The Intersection of COVID-19 and Reproductive Health: A Literature Review on Male Fertility Challenges
Mahmoud Alafifi1,2, Mouad El Badr1,2,*, Adil Kbirou1,2, Amine Moataz1,2, Mohamed Dakir1,2, Adil Debbagh1,2 and Rachid Aboutaieb1,2
1Department of Urology, University Hospital Center IbnRochd Casablanca, Morocco
2Faculty of Medicine and Pharmacy of Casablanca, Morocco
Received Date: 24/03/2024; Published Date: 10/09/2024
*Corresponding author: Mouad El Badr, Department of Urology, University Hospital Center IbnRochd Casablanca, Morocco; Faculty of Medicine and Pharmacy of Casablanca, Morocco
Abstract
Objective: To highlight the impact of viral infections including COVID-19 as well as the need for an evaluation of reproductive function in patients carrying COVID-19.
Materiel and Methods: It is a literature review, narrative and non-systematic of articles published in French and English on the impact of viral infections including Covid-19 on reproductive function.
Results: The 2019 new coronavirus (COVID-19) pandemic was a public health emergency with an enormous impact on human health and life. COVID-19 has a respiratory tropism known by the damage of several other organs; as one can even have a reproductive health problem in humans by impaired reproductive function. Several studies have objectified and demonstrated the presence of viruses in sperm as well as their great durability in this fluid compared to other biological fluids. Also, other studies have confirmed the absence of SARS-CoV in human sperm in the same way as the absence of evidence of expression of ACE2 and TMPRSS2 in terms of expression of the RNA virus. However, more in-depth studies are essential to highlight the impact of Sars-Cov-2 on human reproductive function.
Conclusion: Given this, several more in-depth studies should be essential to measure the short and long term effects of SARS-CoV-2 infection on the human reproductive system.
Keywords: COVID-19; Coronavirus; Viral orchitis; Infertility
Introduction
Male infertility accounts for 50% of infertility cases and affects approximately 15% of couples worldwide, with inflammation and infectious origins being key etiological factors [1,2]. Viral infections have been identified as significant contributors to male reproductive system damage, with viruses like HIV, HBV, MuV, SARS-CoV, and SARS-CoV-2 showing negative effects [2,3].
SARS-CoV-2, the virus responsible for the COVID-19 pandemic since December 2019, has garnered global attention due to its rapid spread and diverse impact on various bodily systems beyond the respiratory tract [4]. Of particular concern is the high expression of ACE2 receptors, crucial for viral entry, in testicular cells, raising questions about potential impacts on male reproductive health and fertility [3,5]. Reports of orchitis in deceased COVID-19 patients and the presence of the virus in seminal fluid further highlight these concerns [2,6].
This literature review aims to delve into the repercussions of SARS-CoV-2 infection on male reproductive health, encompassing aspects such as reproductive system functionality, gametes, and male gonadal function [1,5]. Multiple studies have highlighted the elevated ACE2 expression in various testicular cell types, indicating a susceptibility to direct viral damage [3,5].
Given the known vulnerabilities of the blood-testes barrier to viral invasion, as evidenced by previous cases involving viruses like HIV and hepatitis causing testicular damage and infertility, understanding the potential impact of SARS-CoV-2 on male fertility is crucial [3,7]. This review will explore both the direct effects of the virus on reproductive tissues and the secondary effects stemming from immunological and inflammatory responses [1,6].
Materiel and Methods
Define Research Objectives: Review literature in both French and English languages on the impact of viral infections, with a focus on COVID-19, on reproductive function.
Explore how viral infections, including COVID-19, affect various aspects of reproductive health, such as fertility, gamete function, and hormonal regulation.
Search Strategy: Utilize databases that include French and English articles, such as PubMed, Google Scholar, and specific French-language databases.
Incorporate keywords related to viral infections ("viral infections," "COVID-19 impact on reproductive function," "viral effects on fertility") and reproductive health ("reproductive function," "fertility," "gamete function").
Include additional terms specific to COVID-19 ("COVID-19," "SARS-CoV-2," "coronavirus impact on fertility").
Inclusion and Exclusion Criteria: Include peer-reviewed articles and scholarly literature published in French and English.
Focus on articles that discuss the impact of viral infections, including COVID-19, on reproductive function, fertility, gamete function, and hormonal regulation.
Exclude non-peer-reviewed sources, opinion pieces, and studies not directly addressing the impact of viral infections on reproductive health.
Data Collection and Analysis: Extract relevant data from selected articles, including study objectives, methodologies, key findings related to the impact of viral infections on reproductive function, and specific effects of COVID-19 on fertility and gamete function.
Analyze the collected data to identify patterns, trends, and notable findings across the reviewed literature.
Quality Assessment: Evaluate the quality of included studies based on study design, methodology, sample size, and statistical analysis.
Consider the credibility and reliability of the findings to ensure the validity of the review.
Synthesis and Conclusion: Synthesize the findings from the reviewed literature to provide a narrative overview of the impact of viral infections, including COVID-19, on reproductive function.
Discuss the implications of these findings for understanding viral effects on fertility, gamete function, hormonal regulation, and potential implications for clinical practice and public health.
Results
The 2019 novel coronavirus (COVID-19) pandemic emerged as a significant public health crisis, profoundly impacting human health and well-being. Beyond its well-documented respiratory tropism, COVID-19 has raised concerns about potential effects on various organ systems, including reproductive health. Studies have increasingly focused on investigating the presence of viruses in sperm and their implications for human reproductive function. Notably, recent research has revealed the presence of viruses in sperm and highlighted their extended durability in seminal fluid compared to other biological fluids. However, conflicting findings exist, with some studies reporting the absence of SARS-CoV-2 in human sperm and lacking evidence of ACE2 and TMPRSS2 expression, essential receptors for viral entry and replication.
Despite these initial findings, the impact of SARS-CoV-2 on human reproductive function remains a topic of ongoing research and debate. While some studies suggest a potential risk of impaired reproductive function due to viral presence and persistence in seminal fluid, others emphasize the need for more comprehensive and in-depth investigations. Further studies are essential to elucidate the mechanisms underlying the potential impact of SARS-CoV-2 on human reproductive health, including its effects on gamete function, hormonal regulation, and fertility outcomes.
Methodologically robust studies, incorporating advanced techniques such as viral detection assays, RNA expression analyses, and longitudinal assessments of reproductive parameters, are warranted to provide a clearer understanding of the relationship between COVID-19 and reproductive function. Moreover, consideration of confounding factors such as comorbidities, medications, and immune responses is crucial in interpreting study findings and determining the true extent of any reproductive health implications.
The findings from this literature review underscore the complexity of the interplay between viral infections, such as COVID-19, and human reproductive health. While initial evidence suggests the potential presence of the virus in seminal fluid and its persistence, more rigorous and comprehensive studies are needed to establish causal relationships, assess long-term effects, and inform clinical practice and public health interventions. Collaborative efforts among researchers, clinicians, and public health authorities are paramount in advancing our understanding of COVID-19's impact on reproductive function and ensuring evidence-based strategies for mitigating potential risks and addressing fertility-related concerns in affected individuals.
Discussion
The possibility that COVID-19 virions might interact with human spermatozoa and disrupt male fertility. This prediction was based upon a proteomic analysis of these cells, indicating that they possess the entire repertoire of receptors needed to support angiotensin signaling, including ACE2—the cellular entry point for coronavirus infection. Because the angiotensin system is known to play such an important role in the maintenance of sperm viability and function, it was proposed that contact with the virus would have adverse reproductive consequences for infected males. It was also suggested that active COVID-19 infection dramatically reduced the testosterone-to-LH ratio, suggesting a significant impact on the responsiveness of Leydig cells to LH stimulation. [5,8]
The novel corona viruses belong to Coronaviridae family that are enveloped viruses with a positive-sense single-strand RNA of around 32 kb. The viral molecules comprise 4 main structural proteins including the spike, membrane, envelope protein, and nucleocapsid. The spike protein protrudes from the envelope of the virion that described to play a critical role in the receptor host selectivity and cellular adhesion. Recent studies reported that SARS-CoV and SARS-CoV-2 spike proteins interacted with angiotensin-converting enzyme 2 (ACE-2). In addition, other cellular receptors also reported to play a secondary role in the viral adhesion such as the C-type lectin CD209L and DC-SIGN binds to SARS-CoV. Nevertheless, ACE-2 appears to be the vital functional receptor for the SARS-CoV and probably for SARS-CoV-2. The interaction between the viral protein and its receptor in the cell membrane is a key process in the replication cycle. Moreover, the efficiency of viral infection is significantly dependent on this process [3].
The main pathological mechanism of cellular damage and direct cell infection is through binding to ACE2 receptors and invasion of cells. High ACE2 expression in testis makes testicles and spermatogenesis the potential target sites of the virus, so the virus is assumed to be a potential risk for infertility [4].
Other entry factors have also been found to be expressed throughout male reproductive tissues. CD147 receptors are expressed in all testicular cells, with maximum expression in differentiating gametocytes . Endosomal cysteine proteases such as CTSB and CTSL are abundant in Leydig cells, Sertoli cells, seminal vesicles, the epididymis, and germ cells and participate in the priming of S protein . Expression of TMPRSS2, an important serine protease needed for viral entry into the host, is also evident in primordial germ cells and the acrosomal region of developing spermatogonia . Finally as per the human protein atlas, neuropilin-1 (NRP-1) is expressed in Leydig cells and seminal vesicles [9].
The highest expression of ACE-2 was reported in 30-year-old patients, which is higher than those in their twenties, while 60-year-old patients showed the lowest level of expression of ACE-2. This may suggest that young male patients are at higher risk of testicular dysfunction by COVID-19 infection than older male patients. The severity and mortality rates of COVID-19 progression varied by sex, which may be related to the influence of hormones on the body’s response to SARS-CoV-2 infection [3,10].
Table 1 : Expression of ACE-2 in various human tissues.

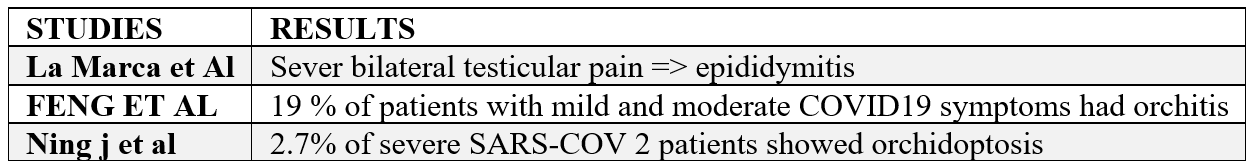
Following the epidemic of SARS-CoV infection in 2002, orchitis was recognized as a complication of SARS. The possible damage to testicles impairs spermatogenesis and extensive spermatogenic cell disruption in SARS. As an abnormal manifestation, La Marca et al. reported severe bilateral testicular pain in the scrotum and inguinal lymph nodes in a 43-year-old man with low-grade fever and delayed onset of dyspnea and other symptoms, related to SARS-COV-2. But, no redness and swelling were reported. They postulated that testicular pain could be related to epididymitis following SARS-COV-2 infection. Similarly, Feng et al. reported that among 6 patients who displayed mild to moderate symptoms of SARS-COV-2 (19%) reported scrotal discomfort and confirmed viral orchitis . In another study, 2.7% of severe SARS-COV-2 patients showed orchidoptosis [4].
Table 2: Results of different studies showing the link between covid 19 and testicular damage.
It was also speculated that infection would lead to a series of detrimental changes in the male tract associated with the creation of significant oxidative stress. These changes include the generation of reactive oxygen species (ROS) by the sperm mitochondria as these cells engage the intrinsic apoptotic pathway ; an increase in local ROS generation as a result of sperm phagocytosis by leukocytes responding to elevated levels of angiotensin II ; and oxidative stress created by the proinflammatory cytokine storm that accompanies COVID-19 infection. The suggested involvement of oxidative stress in mediating the impact of COVID-19 on male reproductive competence has led Marin et al. to advocate the potential benefits of antioxidant (astaxanthin) treatment in the accompanying Letter to the Editor [8].
Most semen studies have involved recovering patients and have found that after 1 month, recovering patients did not have detectable levels of SARS-CoV-2 in their semen . Thus, Temiz et al. could detect a deterioration of sperm morphology following infection, while Ruan et al. found that no SARS-CoV 2 RNA was detected in any of the patients, however, sperm concentration count and motility were reduced [8,11].
By contrast, Gacci et al. concluded that 25% (11/43) of men recovering from COVID-19 infection exhibited a severe disruption of their semen profile, with a majority (8/11) being azoospermic, the degree of spermatogenic disruption being significantly correlated with disease severity. In another small study, Li et al. found that 39% (9/23) of recovering COVID patients were suffering from oligozoospermia, while their semen profile was frequently characterized by elevated levels of leukocytic infiltration [8].
In addition, Erbayand et al. reported the results of sperm tests performed both before and after infection with SARS-CoV-2. Moderately symptomatic individuals had significantly lower total viability and progressive motility results. In the moderately symptomatic group, overall sperm parameters, including sperm volume, were reduced considerably . Twenty-four male COVID-19 survivors were also analyzed by Pazir et al. .Both total sperm motility and total sperm volume were statistically significantly reduced after COVID-19 [10].
Table 3: Different studies showing semen profile damage post SARS-COV 2.

Induction of LH and T production is necessary for spermatogenesis.
Any damage to Sertoli or spermatogenic cells may cause spermatogenic failure and lead to male infertility. Spermatogenesis and androgen secretion are two major roles of the testis [4].
It is suspected that SARS-CoV-2 causes low levels of LH, follicle-stimulating hormone (FSH), and T by triggering inflammatory responses that impair the normal function of the hypothalamic–pituitary–testicular (HPT) axis. In COVID-19 patients, the ratio of T to LH and FSH to LH was significantly reduced, but T levels were comparable. This may be the first definitive evidence that COVID-19 affects testicular sex hormone production. Still, it should be followed up with a more in-depth study of seminal fluid from COVID-19 patients to determine the effects of the virus on sperm quality. There are discrepancies with this notion, however, as recent reports have shown that COVID-19 patients have lower blood T levels, higher LH levels, and lower T to LH ratio than healthy men [10].
The evidence on the effect of SARSCoV- 2 on male sex hormones among young patients that had recovered from SARS-CoV-2 infection. Although serum T did not statistically change, they confirmed that male gonadal function may be impaired through the SARS-CoV-2 infection because a significant increase in serum LH level was observed, which decreased the T/LH ratio and FSH/LH ratio in patients that had recovered, compared to their healthy counterparts [4].
Several studies examined sex hormone levels in COVID-19 patients during recovery and compared them to sex hormone levels during hospitalization or before infection. A study by Karkin et al. found that TT levels were higher and LH levels were lower before COVID-19, while the difference in FSH levels was not statistically significant [10].
Weijie et al. showed that a lower T:LH ratio is correlated with greater AST, CRP, and AMH levels, however only CRP is significantly correlated. Produced by the liver in the acute phase of inflammation throughout the body, a rapid increase in CRP levels were found to occur in severe cases, compared to non-severe cases of SARS-CoV-2. In SARS-CoV-2, elevated CRP levels are accompanied by the production of abnormal cytokines such as interferon, which influences the function of the testes and spermatogenesis . In the early stages of SARS-Cov-2 infection, the CRP levels are positively correlated with lung lesions with demonstrated severity of the condition which is used as a key indicator for disease monitoring. Also, Giulia and
associates reported that in SARS-CoV-2 recovered patients, total testosterone (TT) level was negatively associated with the CRP level [4].
Fevers are the body’s response to systemic inflammation, and over 80% of COVID-19 patients are reported to develop a fever, which tends to be prolonged with an average duration of 10 days [11].
It’s characterized by an oral temperature of 37.7°C and above. Body temperature in a healthy person ranges from 36.1°C to 37.2 and is crucial to maintain the normal physiology of the body. Disruption of thermal homeostasis impairs the proper activity of organs, including those involved in reproductive biology.. Slight changes in testicular temperature can cause incidences of morphological abnormality in developing spermatozoa and spermatids. Additional body temperature increases also curtail the sperm-storage capacity of the cauda epididymidis [9].
Furthermore, it has been reported that a fever of > 39°C for over 3 days can lead to significant reduction in semen concentration and motility. Fever of up to 41.2 °C for 10 days was sufficient enough to impair spermatogenesis in typhoid patients, leading to an azoospermic state [9,11].
Excess temperature could also invite germ cell apoptosis , and Leydig cells are vulnerable to morphological deterioration following exposure to high temperature . Finally, hypothermic states promote the death of Sertoli cells, which might hamper sperm quality and fertility [9].
Conclusion
The COVID-19 pandemic is an ongoing biological disaster affecting the human population through multiple avenues. Reproductive organs are sensitive to pathogenic insult, and hence might be targeted by SARS-CoV-2 infection. SARS-CoV-2-induced ACE-2 shedding, cytokine storm, oxidative stress, and elevated body temperature are all potential threats to reproductive health and physiology
While much is left to be studied, COVID-19 does appear to impact male fertility, at least temporarily. From review of the current literature, it has become evident that COVID-19 can lead to a reduction in testosterone production and a state of temporary hypogonadism . It was originally hypothesized that since the testes are prone to direct infection by the SARS-CoV-2 virus due to their ACE2 expression, male fertility is adversely affected. Although controversy remains, the data supports that a reduction in testosterone production is more likely associated with indirect testicular damage due to systemic or local inflammation. Additionally, with the rapidly evolving pandemic, it is important to maintain vigilance for potential interventions and delays of care that can lead to lasting effects on fertility .
Due to the presence of entry factors for SARS-CoV-2 in various reproductive tissues, virions can invade reproductive organs and might disrupt the fertility efficiency of couples planning to conceive in the near future. Orchitis, low sperm quality and oligozoospermia, are even evident in some COVID-19 cases.
References
- Fijak M, et al. Infectious, inflammatory, and 'autoimmune' male factor infertility: How do rodent models inform clinical practice? Human Reproduction Update, 2018; 24(4): 416–441.
- Weihua Liu, et al. «Viral threat to male fertility». Institute of Basic Medical Sciences, School of Basic Medicine, Chinese Academy of Medical Sciences, Peking Union Medical College, Beijing, China. DOI: 10.1111/and.13140
- Malki MI. COVID-19 and male infertility: An overview of the disease. Medicine (Baltimore), 2022; 101(27): e29401.
- Moshrefi M, et al. The probable destructive mechanisms behind COVID-19 on the male reproductive system and fertility. J Assist Reprod Genet, 2021; 38: 1691–1708.
- Aitken RJ. COVID-19 and human spermatozoa—Potential risks for infertility and sexual transmission? Andrology, 2021; 9: 48–52.
- Sun J, et al. Isolation of infectious SARS-CoV-2 from the urine of a COVID-19 patient. State Key Laboratory of Respiratory Disease, Republic of China. Emerging Microbes & Infections, 2020; 9(1): 991-993. DOI: 10.1080/22221751.2020.1760144.
- Feng Pan, et al. «Aucune preuve de SRAS-CoV-2 dans le sperme de mâles se remettant de COVID-19». Département d'urologie, Union Hospital, Tongji Medical College, Huazhong University of Science et technologie, Wuhan 430022, Chine. Fertilité et stérilité, 2020. doi:https://doi.org/10.1016/j.fertnstert.2020.04.024.
- Aitken RJ. COVID-19 and male infertility: An update. Andrology, 2022; 10: 8-10.
- Prem Rajak, Sumedha Roy, Moumita Dutta, Sayanti Podder, Saurabh Sarkar, Abhratanu Ganguly, et al. Understanding the cross-talk between mediators of infertility and COVID19, Reproductive Biology, 2021; 21(4): 100559.
- Kalfas T, Kaltsas A, Symeonidis EN, Symeonidis A, Zikopoulos A, Moustakli E, et al. COVID-19 and Male Infertility: Is There a Role for Antioxidants? Antioxidants, 2023; 12(8): 1483.
- Alexander B Collins, Lei Zhao, Ziwen Zhu, Nathan T Givens, Qian Bai, Mark R Wakefield, et al. Impact of COVID-19 on Male Fertility, Urology, 2022; 164: Pages 33-39.

