 1–50. https://doi.org/10.3390/nu11122891.
- Namazova Baranova L, Pettoello-Mantovani M, Ehrich J. IS-007 European Paediatric Association Archives of Disease in Childhood, 2014; 99: A2–A3.
- Pietrobelli A, Agosti M. Nutrition in the first 1000 days: ten practices to minimize obesity emerging from Published Science. Int J Environ Res Public Health, 2017. https://doi.org/10.3390/ijerph14121491.
- Pietrobelli A, Agosti M, Zuccotti G. Putting the barker theory into the future: time to act on preventing pediatric obesity. Int J Environ Res Public Health, 2016. https://doi.org/10.3390/ijerph13111151.
- Larqué E, Labayen I, Flodmark C-E, et al. From conception to infancy - early risk factors for childhood obesity. Nat Rev Endocrinol, 2019; 15(8): 456–478. https://doi.org/10.1038/s41574-019-0219-1.
- Bailey RL, West KP, Black RE. The epidemiology of global micronutrient deficiencies. Ann Nutr Metab, 2015; 66: 22–33. https://doi.org/10.1159/000371618.
- Mattei D, Pietrobelli A. Micronutrients and brain development. Curr Nutr Rep, 2019; 8(2): 99–107. https://doi.org/10.1007/s13668-019-0268-z.
- Lockyer F, McCann S, Moore SE. Breast milk micronutrients and infant neurodevelopmental outcomes: a systematic review. Nutrients, 2021; 13(11): 1–16. https://doi.org/10.3390/nu13113848. Further studies are required to inform policy recommendations and practice and maximise the benefits of exclusive breastfeeding for the neurocognitive development of all infants.
- Irvine N, England-Mason G, Field CJ, Dewey D, Aghajafari F. Prenatal folate and choline levels and brain and cognitive development in children: a critical narrative review. Nutrients, 2022; 14(2): 1–22. https://doi.org/10.3390/nu14020364. Maternal folate and choline concentrations during pregnancy may play a role in children’s cognitive development.
- Picone S, Ritieni A, Fabiano A, et al. Lutein levels in arterial cord blood correlate with neuroprotein activin A in healthy preterm and term newborns: A trophic role for lutein? Clin Biochem, 2018; 52: 80–84. https://doi.org/10.1016/j.clinbiochem.2017.11.017.
- Lindbergh CA, Mewborn CM, Hammond BR, Renzi-Hammond LM, Curran-Celentano JM, Miller LS. Relationship of lutein and zeaxanthin levels to neurocognitive functioning: an fMRI study of older adults. J Int Neuropsychol Soc, 2017; 23(1): 11–22. https://doi.org/10.1017/S1355617716000850.
- Gruszecki WI, Strzałka K. Carotenoids as modulators of lipid membrane physical properties. Biochim Biophys Acta, 2005; 1740(2): 108–115. https://doi.org/10.1016/j.bbadis.2004.11.015.
- Wallace TC, Blusztajn JK, Caudill MA, et al. Choline: the underconsumed and underappreciated essential nutrient. Nutr Today, 2018; 53(6): 240–253. https://doi.org/10.1097/NT.0000000000000302.
- Viswanathan M, Treiman KA, Kish-Doto J, Middleton JC, Coker-Schwimmer EJL, Nicholson WK. Folic acid supplementation for the prevention of neural tube defects: updated evidence reports and systematic review for the US preventive services task force. JAMA, 2017; 317(2): 190–203. https://doi.org/10.1001/jama.2016.19193.
- Elmadfa I, Meyer AL. Vitamins for the first 1000 days: preparing for life. Int J Vitam Nutr Res Int Zeitschrift fur Vitamin- und Ernahrungsforschung J Int Vitaminol Nutr, 2012; 82(5): 342–347. https://doi.org/10.1024/0300-9831/a000129.
- Rogne T, Tielemans MJ, Chong MF-F, et al. Associations of maternal vitamin B12 concentration in pregnancy with the risks of preterm birth and low birth weight: a systematic review and meta-analysis of individual participant data. Am J Epidemiol, 2017; 185(3): 212–223. https://doi.org/10.1093/aje/kww212.
- Chittimoju SB, Pearce EN. Iodine deficiency and supplementation in pregnancy. Clin Obstet Gynecol, 2019; 62(2): 330–338. https://doi.org/10.1097/GRF.0000000000000428.
- Levie D, Korevaar TIM, Bath SC, et al. Association of maternal iodine status with child IQ: a meta-analysis of individual participant data. J Clin Endocrinol Metab, 2019; 104(12): 5957–5967. https://doi.org/10.1210/jc.2018-02559.
- Beard JL. Why iron deficiency is important in infant development. J Nutr, 2008; 138(12): 2534–2536. https://doi.org/10.1093/jn/138.12.2534.
- Greminger AR, Lee DL, Shrager P, Mayer-Pröschel M. Gestational iron deficiency differentially alters the structure and function of white and gray matter brain regions of developing rats. J Nutr, 2014; 144(7): 1058–1066. https://doi.org/10.3945/jn.113.187732.
- Zittermann A, Pilz S, Berthold HK. Serum 25-hydroxyvitamin D response to vitamin D supplementation in infants: a systematic review and meta-analysis of clinical intervention trials. Eur J Nutr, 2020; 59(1): 359–369. https://doi.org/10.1007/s00394-019-01912-x. Vitamin D supplementation of 400 IU/day is sufficient for achieving 25OHD concentrations able to prevent nutritional rickets.
- Saggese G, Vierucci F, Prodam F, et al. Vitamin D in pediatric age: consensus of the Italian Pediatric Society and the Italian Society of Preventive and Social Pediatrics, jointly with the Italian Federation of Pediatricians. Ital J Pediatr, 2018; 44(1): 1–40. https://doi.org/10.1186/s13052-018-0488-7.
- Ackland ML, Michalczyk AA. Zinc and infant nutrition. Arch Biochem Biophys, 2016; 611: 51–57. https://doi.org/10.1016/j.abb.2016.06.011.
- Keen CL, Uriu-Hare JY, Hawk SN, et al. Effect of copper deficiency on prenatal development and pregnancy outcome. Am J Clin Nutr, 1998; 67(5 Suppl): 1003S-1011S. https://doi.org/10.1093/ajcn/67.5.1003S.
- Dolk HM, Nau H, Hummler H, Barlow SM. Dietary vitamin A and teratogenic risk: European Teratology Society discussion paper. Eur J Obstet Gynecol Reprod Biol, 1999; 83(1): 31–36. https://doi.org/10.1016/s0301-2115(98)00228-0.
- Guideline: Protecting, Promoting and Supporting Breastfeeding in Facilities Providing Maternity and Newborn Services. Geneva: World Health Organization, 2017.
- Piccoli GB, Clari R, Vigotti FN, et al. Vegan-vegetarian diets in pregnancy: danger or panacea? A systematic narrative review BJOG, 2015; 122(5): 623–633. https://doi.org/10.1111/1471-0528.13280.
- Chouraqui JP. Risk assessment of micronutrients deficiency in vegetarian or vegan children: not so obvious. Nutrients, 2023; 15(9): 2129. https://doi.org/10.3390/nu15092129. Diets that are more restrictive in animal source foods, such as vegan diets, have a greater likelihood of nutritional deficiencies; vegan and macrobiotic diets should be avoided during pregnancy and childhood.
- Chouraqui JP. Risk assessment of micronutrients deficiency in vegetarian or vegan children: not so obvious. Nutrients, 2023; 15(9): 2129. 10.3390/nu15092129. Diets that are more restrictive in animal source foods, such as vegan diets, have a greater likelihood of nutritional deficiencies; vegan and macrobiotic diets should be avoided during pregnancy and childhood.
- Kiely ME. Risks and benefits of vegan and vegetarian diets in children. Proc Nutr Soc, 2021; 80(2): 159–164. doi: 10.1017/S002966512100001X.
- Mariotti F, Gardner CD. Dietary protein and amino acids in vegetarian diets—a review. Nutrients, 2019; 11(11): 1–19. doi: 10.3390/nu11112661.
- Simeone G, Bergamini M, Verga MC, et al. Do vegetarian diets provide adequate nutrient intake during complementary feeding? A systematic review. Nutrients, 2020; 14(17): 1–23. doi: 10.3390/nu14173591.
- Melina V, Craig W, Levin S. Position of the academy of nutrition and dietetics: vegetarian diets. J Acad Nutr Diet, 2016; 116(12): 1970–1980. doi: 10.1016/j.jand.2016.09.025.
- Fewtrell M, Bronsky J, Campoy C, et al. Complementary feeding: a position paper by the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) Committee on Nutrition. J Pediatr Gastroenterol Nutr, 2017; 64(1): 119–132. doi: 10.1097/MPG.0000000000001454.
- Redecilla Ferreiro S, Moráis López A, Moreno Villares JM. Position paper on vegetarian diets in infants and children. Committee on Nutrition and Breastfeeding of the Spanish Paediatric Association. An Pediatr, 2020; 92(5): 306.e1–306.e6. 10.1016/j.anpedi.2019.10.013.
- Hower J, et al. Vitamin D fortification of growing up milk prevents decrease of serum 25-hydroxyvitamin D concentrations during winter: a clinical intervention study in Germany. Eur J Pediatr, 2013; 172(12): 1597–1605.
- Chouraquai J-P, et al. The Role of Young Child Formula in Ensuring a Balanced Diet in Young Children (1–3 Years Old). Nutrients, 2019; 11(9): 2213.
- Mayneris-Perxachs J, Swann JR. Metabolic phenotyping of malnutrition during the first 1000 days of life. Eur J Nutr, 2019; 58(3): 909–930. https:// doi. org/ 10. 1007/ s00394- 018- 1679-0.
- Societa Italiana di Nutrizione Umana. LARN: Livelli di Assunzione di Riferimento di Nutrienti ed energia per la popolazione italiana, IV revision of Dietary Reference Intake for the Italian Population (LARN 2014). Milan: SICS Editore.
- De Giorgis V, Tagliabue A, Bisulli F, Brambilla I, CameriniA, Cusmai R, et al. Ketogenic dietarytherapies in epilepsy: recommendations of the Italian Leagueagainst Epilepsy Dietary Therapy Study Group. Front Neurol, 2023; 14: 1215618. https://doi.org/10. 3389/fneur.

