Advancements in Ascites Management: A Comprehensive Narrative Review of the Alfa Pump System and its Role in Improving Patient Outcomes
Vikash Kumar Karmani1,*, Nabiha Naqvi2, Shahzeb Rehman1, Aima Tahir3, Asma Yasin4, Minha Aslam4 and Farhan Khan5
1Department of Internal Medicine, Jinnah Sindh Medical University, Karachi, Sindh, Pakistan
2Department of Internal Medicine, Federal Medical and Dental College, Islamabad Pakistan
3Department of Internal Medicine, Women Medical and Dental College, Abbottabad, Khyber Pakhtunkhwa, Pakistan
4Department of Internal Medicine, Dow University of Health Science, Karachi, Sindh, Pakistan
5Department of Internal Medicine, Liaquat University of Medical and Health Sciences, Jamshoro, Sindh, Pakistan
Received Date: 09/03/2024; Published Date: 22/07/2024
*Corresponding author: Vikash Kumar Karmani, Department of Internal Medicine, Jinnah Sindh Medical University, Karachi, Sindh, Pakistan
Abstract
Ascites remains a significant challenge in patients with cirrhosis, posing difficulties in management and affecting prognosis. This review examines the current understanding of ascites, including its underlying mechanisms, symptoms, and treatment options, with a specific focus on the innovative Alfa Pump device. The review begins by discussing traditional approaches to managing ascites, while also addressing their limitations and potential complications. It then explores the emergence of the Alfa Pump system, a novel implantable device designed to tackle refractory ascites by continuously draining fluid from the abdomen while minimizing circulatory issues. Through a synthesis of current literature and clinical evidence, this narrative review underscores the importance of a multidisciplinary approach in the management of ascites, with a particular emphasis on the evolving role of the alfapump in improving outcomes and quality of life for patients with refractory ascites.
Keywords: Ascites; Alfa pump; Diuretics; TIPs
Introduction
Ascites comes from a Greek word ‘askos’, meaning a bag or a sac [1]. It is the pathological condition in which there is an accumulation of fluid in the peritoneal cavity of the abdomen [2]. In general, a fluid built-up of 25 milliliters (about 0.85 oz) is considered as having ascites [3]. If severe, it can lead to painful breathing and restricted movement [4]. Ascites is the most common complication of cirrhosis. It manifests in about 50% of patients with cirrhosis [2,5] and has a mortality rate of around 50% over a three-year period [6]. The prognosis is notably poor for refractory ascites, with a survival rate of less than 50% within one year.
In general, males have minimal intraperitoneal fluid, while females typically have around 20 mL, influenced by the phase of their menstrual cycle [2].
Causes
Ascites has two primary categories of causes. Portal hypertension, indicated by a SAAG ratio >1.1, leads to conditions like liver cirrhosis, hepatic congestion, alcoholic hepatitis, fulminant hepatic failure, and massive hepatic metastases. Non-hepatic causes include congestive heart failure, constrictive pericarditis, tricuspid insufficiency, and Budd-Chiari syndrome. Non-portal hypertension causes, with a SAAG ratio <1.1, involve hypoalbuminemia, seen in nephrotic syndrome, protein-losing enteropathy, and severe malnutrition with anasarca. Infections like bacterial peritonitis, tuberculous peritonitis, fungal peritonitis, and HIV-associated peritonitis also contribute, along with malignant conditions such as peritoneal carcinomatosis, primary mesothelioma, pseudomyxoma peritonei, and hepatocellular carcinoma. Other conditions like chylous [7] ascites, pancreatic ascites, nephrogenic ascites, urine ascites, ovarian diseases, familial Mediterranean fever, vasculitis, granulomatous peritonitis, and eosinophilic peritonitis are additional factors. Contributing lifestyle and health factors include viral hepatitis, alcohol use disorder, intravenous drug use, type 2 diabetes mellitus, and hypercholesterolemia [1,3].
Pathophysiology
Ascites pathophysiology involves three interrelated theories [8]. The underfilling theory suggests that portal hypertension induces increased fluid filtration from abdominal and liver blood vessels, reducing circulating blood volume and activating systems like renin and aldosterone, causing kidney retention of sodium and water. The overflow theory posits sodium retention without decreased blood volume, attributed to reduced substances promoting sodium excretion or impaired liver capacity to remove sodium-retaining agents, leading to fluid accumulation in blood vessels [8,9]. The arterial vasodilation theory (Figure 1) combines elements of both theories, proposing that vasodilation reduces blood volume, triggering sodium retention and increased plasma volume, resulting in overflow into the peritoneal cavity. An updated version, the forward theory (Figure 2), integrates underfilling with increased splanchnic capillary pressure, filtration rate, and elevated lymph formation as contributors to ascites [8,10]. Excessive vasodilators exacerbate ascites, while reduced atrial natriuretic peptide response leads to sodium accumulation in advanced cirrhosis stages [11-14].
Manifestation
Signs and symptoms of ascites vary based on the etiology. In general, patients present with abdominal distension and discomfort accompanied by weight gain, early satiety, dyspnea, and shortness of breath [2]. Patients develop fever, abdominal tenderness, and confusion in case of bacterial infection. Malignant ascites may present with generalized abdominal pain and weight loss, shifting dullness, and tender abdomen upon examination. peritoneal carcinoma can lead to a Sister Mary Joseph nodule that can be palpated [15] and in case of upper abdominal malignancy virchow node would be present [1]. Patients with ascites due to heart failure present with dyspnea and orthopnea alongside peripheral edema, jugular venous congestion, and chest crepitations on examination. Patients with chylous ascites complain of diarrhea accompanied by steatorrhea, night sweats, fever, nausea, and early satiety alongside a malnourished appearance with edema and enlarged lymph nodes [16]. Hepatic diseases, cirrhosis, in particular, are one of the leading causes of ascites. Patients are often jaundiced with spider angiomata and palmar erythema, Muscle wasting, visible abdominal collaterals, and gynecomastia.
Treatment
Ascites can manifest in various forms, and the approach to treatment and management is contingent upon its type, severity [17], and degree of liver failure [18].
Patients with uncomplicated ascites are normally managed with salt restriction, diuretic therapy, and therapeutic paracentesis. Patients may also be referred for liver transplantation in case of liver cirrhosis.
Complicated ascites occurs when one or more complications such as spontaneous bacterial peritonitis, hepatorenal syndrome or hyponatremia occurs. Management should focus on treating these conditions in addition to reducing the ascites [19].
Refractory ascites, according to the International Ascites Club, is defined as ascites that cannot be mobilized or the early recurrence of which cannot be satisfactorily prevented by medical therapy [20]. However, a spectrum of treatment options exists to address this condition. These include well-established approaches such as large volume paracentesis, Transjugular Intrahepatic Portosystemic Shunt (TIPS), peritoneovenous shunt, liver transplantation, and the use of vasopressin receptor antagonists (vaptans). In addition to these established methods, a promising and beneficial novel treatment option has emerged in the form of the Automated Low Flow Ascites (ALFA) pump, offering a new dimension in managing refractory ascites [21,22].
Large-volume paracentesis (LVP) is the first-line treatment of refractory ascites. Plasma volume expansion is needed to prevent post-paracentesis dysfunction [23].
TIPS placement induces decompression of the portal circulation by shunting an intrahepatic portal branch into a hepatic vein [20]. This causes a decrease in portal pressure which [24,25] leads to improvement in systemic hemodynamics and increased effective blood volume, thereby, improving renal perfusion, favoring salt and water excretion as early as four weeks after TIPS insertion [24].
Liver Transplant (LT) should be discussed in all patients with refractory ascites [20]. Following LT, clinical ascites can be present for three to four months. It is therefore crucial that patients continue to adhere to their low-sodium diet post-transplantation until ascites is eliminated [26].
Tolvaptan, a vaptan, is a V2- receptor blocker. It reduces the expression of aquaporin-2 and inhibits water reabsorption in the collecting ducts [27]. Recent studies and meta-analysis show that tolvaptan is effective in the treating refractory cirrhosis particularly in those with underlying liver cirrhosis and hepatitis C. However, further studies need to be carried out to establish greater efficacy of tolvaptan [27,28].
Automated low-flow ascites pump (ALFA pump)
Background:
Alfa pump is a class III medical device that is implanted and designed to facilitate the automated movement of ascitic fluid from the peritoneal space to the urinary bladder, where it is subsequently expelled. The device can be implanted within one hour under general or local anaesthesia, and once implanted, it can be charged wirelessly through a hand-held charger that also allows for the personalized programming and monitoring of the device. The charger is placed positioned over the pump area twice daily for a maximum duration of 20 minutes each time [29-33].
In 1998, Rozenblit and coauthors introduced the initial mechanical device aimed at actively transferring ascites from the peritoneal cavity to the urinary bladder. Despite this, none of these systems has achieved widespread clinical applicability primarily due to technical challenges [33,34]. The inaugural prototypes were developed in 2005, and the founders illustrated the technical viability of the method.
The ALFA pump made its debut in 2011 [35]. The device has also acquired the CE mark, and it is presently accessible in all countries that recognize the specified standard and provide instructions for use in their local languages, including Arabic, Danish, Dutch, English, French, German, Hebrew, Italian, Norwegian, Polish, Spanish, Swedish, and Turkish. A pivotal clinical trial for market introduction is currently in progress in the USA and Canada (POSEIDON; NCT03973866), with an expected completion date in 2024. The device enjoys full reimbursement in Switzerland and holds innovative treatment status (NUB status 1) under specific conditions in Germany. In Israel, reimbursement is available under certain circumstances. In the UK, usage is recommended with special considerations for clinical governance, consent, audit, or research [37,38].
Mechanism:
The alfa pump is an implantable pump system that is designed to move ascitic fluid from the abdominal cavity to the urinary bladder through catheters specially routed into the peritoneal and bladder. Alfa pump removes 500ml to 2.5L per day [35]. Four pressure sensors within the alfa pump monitor abdominal and bladder pressure, offering insights into flow rate and system dynamics. The pump initiates a pumping cycle only when the bladder pressure falls below a specific threshold. Simultaneously, pumping ceases promptly if the peritoneal cavity pressure experiences a significant drop, indicating insufficient accessible fluid for the alfa pump [36] (Figure 3).
The alfa pump employs a gear pump, where fluid is propelled forward between rotating gears to achieve the desired volume. This requires a specific number of motor turns, coupled with motor speed, determining the alfa pump's flow rate. As ascites is transported, it passes through multiple pressure sensors, with changes in their values confirming the active movement of fluid.
The overseeing physician for a patient with an implanted alfa pump utilizes the alfa pump programmer—a computer equipped with Flow Control software. Flow Control allows for the programming of the target daily volume, pumping schedule, frequency, and the ability to toggle the alfa pump on and off [39].
It is important to provide pre- and post- implantation care to the patients. Mostly, the patient undergoes hospitalization 24-48 hours before the implantation procedure. Paracentesis is carried out to confirm the absence of ongoing spontaneous bacterial peritonitis and to drain the abdomen. It is essential to retain 1-2 liters of ascites before implantation to verify the proper functioning of the pump before surgical closure and to reduce the risk of ascitic fluid leakage. Intravenous antibiotic prophylaxis is initiated on the day of implantation and continued for 48 to 72 hours. The consideration of non-selective beta-blockers should also be reassessed in each patient who is pre-selected for alfa pump implantation in accordance with existing guidelines [40,41].
Large-volume paracentesis (LVP) provides only transient alleviation of symptoms associated with ascites. It can also lead to circulatory dysfunction after paracentesis requiring albumin infusions. Moreover, the need for frequent hospital visits associated with LVP results in a diminished quality of life and substantial costs. It had been shown that compared to large volume paracentesis, TIPS resulted in a greater reduction in the ascites volume and the need for repeated paracentesis. However, it was associated with hepatic encephalopathy and cardiac decompensation [42].
Effectiveness:
The alfa pump is frequently regarded as an effective treatment option for refractory ascites in patients who are not suitable candidates for TIPS and LVP, particularly those awaiting liver transplant. Among patients who had underwent alfa pump implantation, 62% no longer required paracentesis, showcasing a noteworthy decrease in the need for this procedure over time (average follow-up time ranging from 6 to 24 months) [43]. Notably, the reduced reliance on paracentesis correlated with an early and sustained enhancement in nutritional status. In the study conducted by Bureau et al., a significant improvement in brachial circumference, tricipital skinfold thickness, and hand grip strength was observed during the initial 3 months following alfa pump placement when compared to the control group [44-46].
Survival outcomes with the alfa pump system have not been explicitly evaluated in the existing published studies. However, a recent meta-analysis indicates that survival is at least comparable to that of patients undergoing large-volume paracentesis (LVP) [47,48]. In a prospective study documenting the real-world, long-term (24-month) use of the alfa pump in 106 patients ineligible for TIPS insertion, the median survival was reported as 10.1 months [49].
Alfa pump insertion is generally safe and successful, thereby, improving nutrition and quality of life of patients [46,50]. 20 -30% of the patients may develop acute kidney injury (AKI), spontaneous bacterial peritonitis (SBP) and/or urinary tract infection (UTI) [29,36,42,50]. There is also an elevated risk of developing electrolyte abnormalities and post-operative bleeding. Additionally, patients may encounter issues related to device malfunction including catheter and pump dysfunction, potentially necessitating re-intervention [29,43,45,52].
Contraindications:
The implantation of the alfa pump device is absolutely contraindicated in cases of loculated ascites, untreatable obstructive uropathy, the presence of an active bacterial infection during implantation (especially spontaneous bacterial peritonitis, urinary infection, or abdominal skin infection), and an expected survival of less than 3 months. It is crucial to exercise special caution with frail patients, and their nutritional status should be thoroughly evaluated and optimized before considering implantation [53].
Cost:
The expenses associated with the alfa pump encompass the device's current cost (EUR22,500), operating room fees, and the costs related to a brief hospital stay [54].
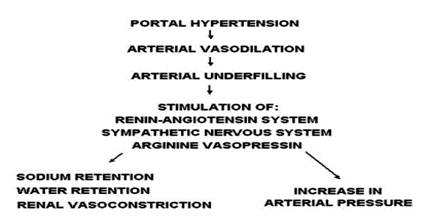
Figure 1: Theory of Arterial Vasodilation.
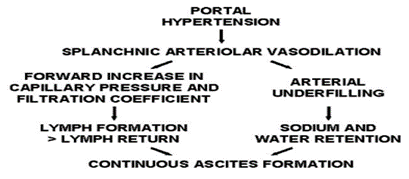
Figure 2: Forward Theory of Ascites Formation.
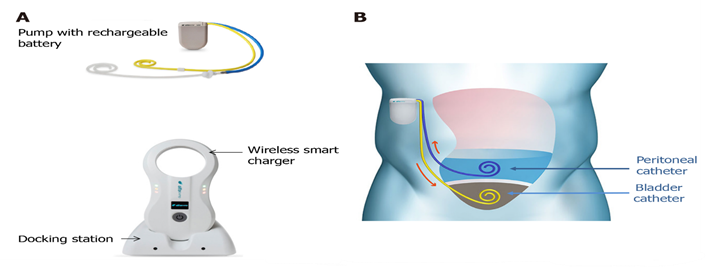
Figure 3: “Alfapump® device and principles of its implantation. A: The system consists of: (1) A pump, which contains a rechargeable battery and is connected to a peritoneal catheter and a bladder catheter; and (2) Charging accessories. The charger collects information and charges the pump through transduction; the docking station must be connected to the electrical network; B: The pump is positioned subcutaneously, under the costal margin (preferably on the right side), so that the patient is not hindered when sitting. The bladder must be full at the time of insertion of the bladder catheter; conversely, only a small amount of ascites is left in place for insertion of the peritoneal catheter, so that the pump can be tested before parietal closure. Images courtesy of Sequana Medical”.
Conclusion
Ascites can present with a wide array of symptoms, from simple to complex cases, thereby offering a spectrum of available treatment options. The newly implemented alfa pump has since shown promise for the treatment of refractory ascites. Its ability to improve fluid management may result in a tailored-treatment approach by resulting in fewer hospital stays and a higher quality of life, all while supporting optimal nutritional status. Additionally, the device's prospective cost savings, even after initial implementation, further boost its appeal as an encompassing ascites management solution. However, it is important to note that despite its emerging success, additional research is needed to effectively evaluate its long-term efficacy and safety in various diverse patient populations. Furthermore, factors including affordability and accessibility might have an impact on how widely the alfa pump may be used in existing healthcare environments. As we conclude, the beneficial aspects highlighted by current findings should be balanced with a commitment to addressing these limitations to allow a thorough and well-informed integration of the alfa pump in clinical practice.
Authors Contribution: All authors contributed to and reviewed the publication, critically revised the manuscript, approved the final version to be published, and agreed to be accountable for all aspects of the work
Conflict of interest: The authors declare no conflict of interest.
Author Declaration: The authors declare that this work is original and backed by scientific research and facts.
Data Availability Statement: All data analyzed during this study are included in the published article.
Funding: The authors have received no funding for this article.
References
- Shah R. Ascites: Background, Pathophysiology, Etiology. eMedicine, 2023.
- Chiejina M, Hrishikesh Samant. Ascites. Nih.gov. StatPearls Publishing, 2019.
- Watson S. What Are Ascites and Paracentesis? WebMD. WebMD, 2016.
- https://www.hopkinsmedicine.org/health/conditions-and-diseases/ascites
- Moore KP, Aithal GP. Guidelines on the management of ascites in cirrhosis. Gut, 2006; 55(suppl_6): vi1–12.
- Wint C. Ascites Causes and Risk Factors. Healthline. Healthline Media, 2015.
- Weniger M, D’Haese JG, Angele MK, Kleespies A, Werner J, Hartwig W. Treatment options for chylous ascites after major abdominal surgery: a systematic review, 2016.
- Amer MO, Elsiesy H. Ascites: Causes, Diagnosis, and Treatment. IntechOpen, 2017.
- Kistler C Andrew. Malignant Ascites: Diagnosis and Management. Cancer Therapy Advisor, 2015.
- Girgrah N. Haemodynamic, renal sodium handling, and neurohormonal effects of acute administration of low dose losartan, an angiotensin II receptor antagonist, in preascitic cirrhosis. Gut, 2000; 46(1): 114–120.
- Arroyo V, Colmenero J. Ascites and hepatorenal syndrome in cirrhosis: pathophysiological basis of therapy and current management. Journal of Hepatology, 2003; 38: 69–89.
- Wiest R. Splanchnic and Systemic Vasodilation. Journal of Clinical Gastroenterology, 2007; 41(Supplement 3): S272–287.
- Iwakiri Y, Groszmann RJ. Vascular endothelial dysfunction in cirrhosis. Journal of Hepatology, 2007; 46(5): 927–934.
- Ferguson JW. Inducible nitric oxide synthase activity contributes to the regulation of peripheral vascular tone in patients with cirrhosis and ascites. Gut, 2006; 55(4): 542–546.
- Gerbes AL, Wernze H, Arendt R, Riedel A, Tilman Sauerbruch, Gustav Paumgartner. Atrial natriuretic factor and renin-aldosterone in volume regulation of patients with cirrhosis. Hepatology, 1989; 9(3): 417–422.
- Al-Busafi SA, Ghali P, Deschênes M, Wong P. Chylous Ascites: Evaluation and Management. ISRN Hepatology, 2014; 2014: 1–10.
- Ali B, Salim A, Alam A, Zuberi BF, Junejo ZA, Azam Z, et al. HEP-Net opinion on the management of ascites and its complications in the setting of decompensated cirrhosis in the resource constrained environment of Pakistan. Pakistan Journal of Medical Sciences, 2020; 36(5).
- Reynolds TB, Geller HM, Kuzma OT, Redeker AG. Spontaneous decrease in portal pressure with clinical improvement in cirrhosis. N Engl J Med, 1960; 263: 734–739.
- Gallo A, Dedionigi C, Civitelli C, Panzeri A, Corradi C, Squizzato A. Optimal management of cirrhotic ascites: A review for internal medicine physicians. Journal of Translational Internal Medicine, 2020; 8(4): 220–236.
- Rudler M, Mallet M, Sultanik P, Bouzbib C, Thabut D. Optimal management of ascites. Liver Int, 2020; 40 Suppl 1: 128-135. doi: 10.1111/liv.14361. Erratum in: Liver Int, 2020; 40(5): 1247.
- Zhao R, Lu J, Shi Y, Zhao H, Xu K, Sheng J. Current management of refractory ascites in patients with cirrhosis. The Journal of International Medical Research, 2018; 46(3): 1138–1145.
- Neong SF, Adebayo D, Wong F. An update on the pathogenesis and clinical management of cirrhosis with refractory ascites. Expert Review of Gastroenterology & Hepatology, 2018; 13(4): 293-305.
- Lenz K, Buder R, Kapun L, Voglmayr M. Treatment and management of ascites and hepatorenal syndrome: an update. Therapeutic Advances in Gastroenterology, 2014; 8(2): 83–100.
- Wong F, Sniderman K, Liu P, et al. Transjugular intrahepatic portosystemic stent shunt: effects on hemodynamics and sodium homeostasis in cirrhosis and refractory ascites. Ann Intern Med, 1995; 122: 816–822.
- Rossle M. TIPS: 25 years later. J Hepatol, 2013; 59: 1081–1089.
- Alessandria C, Ozdogan O, Guevara M, et al. MELD score and clinical type predict prognosis in hepatorenal syndrome: relevance to liver trans- plantation. Hepatology, 2005; 41: 1282–1289.
- Bellos I, Kontzoglou K, Psyrri A, Pergialiotis V. Tolvaptan Response Improves Overall Survival in Patients with Refractory Ascites: A Meta-Analysis. Digestive Diseases, 2019; 38(4): 320–328.
- Tahara T, Mori K, Mochizuki M, Ishiyama R, Noda M, Hoshi H, et al. Tolvaptan is effective in treating patients with refractory ascites due to cirrhosis. Biomedical Reports, 2017; 7(6): 558-562.
- Niels Kristian Aagaard, Massimo Malagó, Andrea De Gottardi, Thomas M, Sauter G, Engelmann C, et al. Consensus care recommendations for alfapump® in cirrhotic patients with refractory or recurrent ascites. BMC Gastroenterology, 2022; 22(1).
- How it works? - alfapump®. Alfapump, 2024.
- Alfapump® system. Sequana Medical, 20024.
- Bellot P, Welker MW, Soriano G, Markus von Schaewen, Beate Appenrodt, Wiest R, et al. Automated low flow pump system for the treatment of refractory ascites: A multi-center safety and efficacy study. Journal of Hepatology, 2013; 58(5): 922–927.
- Stirnimann G, Banz V, Storni F, De Gottardi A. Automated low-flow ascites pump for the treatment of cirrhotic patients with refractory ascites. Therapeutic Advances in Gastroenterology, 2017; 10(2): 283–292.
- Grigory Rozenblit, Louis, Rundback JH, Poplausky MR, Lebovics E. Peritoneal-Urinary Drainage for Treatment of Refractory Ascites: A Pilot Study. Journal of Vascular and Interventional Radiology, 1998; 9(6): 998–1005.
- Kasztelan-Szczerbinska B, Cichoz-Lach H. Refractory ascites the contemporary view on pathogenesis and therapy. PeerJ, 2019; 7: e7855.
- Weil-Verhoeven D, Di Martino V, Stirnimann G, Cervoni JP, Nguyen-Khac E, Thévenot T. Alfapump®implantable device in management of refractory ascites: An update. World journal of hepatology, 2022; 14(7): 1344.
- Aithal GP, Palaniyappan N, China L, Härmälä S, Macken L, Ryan JM, et al. Guidelines on the management of ascites in cirrhosis. Gut, 2020; 70(1): gutjnl-2020-321790.
- 1 Recommendations | Subcutaneous automated low-flow pump implantation for refractory ascites caused by cirrhosis | Guidance | NICE, 2018.
- Stirnimann G, Berg T, Spahr L, Zeuzem S, McPherson S, Lammert F, et al. Treatment of refractory ascites with an automated low-flow ascites pump in patients with cirrhosis. Alimentary Pharmacology & Therapeutics, 2017; 46(10): 981–991.
- Dembinski J, Aranovich D, Banz V, Ehmann T, Klein I, Massimo Malagó, et al. Surgical technique for placement of the automated low flow ascites pump (Alfapump). Langenbeck’s Archives of Surgery, 2020; 405(1): 117–123.
- Angeli P, Bernardi M, Villanueva C, Francoz C, Mookerjee RP, Trebicka J, et al. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. Journal of Hepatology, 2018; 69(2): 406–460.
- Storni F, Stirnimann J, Banz V, De Gottardi A, Stirnimann G. Treatment of refractory ascites with an automated low flow ascites pump in patients awaiting liver transplantation. Journal of Liver Transplantation, 2021; 4: 100037.
- Lepida A, Marot A, Trépo E, Degré D, Moreno C, Deltenre P. Systematic review with meta-analysis: automated low-flow ascites pump therapy for refractory ascites. Alimentary Pharmacology & Therapeutics, 2019; 50(9): 978–987.
- Solbach P, Christoph, Freya Wellhöner, Richter N, Heidrich B, Lenzen H, et al. Automated low-flow ascites pump in a real-world setting: complications and outcomes. European Journal of Gastroenterology & Hepatology, 2018; 30(9): 1082–1089.
- Wong F, Bendel E, Sniderman K, Frederick T, Haskal ZJ, Sanyal A, et al. Improvement in Quality of Life and Decrease in Large‐Volume Paracentesis Requirements With the Automated Low‐Flow Ascites Pump. Liver Transplantation, 2020; 26(5): 651–661.
- Bureau C, Adebayo D, Chalret de Rieu M, Elkrief L, Valla D, Peck-Radosavljevic M, et al. Alfapump® system vs. large volume paracentesis for refractory ascites: A multicenter randomized controlled study. Journal of Hepatology, 2017; 67(5): 940-949.
- Will V, Rodrigues SG, Berzigotti A. Current treatment options of refractory ascites in liver cirrhosis – A systematic review and meta-analysis. Digestive and Liver Disease, 2022.
- Mustapha S. Cirrhotic ascites: A review of pathophysiology and management. Nigerian Journal of Gastroenterology and Hepatology, 2020; 12(1): 3.
- Guido Stirnimann, Florian van Bömmel, Spahr L, Zeuzem S, McPherson S, Lammert F, et al. Final safety and efficacy results from a 106 real‐world patients registry with an ascites‐mobilizing pump. Liver International, 2022; 42(10): 2247–2259.
- Fukui H, Kawaratani H, Kaji K, Takaya H, Yoshiji H. Management of refractory cirrhotic ascites: challenges and solutions. Hepatic Medicine: Evidence and Research, 2018; 10: 55–71.
- Solà E, Sanchez-Cabús S, Rodriguez E, Elia C, Cela R, Moreira R, et al. Effects of alfapumpTM system on kidney and circulatory function in patients with cirrhosis and refractory ascites. Liver Transplantation, 2017; 23(5): 583-593.
- Bendel EC, Sniderman KW, Shaw CJ, Frederick R, Wong F, Sanyal AJ, et al. Feasibility and Procedural Safety of alfapump System Implantation by IR: Experience from the MOSAIC Study, a Multicenter, Open-Label Prospective Study in Cirrhotic Patients with Refractory Ascites. Journal of Vascular and Interventional Radiology, 2020; 31(8): 1256-1262.e3.
- Weil D, Vincent Di Martino, Guido Stirnimann, Jean Paul Cervoni, Éric Nguyen-Khac, Thierry Thévenot. Alfapump®implantable device in management of refractory ascites: An update. World Journal of Hepatology, 2022; 14(7): 1344–1356.
- Stirnimann G, Banz V, Storni F, De Gottardi A. Automated low-flow ascites pump for the treatment of cirrhotic patients with refractory ascites. Therapeutic Advances in Gastroenterology, 2017; 10(2): 283–292.

