Revolutionizing Tibial Fracture Management: The Therapeutic Potential of IL-1ra Solutions
Martin D Tanhaei1,2,3,*
1Biomedical Engineering, University of California, USA
2Biomedical Engineering, Johns Hopkins University, USA
3School of Medicine, St. George's University, GRD
Received Date: 17/02/2024; Published Date: 27/06/2024
*Corresponding author: Martin D Tanhaei, MS, Biomedical Engineering, University of California, Irvine, USA; Johns Hopkins University, Baltimore, USA; School of Medicine, St. George's University School of Medicine, True Blue, GRD
Abstract
The management of tibial fractures, prevalent among adult leg injuries, necessitates advancements beyond conventional casts, splints, and metal implants, which often fall short in preventing complications like inadequate osseointegration and implant rejection due to fibrous scar tissue formation. This study explores the use of interleukin-1 receptor antagonist (IL-1ra) solutions, capitalizing on IL-1ra's anti-inflammatory properties to hypothesize improvements in cartilage formation and bone regeneration by reducing inflammation and fostering bone-implant integration. Through an examination of IL-1ra's molecular mechanisms and regenerative capabilities, supported by immunological and bone density analyses, we demonstrate its potential in mitigating IL-1-mediated inflammation and enhancing bone density in treating tibial fractures. The findings advocate for further clinical exploration of IL-1ra-based solutions as a promising avenue for revolutionizing tibial fracture management by addressing common post-surgical complications.
Categories: Allergy/Immunology; General Surgery; Orthopedics
Keywords: Anti-inflammatory therapy; Osseointegration; Bone regeneration; Il-1ra; Tibial fractures
Introduction and Background
In the realm of orthopedic medicine, tibial fractures notably account for a significant portion of adult leg fractures, emphasizing the need for advanced treatment modalities [1,2]. Annually impacting nearly half a million individuals in the United States alone, these injuries impose substantial economic burdens, with costs anticipated to surpass $2.5 billion [1,2]. Despite the prevalence of conventional treatments like casts, splints, and metal implants, challenges such as inadequate osseointegration and the risk of implant failure persist, highlighting a critical area for innovation [1,3]. This backdrop sets the stage for the exploration of IL-1ra solutions, a novel approach poised to revolutionize the treatment landscape by enhancing bone healing and reducing complications associated with tibial fractures.
Bone is one of few tissues that can heal without creating fibrous scar tissue. While bone healing typically follows the steps of bone development, insufficient bone growth and osseointegration due to fibrous scar formation around the fixation site can induce fracture fixation failures. Surrounding fibrous scar formation leads to foreign body response and rejection of implant and site inflammation. Therefore, integrating scar tissues into the fixation implant’s design is key to reducing scar formation around the implant [4].
Minor damage during surgery and implantation may start a possible cascade of implant failure. Within 7-10 days of many surgeries, secondary bleeding can occur and is not necessarily restricted to the surgical wound. Current research highlights that secondary blood loss from surgical fixation increases the risk of postoperative anemia [5]. Additionally, Schell et al. found that removing hematoma in the open tibial fracture model in 4 or 7 days will significantly reduce bone formation in two weeks. Repetitive surgeries and procedures that lead to the removal of hematoma will therefore reduce bone healing time and should be avoided when possible [6].
Post-operative fracture fixation (POFF) carries a significant risk of poor bone healing and loss of bone function. POFF infection requires interdisciplinary care, combined surgical and antibiotic treatment, as well as intelligent and early detection. POFF infections are the primary source of morbidity and mortality and cause significant economic damage. The success rate of POFF infection treatment is between 70% and 90%, which varies between opened and closed fractures. In open fractures, POFF infection incidents can reach up to 30%. The main goal of POFF infection treatment is to eradicate the infection and place a sterile implant with consideration for healing the fracture and avoiding chronic osteomyelitis [3]. POFF infections are the most challenging complications in orthopedic trauma surgery, and bone-integrative solutions are needed to modify the metal implant fixtures.
The inflammatory response is essential for regeneration, creating a signaling cascade for the immune- mediated response. Inflammation resolution is controlled by immune cells through cytokine-mediated responses. They overall contribute to immune resolution, and the mechanism is not fully understood. Inflammation resolution is a pivotal point in the induction of osteogenesis and angiogenesis. Angiogenesis sets up bone regeneration capacity by providing the mediators to support bone regeneration [7]. Foundation for bone regeneration is also induced by collagen matrix formation by chondrocytes during the regeneration step that helps guide osteogenesis. However, if the inflammation is not resolved due to infection in the case of POFF, the lack of inflammation resolution interrupts bone regeneration [3].
Interleukin-1 (IL-1) is a pro-inflammatory mediated cytokine that is elevated in traumatic tissue injury. Current research findings show IL-1 increases chondrocyte apoptosis, reduces cartilage matrix formation, and blocks regenerative steps during the prolonged inflammation response. Chronic inflammation, therefore, creates a significant reduction in cartilage formation that disrupts the foundation for osteogenesis and further bone formation. In light of these discoveries, the IL-1 receptor antagonist (IL-1ra) in cartilage repair may be adequate to suppress IL-1α-induced GAG loss, collagen loss, and NO synthesis, which overall are a backbone for bone formation [8].
Tissue engineering for restoring the original integrity of tibial bone is a focus of much ongoing research and bone scaffolds have been developed to enhance the recovery of bone integrity. In recent studies, bone scaffolds have been shown to stimulate bone formation and enhance bone-implant integration in tibial fracture. The scaffolds improve tissue repairing capacity and support regeneration at the bone-implant interface biomaterial [2]. Given these findings, this report hypothesizes that IL-1ra will potentially improve cartilage formation in bone regeneration through multiple pathways involving signaling cascades, reduction of inflammation, and improving the stability of the cartilage foundation that underlies bone regeneration. Eventually, improvement in cartilage formation provides a stronger basis for bone formation, and ultimately healing, in tibial fracture repair.
Review
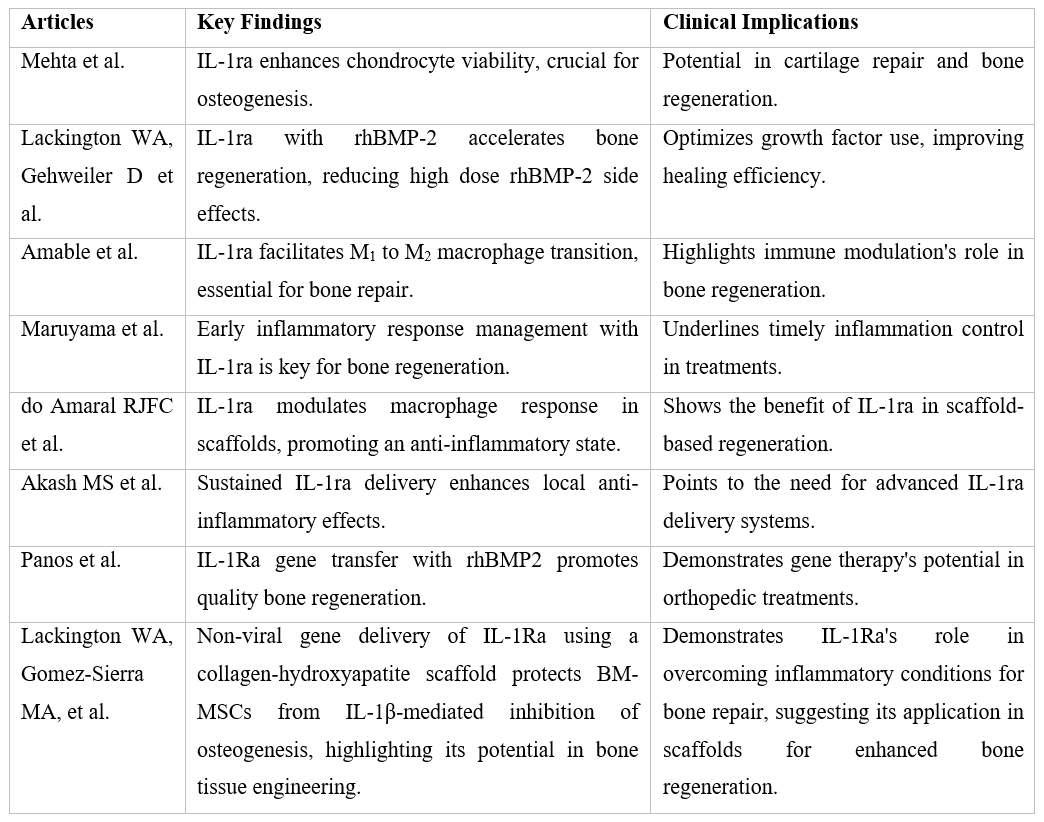
IL-1ra plays a critical role in moderating inflammatory responses, a pivotal factor in bone regeneration and healing. Innovative studies have explored the efficacy of IL-1ra, particularly in conjunction with lower doses of recombinant human Bone Morphogenetic Protein-2 (rhBMP-2), in a femoral fracture model, demonstrating accelerated bone regeneration and improved mechanical strength [9]. This synergy not only marks a potential advancement in reducing rhBMP-2-related side effects but also broadens the scope of IL-1ra’s applications, including its role in mitigating inflammatory responses that contribute to conditions such as diabetes mellitus, thereby showcasing its therapeutic versatility [9].
The macrophage activation pathway, modulated by IL-1ra, plays a crucial role in the healing process. The transition from the M1 pathway, characterized by pro-inflammatory cytokine secretion, to the M2 pathway, which promotes anti-inflammatory agents including IL-1ra, is essential for bone healing. This shift facilitates the homing of bone marrow-derived mesenchymal stem cells (BM-MSCs), creating a conducive environment for bone repair. However, an imbalance or delay in this transition can lead to excessive fibrous tissue formation, potentially resulting in fracture fixation failure. The modulation of this pathway, mediated by IL-1ra, is therefore instrumental in promoting osteogenesis and angiogenesis, essential processes for bone recovery [10]. Furthermore, the efficacy of bone regeneration is intricately linked to the regulation of the inflammatory response, with studies highlighting those inadequacies in managing the initial inflammatory stage can detrimentally affect the healing process [11].
The potential for wound healing is notably enhanced through angiogenesis and vascularization processes, critical in the early stages of healing. Platelet-Rich Plasma (PRP) treatment, in its pro-inflammatory stage, induces macrophages to release macrophage inflammatory proteins (MIP-1α) and higher levels of anti-inflammatory markers such as IL-1ra when treated with composite scaffolds [12]. This response is essential for creating an optimal environment for keratinocytes and fibroblasts, promoting the formation of an epidermal-like layer conducive to skin regeneration. The modulation of macrophage responses by composite scaffolds, encouraging a shift from a pro-inflammatory to an anti-inflammatory state, is pivotal for the healing process, highlighting the importance of IL-1ra in this transition [12].
Current strategies for enhancing vascularization in tissue engineering include modifications to scaffolds, introduction of growth factors, and the combined implantation of endothelial progenitor cells. The increased surface roughness of materials has been shown to promote bone regeneration and facilitate the macrophage differentiation tendency towards the M2 stage, associated with the release of IL-1ra. While a single dose of IL-1ra may be ineffective, sustained doses are essential for significantly suppressing IL-1α-induced catabolism, highlighting the importance of continuous delivery systems for therapeutic efficacy [8].
Moreover, the biological crosstalk between cartilage and synovium is significant in the context of traumatic injury, where IL-1 is elevated, stimulating cartilage degradation and inhibiting matrix biosynthesis. IL-1ra's role in suppressing cytokine-induced catabolism in both cartilage and cartilage-synovium co-cultures demonstrates its therapeutic potential, emphasizing the necessity for sustained delivery mechanisms to maintain therapeutic levels of IL-1ra at the site of injury [8, 13]. The groundbreaking research conducted by Panos et al. [14] marks a significant milestone in the field of orthopedic medicine, particularly in the treatment of bone fractures. Their study meticulously demonstrates how the strategic application of IL-1ra gene transfer, in combination with reduced doses of rhBMP2, profoundly enhances bone healing processes through endochondral ossification. This novel approach not only circumvents the negative repercussions associated with the traditionally high doses of rhBMP2, such as severe inflammation, adverse side effects, and the formation of morphologically abnormal bone but also fosters the development of bone structures that more accurately replicate the natural, healthy state of bone tissue. By leveraging the body's innate healing mechanisms and redirecting osteogenesis towards a more natural pathway of bone formation, Panos et al. illuminate a path forward that could revolutionize the management of bone fractures. The study by Lackington et al. [15] explores the use of non-viral gene delivery of IL-1ra via a collagen-hydroxyapatite scaffold to protect rat BM-MSCs from IL-1β-mediated inhibition of osteogenesis. This innovative approach also underscores the potential of IL-1ra in enhancing osteogenic differentiation in an inflammatory environment, highlighting its therapeutic promise in bone tissue engineering.
The review of IL-1ra’s role in bone healing and regeneration elucidates its significant potential in orthopedic treatments. By mitigating the inflammatory response and enhancing the healing processes at a cellular level, IL-1ra presents a promising therapeutic avenue. The insights from these studies presented in this review underscore the necessity for further research into optimized delivery systems and the exploration of IL-1ra’s full potential in clinical applications, particularly in the management of tibial fractures. This comprehensive overview, bolstered by the groundbreaking findings of Panos et al. and Lackington et al., highlights the critical role of IL-1ra in advancing orthopedic medicine, pointing towards a future where innovative treatments like IL-1ra solutions can significantly improve outcomes for patients with tibial fractures. Exploring alternative viewpoints of these solutions, it is crucial to address skepticism regarding Immunoengineering solutions, particularly concerns over potential immune system over-modulation, the long-term viability and integration of bioengineered materials, and the economic implications of adopting these advanced treatments in standard clinical practice.
Table 1: Research Summary.
Conclusion
The investigation into IL-1ra solutions for tibial fracture treatment underscores the imperative role of targeted anti-inflammatory strategies in augmenting bone healing processes. By examining IL-1ra's modulation of inflammatory responses and its impact on bone regeneration, we highlight a promising therapeutic avenue that addresses key challenges in fracture management, including fibrous scar formation and implant rejection. These findings not only reinforce the therapeutic potential of IL-1ra solutions in orthopedics but also emphasize the need for continued research to refine delivery mechanisms and evaluate patient-centered outcomes, paving the way for innovative solutions in clinical practice.
Disclosures
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following
Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work.
Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work.
Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
References
- Grütter R, Cordey J, Bühler M, et al. The epidemiology of diaphyseal fractures of the tibia. Injury,200031; 3: 64-67. doi: 10.1016/s0020-1383(00)80035-2.
- Amini AR, Laurencin CT, Nukavarapu SP. Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng, 2012; 40: 363-408. doi: 10.1615/critrevbiomedeng.v40.i5.10.
- Steinmetz S, Wernly D, Moerenhout K, et al. Infection after fracture fixation. EFORT Open Rev, 2019; 468-475. doi: 10.1302/2058-5241.4.180093.
- Noskovicova N, Hinz B, Pakshir P. Implant Fibrosis and the Underappreciated Role of Myofibroblasts in the Foreign Body Reaction. Cells, 2021; 1794-2021. doi: 10.3390/cells10071794.
- Gibbs VN, Champaneria R, Novak A, et al. Pharmacological interventions for the prevention of bleeding in people undergoing definitive fixation of hip, pelvic and long bone fractures: a systematic review and network meta‐analysis. Cochrane Database Syst Rev, 2019. doi: 10.1002/14651858.CD013499
- Schell H, Duda GN, Peters A, et al. The haematoma and its role in bone healing. J Exp Orthop, 2017; 4: 5. doi: 10.1186/s40634-017-0079-3.
- Newman H, Shih YV, Varghese S. Resolution of inflammation in bone regeneration: From understandings to therapeutic applications. Biomaterials, 2021; 277: 121114. doi: 10.1016/j.biomaterials.2021.121114.
- Mehta S, Akhtar S, Porter RM, et al. Interleukin-1 receptor antagonist (IL-1Ra) is more effective in suppressing cytokine-induced catabolism in cartilage-synovium co-culture than in cartilage monoculture. Arthritis Res Ther, 2019. doi: 10.1186/s13075-019-2003-y.
- Lackington WA, Gehweiler D, Zhao E, et al. Interleukin-1 receptor antagonist enhances the therapeutic efficacy of a low dose of rhBMP-2 in a weight-bearing rat femoral defect model. Acta Biomater, 2022; 1: 189- 197. doi: 10.1016/j.actbio.2022.07.012
- Amable PR, Teixeira MVT, Carias RBV, et al. Protein synthesis and secretion in human mesenchymal cells derived from bone marrow, adipose tissue and Wharton’s jelly. Stem Cell Res Ther, 53. doi: 10.1186/scrt442.
- Maruyama M, Rhee C, Utsunomiya T, et al. Modulation of the Inflammatory Response and Bone Healing. Front Endocrinol (Lausanne), 2020; 11: 386. Published 2020 Jun 11. doi:10.3389/fendo.2020.00386.
- do Amaral RJFC, Zayed NMA, Pascu EI, et al. Functionalising collagen-based scaffolds with platelet-rich plasma for enhanced skin wound healing potential. Frontiers, 2019. doi: 10.3389/fbioe.2019.00371.
- Akash MS, Rehman K, Sun H, et al. Sustained delivery of IL-1Ra from PF127-gel reduces hyperglycemia in diabetic GK-rats. PloS, 8: 55925. doi: 10.1371/journal.pone.0055925
- Panos JA, Coenen MJ, Nagelli CV, et al. IL-1Ra gene transfer potentiates BMP2-mediated bone healing by redirecting osteogenesis toward endochondral ossification. Molecular therapy: the journal of the American Society of Gene Therapy, 2023; 31: 420-434. doi: 10.1016/j.ymthe.2022.10.007.
- Lackington WA, Gomez-Sierra MA, Gonzalez-Vazquez A, et al. Non-viral Gene Delivery of Interleukin-1 Receptor Antagonist Using Collagen-Hydroxyapatite Scaffold Protects Rat BM-MSCs from IL-1β-Mediated Inhibition of Osteogenesis. Front Bioeng Biotechnol, 2020; 8: 582012. doi: 10.3389/fbioe.2020.582012.

