Restenosis in Patients with Percutaneous Coronary Intervention, Etiologies and Remedy. A Comprehensive Review of Current Literature
Ovie Okorare1,*, Akanimo Antia2,Gabriel Alugba3, Jumbo Unwam4, Emmanuel Daniel5, Daniel Ubokudum6, Ogenetejiri Gbegbaje7, Bright Chizitere Nwatamole8 and Endurance Evbayekha9
1Resident Physician, Department of Internal Medicine, Vassar Brothers Medical Center, Nuvance Health Poughkeepsie, USA
2Lincoln Medical and Mental health Center, USA
3Hackensack University Medical Center/Englewood Hospital and Medical Center, USA
4Thomas Hospital, USA
5Trinity Health Ann Arbor, USA
6Thomas Hospital, USA
7Hackensack University Medical Center/Englewood Hospital and Medical Center, USA
8St. James's University Teaching Hospital Leeds, United Kingdom
9St. Luke’s Hospital, USA
Received Date: 10/01/2024; Published Date: 24/05/2024
*Corresponding author: Ovie Okorare MBBS, Resident Physician, Department of Internal Medicine, Vassar Brothers Medical Center, Nuvance Health, Poughkeepsie, New York, 12601
Abstract
Coronary artery disease remains one of the leading causes of cardiovascular mortality worldwide. With advancement of medical therapy, percutaneous coronary intervention with stenting has become a cornerstone in the management of acute coronary syndrome. However, complications such as restenosis of stent has become a bane in the treatment of coronary artery disease. Factors such as sedentary lifestyle, nonadherence to medication, mechanical factors such as stent expansion, stent trauma, stent allergy, genetic factors have been implicated in the occurrence of in-stent restenosis. Patients often present in overt symptomatic acute coronary syndrome. Coronary angiography remains the mainstay of diagnosis. Treatments such as placement of drug-eluting stents, drug coated balloons, vascular brachytherapy and balloon angioplasty have been employed in the management.
Keywords: Coronary artery disease; In-stent restenosis; Acute coronary syndrome
Introduction
Coronary Artery Disease (CAD) results from accumulation of fat plaques within the walls of the arteries that supply the heart myocardium [1]. Globally, CAD is one of the leading causes of death [1]. Numerous clinical studies have shown that atherosclerosis is the most crucial cause of CAD which comprises lipid adherence to the arterial wall thus inducing inflammation and endothelial dysfunction, resulting in the proliferation and migration of vascular smooth muscle cells and eventually intimal hyperplasia [2].
Since its inception, Percutaneous Coronary Intervention (PCI) has been effective in the primary treatment of complex CAD which has helped in the management of Myocardial Infarction (MI), and death related to acute coronary syndrome (ACS) [3]. However, PCI may cause a few types of complications such as traumatic coronary artery dissection, coronary artery perforation, coronary artery restenosis, and iatrogenic coronary artery thrombosis [3]. Restenosis in coronary arteries typically occurs within 6 months to 12 months post-PCI with about 50% of the artery occluded and this scenario becomes more prominent with high morbidity if a drug eluting stent was placed [4]. With the use of modern drug-eluting stents (DES), in-stent restenosis (ISR) happens in 2-10% of PCI cases [5].
Multiple factors have been implicated in the science and mechanism of coronary artery restenosis after PCI such as endothelial progenitor cells (EPCs), Specificity Protein 1 (SP1), Calcineurin-Like Phosphodiesterase Domain containing 1 (CPPED1), medication non-compliance (Statins, and renin–angiotensin–aldosterone system blockers reduce in-stent restenosis), high levels of HB-EGF, interleukin-10 and interleukin-18, and sedentary lifestyle [1,6-10]. A study also argued there may be an association between the occurrence of ISR and anemia thus recommending that anemia should be assessed at the follow up clinic visits [9]. The etiology and management of ISR has been a major niche in the field of interventional cardiology, our study aims to review the current literatures on the factors, causes, etiologies, and remedies available for the prevention of restenosis in patients post-PCI.
Review
Methodology: We based our study on a defined set of inclusion and exclusion criteria.
Inclusion Criteria: We included studies within the last 10 years. We searched PubMed, Google Scholar, EMBASE
and the Cochrane databases for relevant studies. We included articles from 2013 to 2023 and considered systematic reviews, meta-analyses, randomized control trials, and clinical trials. Keywords for the search included “restenosis” and “percutaneous coronary intervention”; we combined the keywords in every combination to generate all possible articles for screening. Our keyword combinations and search results generated a total of 800 articles. We read the abstracts with our objectives, the inclusion criteria, and the exclusion criteria (below) in mind, which narrowed them down to 80 full-text articles. Ultimately, we included 67 articles in our review (Figure 1).
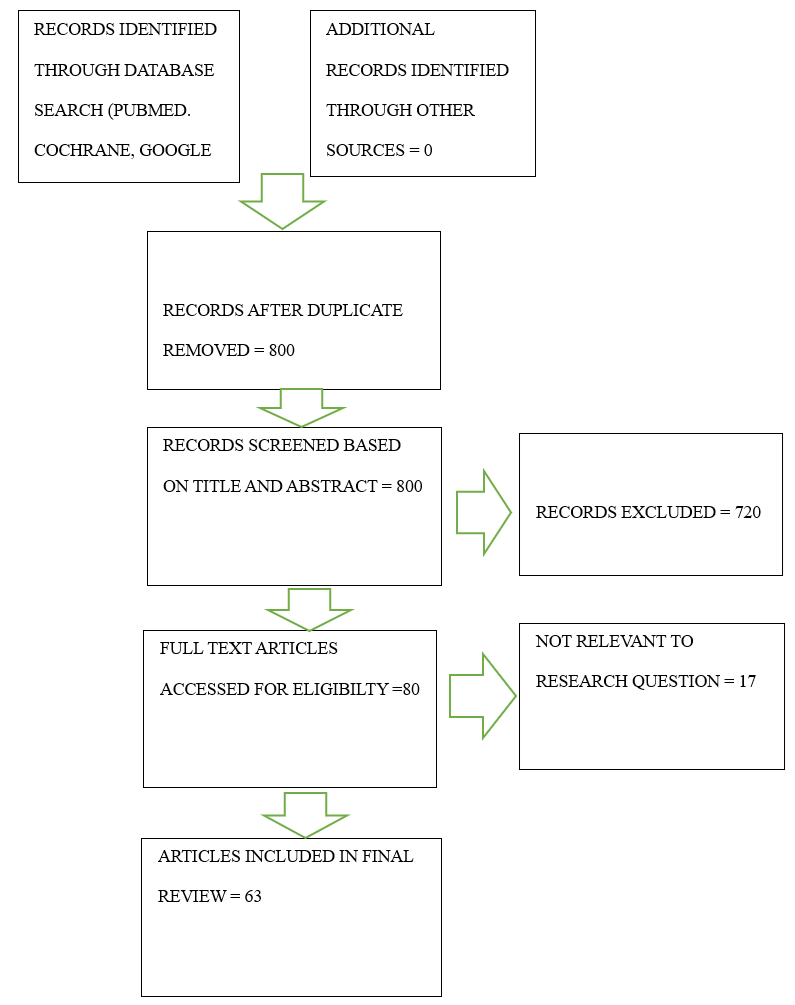
Figure 1: PRISMA chart detailing the systematic search.
Exclusion Criteria: We excluded all studies case reports, case series, articles in languages other than English, and non-full-text articles.
Discussion
Epidemiology of modern-day percutaneous coronary intervention
There are several articles postulating varying degrees of change as regards the utilization of PCI over the years. Interestingly, the prevalence of PCI when stratified as urgent, elective, in-patient or outpatient differs [11]. A somewhat recent retrospective study using data from 4 states in the US showed that between 2010 to 2017, there was a 14.9% increase in the rates of urgent PCI performed but a somewhat stable rate of elective PCI [11]. It also showed a decrease in inpatient PCIs but a noteworthy 97.3% increase in the prevalence of outpatient PCI [11]. All trends in this study were statistically significant [11]. There have also been reports of increased trends in incidence of complex PCI cases [12] explained by the continuous evolution of PCI and the emergence of data showing comparable outcomes in selected complex cases when compared to CABG [13,14]. The fact that the PCI is a less invasive and more tolerable procedure has increased its utilization and could also play a part, seeing the increasing age, co-morbidity index and changes to the risk-profile of the current patient population [15]. A similar trend was replicated by a study that analyzed the New South Wales Admitted Patient Data Collection (NSW APDC) showing a 35% increase in the annual PCI rate between 2008 and 2019 [15].
It is also important to note the significant increase in the number of hospitals with the capacity to perform PCI procedures [16]. Between 2003 and 2011, there was a 21.2% increase in the number of PCI capable centers and the advent of the new CMS rule allowing PCI in ambulatory surgical centers following data supporting the possibility of same-day-discharge and lower cost incurred might further this trend [16,17]. On another note, there was a noteworthy longer delay in symptoms to first medical contact and the door to balloon time during the peak of the COVID 19 pandemic in 2020 but fortunately, this had no significant effect on major adverse cardiac events (MACE) depicting the perseverance of esteemed quality in PCI management [18].
Pathophysiology of restenosis in coronary artery disease
Mechanism of restenosis in PCI In-Stent Restenosis (ISR) has been known to be a major complication reducing the long-term efficacy of coronary artery stenting, for which Drug-Eluting Stents (DES) were developed to overcome [19]. Restenosis results from vascular injury caused by balloon dilatation and stent implantation, arising from inflammatory responses triggered by endothelial denudation, mechanical stretch, and subintimal hemorrhage [20]. This culminates in a cascade of various proliferative processes [20]. Vascular smooth muscle cell activation such as proliferation, differentiation, migration, extracellular matrix synthesis, and migration of matrix metalloproteinase results in the formation of neointimal hyperplasia [21,22]. DES acts by releasing adequate amounts of anti-inflammatory, immunomodulatory, or antiproliferative agents, well distributed at the site of vascular injury during the early phase of healing [20]. Although various notable predictors for ISR have been recognized, the precise reasons for DES restenosis are still not fully understood [23]. Multiple factors such as biological, mechanical, technical, and genetic have been shown to play key roles in DES restenosis [23].
Biological Factors
Inflammation
Inflammation causes several proliferative processes, thus playing a vital role in the pathogenesis of ISR, promoting neointimal proliferation [24]. C-reactive protein (CRP), a commonly used inflammatory biomarker, has been shown to predict ISR [20]. An increase in baseline and post-procedural CRP levels is associated with Bare-Metal Stent (BMS) restenosis [25,26]. However, CRP levels do not significantly predict the risk of DES restenosis as the eluted drug can eliminate local inflammatory responses that could lead to ISR in patients with enhanced systemic inflammatory response [27,28]. Although, increased CRP is associated with an increased risk of DES thrombosis [29]. Other significant inflammatory markers that have been evaluated for increased risk of DES include complements C3a and C5a, plasminogen activator inhibitors, and matrix metalloproteinases (MMPs) [30-32]. MMPs are involved in the migration of vascular smooth muscle cell matrix remodeling [33]. Some studies have shown an association between MMPs and the occurrence of DES restenosis [33].
Neo atherosclerosis
Neo atherosclerosis is due to the accumulation of lipid foamy macrophage in the neointimal, with or without necrotic core and calcium [34,35]. In-stent neo-atherosclerosis has been reported to be an important factor in late vascular complications seen in PCI, which includes late ISR and late stent thrombosis [20]. In BMS, it was seen that restenosis with neo-atherosclerosis emerged after 3 years with a prevalence of 15.4%, while in DES, ISR with neo-atherosclerosis was seen at an earlier time with subsequent increase with time [20]. Neo atherosclerosis is suggested to be accelerated dysfunctional and incompetent endothelial coverage of the stented segment, especially in DES [20]. Optical Coherence Tomography (OCT) is the most suitable modality in assessing neo-atherosclerosis and helps in distinguishing it from neointimal hyperplasia [20]. Neo atherosclerosis on OCT is characterized by a heterogeneous composition with an in-stent necrotic core with a thin fibrous cap, lipid or calcification, and foamy macrophage accumulation, while neointimal hyperplasia is seen as a homogeneous bright layer on OCT [20,36].
Medication Resistance
Resistance to antiproliferative drugs has been suggested to be a cause of restenosis [20]. One such is that of resistance to drugs acting on mTOR receptors [20]. Sirolimus and other drugs in its class inhibit the function of the mammalian Target of Rapamycin (mTOR) causing suppression of smooth muscle cell migration and proliferation by arresting cells in the G1 phase or even inducing apoptosis of cells [20]. However, mutations of mTOR or FKBP12 prevent rapamycin from binding to mTOR [37,38]. Likewise, mutations or defects of mTOR-regulated proteins, including S6K1,4E-BP1, PP2A-related phosphatase, and p27 (Kip1) contribute to rapamycin insensitivity [20]. In the same vein, resistance to Paclitaxel, which binds β-tubulin subunits of microtubules causing interference with microtubule dynamics and preventing their depolymerization, is also suggested to cause restenosis20. Paclitaxel resistance is due to increased expression of the mdr-1 gene and its products [20].
Stent Allergy
Hypersensitivity reactions to any component of DES including the anti-stenotic drug, drug carrier vehicle(polymer), and the stent platform may lead to restenosis after implantation [20]. Previously, allergic reactions to nickel and molybdenum released from BMS were one of the triggering mechanisms for ISR [20].
Mechanical Factors
Stent Expansion
Stent under-expansion or over-dilation of an undersized stent is associated with DES restenosis. Stent under-expansion results from poor expansion mainly due to calcified lesions and chronic stent recoil [20]. Stent under-expansion can be visualized in cross-sectional intravascular ultrasound or OCT image [20]. Multiple studies using intravascular ultrasound have revealed that stent under-expansion is an important predictor of restenosis after DES implantation [23,29]. This is likely explained as when the minimum stent area is small at baseline, the expected neointimal hyperplasia is assumed to be significant, compared to when the minimum stent area is large, the same amount of neointimal hyperplasia would be less in causing ISR [20]. There is also low shear stress and flow reversal with stent under-expansion as it disrupts blood flow. This cascades into multiple progressive events leading to neointimal growth [40].
Paradoxically, over-dilatation has been suggested to cause restenosis, this is due to extreme post-dilatation, impairing the effectiveness of DES by enhancing tissue proliferation. In response to greater vessel injury, altering the mechanical properties of the stent and disrupting the polymer coating [41].
Stent Trauma
Restenosis can arise from stent fracture due to local trauma exerted on the vessel and the movement of the stent edge [42]. Furthermore, there is a decrease in local drug delivery at the fracture area and this increases the growth of neointimal tissues [20]. Predictors of stent trauma and subsequent fracture include the length of the implanted stent, saphenous vein graft location, and right coronary artery location [20].
Other mechanical factors include stent gap, polymer damage, non-uniform stent strut distribution, and non-uniform drug deposition [20,28,43,44].
Genetic Factors
Genetic factors play a vital role in inflammatory response [45], this indirectly contributes to the development of neointimal tissues. GENDER study revealed a variant of β2 adrenergic receptor to be associated with elevated risk of ISR [46]. However, rare alleles of CSF2, CD14, and CCL11 as well as polymorphism in the TNF gene were associated with decreased risk of ISR [47]. In addition, polymorphism in the gene of platelet glycoprotein IIIa, Factor V Leiden, and P2Y12 receptors affect the risk of restenosis [48].
Clinical approach to patients with restenosis
Presentation with symptoms of myocardial ischemia in patients post-PCI should raise concern for restenosis [49]. Patients can present with stable angina, unstable angina, NSTEMI, and STEMI [49]. Recent data suggest that ISR-PCI accounts for 5-10% of all PCI procedures performed in current clinical practice [50]. ISR was previously recognized as a pathological process. However, it is now increasingly recognized that ISR is not benign and can commonly presents as an ACS [51]. In a retrospective analysis of the Cath-PCI registry it showed that about 25% of the patients with ISR will present with an NSTEMI (15.5%) or STEMI (7.8%) caused by the ISR lesion [52]. Patients with ISR PCI were more likely to have hypertension, dyslipidemia, end-stage renal disease, diabetes, concomitant cerebrovascular disease, peripheral arterial disease, and chronic lung disease [52]. It is important to take a thorough history of how well-controlled these co-morbidities are in patients suspected of restenosis [52]. It is also important to gather information about adherence to anti-platelet therapy, and lipid-lowering medications. Patients who come in with chest pain should get an electrocardiogram and high sensitivity troponin [52]. The gold standard for diagnosis of ISR is coronary angiography with the aid of intracoronary imaging [53].
Treatment
The European Society of Cardiology recommends the implementation of Drug Eluting Stents (DES) and treatment with Drug Coated Balloons (DCB) due to their demonstrated superiority and favorable outcomes in ISR treatment [54-56].
DES stands out as the most effective therapeutic option owing to its potent anti-proliferative properties [57]. It has exhibited superior efficacy compared to DCB in pivotal trials and network meta-analyses, substantiating its status as the preferred choice [57,39]. Nevertheless, there is currently no definitive evidence guiding the selection of a specific DES type for DES-ISR treatment, nor is there consensus regarding the need for stent-type modification during additional DES implantation for DES-ISR [57,58].
DCBs function by delivering antiproliferative therapy to the vessel wall, eliminating the need for an additional metallic scaffold-like DES [56]. The balloon coating typically comprises lipophilic active drugs and a spacer that facilitates drug transfer from the balloon surface to the vessel wall [59]. Commonly employed medications include paclitaxel and sirolimus, although recent concerns regarding increased mortality have been raised with paclitaxel usage in peripheral interventions [60].
Vascular brachytherapy, a technique involving the delivery of radiation to inhibit neointimal formation within the stent and impede neointimal cell growth in the targeted area without damaging the surrounding tissue, has seen limited usage since the advent of DES [61,62]. However, some observational analyses suggest that intravascular brachytherapy (IVBT) may play a role in managing recurrent ISR [61,62].
Additional treatment modalities, such as balloon angioplasty, cutting and scoring balloons, ablative therapy, and bioresorbable scaffolds, have demonstrated inferior efficacy when compared to DES and DCB, or have been associated with complications. Consequently, they are not routinely employed except as adjunctive therapies [57,58].
In complex cases, such as ISR of the Left Main Stem (LMS), recalcitrant ISR in a major vessel, multivessel disease, or ISR located in the ostial Left Anterior Descending (LAD) artery, Coronary Artery Bypass Grafting (CABG) represents a viable treatment option [63].
Prevention
In-stent restenosis remains a challenge in patients undergoing PCI, requiring various mechanisms from increased vascular proliferation to increased platelet activation which increases the risk of thrombosis. Paclitaxel, a microtubule-stabilizing drug has demonstrated efficacy in preventing stent restenosis due to its antiproliferative, antiplatelet, and antithrombotic properties [62]. A Combination of metformin and atorvastatin a lipid-lowering drug that reduces LDL has been shown to decrease restenosis in a dose-dependent fashion with typical doses ranging from metformin 1.5gram per day plus atorvastatin 20 milligram per night and metformin 1.5gram per day +atorvastatin 40 milligram per night. Metformin reduces ISR by inhibiting the concentration of oxandrolone and steroids [63].
Conclusion
In-stent restenosis is an established but rare complication post-PCI. The use of modern-day drug eluting stent has helped to decrease the incidence of ISR. Etiology remains multifactorial, considering the genetic and biologic risk factors. Patients often present with symptoms of acute coronary syndrome, with coronary angiography being the gold standard for diagnosis. Preventive measures with lipid lowering therapy are highly encouraged, as well as the use of DES and DCB for treatment.
Declaration of interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding: None
References
- Jiang H, Liu W, Liu Y, Cao F. High levels of HB-EGF and interleukin-18 are associated with a high risk of in-stent restenosis. Anatol J Cardiol, 2015; 15(11): 907-912. doi: 10.5152/akd.2015.5798.
- Libby P, Okamoto Y, Rocha VZ, Folco E. Inflammation in atherosclerosis: transition from theory to practice. Circ J, 2010; 74: 213–220.
- Kandan SR, Johnson TW. Management of percutaneous coronary intervention complications. Heart, 2019; 105(1): 75-86.
- Wu J, Zhao L, Lin K, Lu L, Luo C. Chinese Herbal Medicines for Restenosis After Percutaneous Coronary Intervention: A Meta-Analysis of Randomized Controlled Trials. J Altern Complement Med, 2019; 25(10): 983-992. doi: 10.1089/acm.2018.0516.
- Ullrich H, Olschewski M, Münzel T, Gori T. Coronary In-Stent Restenosis: Predictors and Treatment. Dtsch Arztebl Int, 2021; 118(38): 637-644. doi: 10.3238/arztebl.m2021.0254.
- Yoshikawa M, Nakamura K, Nagase S, Sakuragi S, Kusano KF, Matsubara H, et al. Effects of combined treatment with angiotensin II type 1 receptor blocker and statin on stent restenosis. J Cardiovasc Pharmacol, 2009; 53: 179–186.
- Kamishirado H, Inoue T, Sakuma M, Tsuda T, Hayashi T, Takayanagi K, et al. Effects of statins on restenosis after coronary stent implantation. Angiology, 2007; 58: 55–60.
- Wang Z, Shao L, Cai X, Zhou Y, Hong L, Li S. The potential function of SP1 and CPPED1 in restenosis after percutaneous coronary intervention. J Card Surg, 2022; 37(12): 5111-5119. doi: 10.1111/jocs.17218.
- Hu H, Wang S, Tang G, Zhai C, Shen L. Impact of anemia on in-stent restenosis after percutaneous coronary intervention. BMC Cardiovasc Disord, 2021; 21(1): 548. doi: 10.1186/s12872-021-02355-1.
- Eyüboglu M. In stent restenosis after percutaneous coronary intervention. Anatol J Cardiol, 2016; 16(1): 73. doi: 10.14744/AnatolJCardiol.2015.6775.
- Almarzooq ZI, Wadhera RK, Xu J, Yeh RW. Population Trends in Rates of Percutaneous Coronary Interventions, 2010 to 2017. JAMA Cardiol, 2021; 6(10): 1219–1220. doi:10.1001/jamacardio.2021.2639.
- Kheifets M, Vons SA, Bental T, Vaknin-Assa H, Greenberg G, Samara A, et al. Temporal Trends in Complex Percutaneous Coronary Interventions. Front Cardiovasc Med, 2022; 9: 913588. doi: 10.3389/fcvm.2022.913588.
- Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med, 2009; 360: 961–972. doi: 10.1056/NEJMoa0804626.
- Stone GW, Kappetein AP, Sabik JF, Pocock SJ, Morice MC, Puskas J, et al. Five-year outcomes after PCI or CABG for left main coronary disease. N Engl J Med, 2019; 381: 1820–1830. doi:10.1056/NEJMoa1909406.
- Shawon MSR, Falster MO, Hsu B, Yu J, Ooi SY, Jorm L. Trends and Outcomes for Percutaneous Coronary Intervention and Coronary Artery Bypass Graft Surgery in New South Wales from 2008 to 2019. Am J Cardiol, 2023; 187: 110-118. doi: 10.1016/j.amjcard.2022.10.047.
- Langabeer James R, et al. Growth in percutaneous coronary intervention capacity relative to population and disease prevalence. Journal of the American Heart Association, 2013; 2(6): e000370.
- Box LC, Blankenship JC, Henry TD, et al. SCAI position statement on the performance of percutaneous coronary intervention in ambulatory surgical centers. Catheter Cardiovasc Interv, 2020; 96: 862– 870. https://doi.org/10.1002/ccd.28991.
- Gong X, Zhou L, Dong T, Ding X, Zhao H, Chen H, et al. Impact of COVID-19 pandemic on STEMI undergoing primary PCI treatment in Beijing, China. Am J Emerg Med, 2022; 53: 68-72. doi:10.1016/j.ajem.2021.11.034.
- Serruys PW, Kutryk MJ, Ong AT. Coronary-artery stents. N Engl J Med, 2006; 354(5): 483-495. doi: 10.1056/NEJMra051091.
- Aoki J, Tanabe K. Mechanisms of drug-eluting stent restenosis. Cardiovasc Interv Ther, 2021; 36(1): 23-29. doi: 10.1007/s12928-020-00734-7.
- Hamon M, Bauters C, McFadden EP, Wernert N, Lablanche JM, Dupuis B, et al. Restenosis after coronary angioplasty. Eur Heart J, 1995; 16 Suppl I: 33-48. doi: 10.1093/eurheartj/16.suppl_i.33.
- Jukema JW, Verschuren JJ, Ahmed TA, Quax PH. Restenosis after PCI. Part 1: pathophysiology and risk factors. Nat Rev Cardiol, 2011; 9(1): 53-62. doi: 10.1038/nrcardio.2011.132.
- Dangas GD, Claessen BE, Caixeta A, Sanidas EA, Mintz GS, Mehran R. In-stent restenosis in the drug-eluting stent era. J Am Coll Cardiol, 2010; 56(23): 1897-907. doi: 10.1016/j.jacc.2010.07.028.
- Niccoli G, Montone RA, Ferrante G, Crea F. The evolving role of inflammatory biomarkers in risk assessment after stent implantation. J Am Coll Cardiol, 2010; 56(22): 1783-1793. doi: 10.1016/j.jacc.2010.06.045.
- Walter DH, Fichtlscherer S, Sellwig M, Auch-Schwelk W, Schächinger V, Zeiher AM. Preprocedural C-reactive protein levels and cardiovascular events after coronary stent implantation. J Am Coll Cardiol, 2001; 37(3): 839-846. doi: 10.1016/s0735- 1097(00)01193-1.
- Ferrante G, Niccoli G, Biasucci LM, Liuzzo G, Burzotta F, Galiuto L, et al. Association between C-reactive protein and angiographic restenosis after bare metal stents: an updated and comprehensive meta-analysis of 2747 patients. Cardiovasc Revasc Med, 2008; 9(3): 156-165. doi: 10.1016/j.carrev.2008.01.003.
- Dibra A, Mehilli J, Braun S, Hadamitzky M, Baum H, Dirschinger J, Schühlen H, Schömig A, Kastrati A. Inflammatory response after intervention assessed by serial C- reactive protein measurements correlate with restenosis in patients treated with coronary stenting. Am Heart J, 2005; 150(2): 344-350. doi: 10.1016/j.ahj.2004.09.030.
- Park DW, Lee CW, Yun SC, Kim YH, Hong MK, Kim JJ, et al. Prognostic impact of preprocedural C reactive protein levels on 6-month angiographic and 1-year clinical outcomes after drug-eluting stent implantation. Heart, 2007; 93(9): 1087-1092. doi: 10.1136/hrt.2006.099762.
- Park DW, Yun SC, Lee JY, Kim WJ, Kang SJ, Lee SW, et al. C-reactive protein and the risk of stent thrombosis and cardiovascular events after drug-eluting stent implantation. Circulation, 2009; 120(20): 1987-1995. doi:10.1161/CIRCULATIONAHA.109.876763.
- Speidl WS, Katsaros KM, Kastl SP, Zorn G, Huber K, Maurer G, et al. Coronary late lumen loss of drug eluting stents is associated with increased serum levels of the complement components C3a and C5a. Atherosclerosis, 2010; 208(1): 285-289. doi: 10.1016/j.atherosclerosis.2009.07.030.
- Katsaros KM, Speidl WS, Kastl SP, Zorn G, Huber K, Maurer G, et al. Plasminogen activator inhibitor-1 predicts coronary in-stent restenosis of drug- eluting stents. J Thromb Haemost, 2008; 6(3): 508-513. doi: 10.1111/j.1538-7836.2007.02884.x.
- Katsaros K, Kastl S, Zorn G, et al. Increased Restenosis Rate After Implantation of Drug-Eluting Stents in Patients With Elevated Serum Activity of Matrix Metalloproteinase-2 and -9. J Am Coll Cardiol Intv, 2010; 3(1): 90–97. https://doi.org/10.1016/j.jcin.2009.10.023.
- Farooq V, Gogas BD, Serruys PW. Restenosis: delineating the numerous causes of drug-eluting stent restenosis. Circ Cardiovasc Interv, 2011; 4(2): 195-205. doi: 10.1161/CIRCINTERVENTIONS.110.959882.
- Park SJ, Kang SJ, Virmani R, Nakano M, Ueda Y. In-stent neoatherosclerosis: a final common pathway of late stent failure. J Am Coll Cardiol, 2012; 59(23): 2051-2057. doi: 10.1016/j.jacc.2011.10.909.
- Otsuka F, Byrne RA, Yahagi K, Mori H, Ladich E, Fowler DR, et al. Neoatherosclerosis: overview of histopathologic findings and implications for intravascular imaging assessment. Eur Heart J, 2015; 36(32): 2147-2159. doi: 10.1093/eurheartj/ehv205.
- Shlofmitz E, Iantorno M, Waksman R. Restenosis of Drug-Eluting Stents: A New Classification System Based on Disease Mechanism to Guide Treatment and State-of-the-Art Review. Circ Cardiovasc Interv, 2019; 12(8): e007023. doi:10.1161/CIRCINTERVENTIONS.118.007023. Erratum in: CircCardiovasc Interv. 2019; 12(10): e000044.
- Huang S, Houghton PJ. Mechanisms of resistance to rapamycins. Drug Resist Update, 2001; 4(6): 378-391. doi: 10.1054/drup.2002.0227.
- Costa MA, Simon DI. Molecular basis of restenosis and drug-eluting stents. Circulation, 2005; 111(17): 2257-2273. doi: 10.1161/01.CIR.0000163587.36485.A7.
- Köster R, Vieluf D, Kiehn M, Sommerauer M, Kähler J, Baldus S, et al. Nickel and molybdenum contact allergies in patients with coronary in-stent restenosis. Lancet, 2000; 356(9245): 1895-1897. doi: 10.1016/S0140-6736(00)03262-1. Erratum in: Lancet 2001; 357(9252): 316.
- Fujii K, Mintz GS, Kobayashi Y, Carlier SG, Takebayashi H, Yasuda T, et al. Contribution of stent underexpansion to recurrence after sirolimus-eluting stent implantation for in-stent restenosis. Circulation, 2004; 109(9): 1085-1088. doi: 10.1161/01.CIR.0000121327.67756.19.
- Koskinas KC, Chatzizisis YS, Antoniadis AP, Giannoglou GD. Role of endothelial shear stress in stent restenosis and thrombosis: pathophysiologic mechanisms and implications for clinical translation. J Am Coll Cardiol, 2012; 59(15): 1337-1349. doi: 10.1016/j.jacc.2011.10.903.
- Saia F, Lemos PA, Arampatzis CA, Hoye A, McFadden E, Sianos G, et al. Clinical and angiographic outcomes after over dilatation of undersized sirolimus-eluting stents with largely oversized balloons: an observational study. Catheter Cardiovasc Interv, 2004; 61(4): 455-460. doi: 10.1002/ccd.20001.
- Nakazawa G, Finn AV, Vorpahl M, Ladich E, Kutys R, Balazs I, et al. Incidence and predictors of drug-eluting stent fracture in human coronary artery a pathologic analysis. J Am Coll Cardiol, 2009; 54(21): 1924-1931. doi:10.1016/j.jacc.2009.05.075.
- Kereiakes DJ, Wang H, Popma JJ, Kuntz RE, Donohoe DJ, Schofer J, et al. Periprocedural and late consequences of overlapping Cypher sirolimus-eluting stents: pooled analysis of five clinical trials. J Am Coll Cardiol, 2006; 48(1): 21-31. doi: 10.1016/j.jacc.2006.02.058.
- Wiemer M, Butz T, Schmidt W, Schmitz KP, Horstkotte D, Langer C. Scanning electron microscopic analysis of different drug eluting stents after failed implantation: from nearly undamaged to major damaged polymers. Catheter Cardiovasc Interv, 2010; 75(6): 905-911. doi: 10.1002/ccd.22347.
- Takebayashi H, Mintz GS, Carlier SG, Kobayashi Y, Fujii K, Yasuda T, et al. Nonuniform strut distribution correlates with more neointimal hyperplasia after sirolimus-eluting stent implantation. Circulation, 2004; 110(22): 3430-3434. doi: 10.1161/01.CIR.0000148371.53174.05.
- Monraats PS, Pires NM, Agema WR, Zwinderman AH, Schepers A, de Maat MP, et al. Genetic inflammatory factors predict restenosis after percutaneous coronary interventions. Circulation, 2005; 112(16): 2417-2425. doi: 10.1161/CIRCULATIONAHA.105.536268.
- Kastrati A, Schömig A, Seyfarth M, Koch W, Elezi S, Böttiger C, et al. PlA polymorphism of platelet glycoprotein IIIa and risk of restenosis after coronary stent placement. Circulation, 1999; 99(8): 1005-1010. doi:10.1161/01.cir.99.8.1005.
- Magalhaes MA, Minha S, Chen F, et al. Clinical Presentation and Outcomes of Coronary In-Stent Restenosis Across 3-Stent Generations. Circ Cardiovasc Interv, 2014; 7(6): 768-776. doi: 10.1161/CIRCINTERVENTIONS.114.001341
- Alfonso F, Coughlan JC, Giacoppo D, Kastrati A, Byrne RB. Management of in-stent restenosis. EuroIntervention, 2022; 18(2): e103-e123. doi: 10.4244/EIJ-D-21-01034
- Moussa ID, Mohananey D, Saucedo J, et al. Trends and Outcomes of Restenosis After Coronary Stent Implantation in the United States. J Am Coll Cardiol, 2020; 76(13): 1521-1531. doi: 10.1016/j.jacc.2020.08.002
- Wang P, Qiao H, Wang R, Hou R, Guo J. The characteristics and risk factors of in-stent restenosis in patients with percutaneous coronary intervention: what can we do. BMC Cardiovasc Disord, 2020; 20(1): 510. doi: 10.1186/s12872-020-01798-2
- Fernando Alfonso, Coughlan JJ, Daniele Giacoppo, Adnan Kastrati, Robert A Byrne. Management of in-stent restenosis.
- Giacoppo D, Gargiulo G, Aruta P, Capranzano P, Tamburino C, Capodanno D. Treatment strategies for coronary in-stent restenosis: systematic review and hierarchical Bayesian network meta-analysis of 24 randomised trials and 4880 patients. BMJ, 2015; 351: h5392.
- Siontis GC, Stefanini GG, Mavridis D, Siontis KC, Alfonso F, Pérez-Vizcayno MJ, et al. Percutaneous coronary interventional strategies for treatment of in-stent restenosis: a network meta-analysis. Lancet, 2015; 386: 655–64.
- Byrne RA, Joner M, Alfonso F, Kastrati A. Drug-coated balloon therapy in coronary and peripheral artery disease. Nat Rev Cardiol, 2014; 11: 13–23.
- Katsanos K, Spiliopoulos S, Kitrou P, Krokidis M, Karnabatidis D. Risk of death following application of paclitaxel-coated balloons and stents in the femoropopliteal artery of the leg: a systematic review and meta-analysis of randomized controlled trials.J Am Heart Assoc, 2018; 7: e011245. doi:10.1161/JAHA.118.011245.
- Teirstein PS, Massullo V, Jani S, Popma JJ, Mintz GS, Russo RJ, et al. Catheter-based radiotherapy to inhibit restenosis after coronary stenting. N Engl JMed, 1997; 336: 1697–1703.
- Negi SI, Torguson R, Gai J, Kiramijyan S, Koifman E, Chan R, et al. Intracoronary Brachytherapy for Recurrent Drug-Eluting Stent Failure. JACC Cardiovasc Interv, 2016; 9: 1259–1265.
- Megaly M, Glogoza M, Xenogiannis I, Vemmou E, Nikolakopoulos I, Omer M, et al. Coronary Intravascular Brachytherapy for Recurrent Coronary Drug-Eluting Stent In-Stent Restenosis: A Systematic Review and Meta-Analysis. Cardiovasc Revasc Med, 2021; 23: 28–35.
- Shlofmitz E, Iantorno M, Waksman R. Restenosis of Drug-Eluting Stents: A New Classification System Based on Disease Mechanism to Guide Treatment and State-of-the-Art Review. Circ Cardiovasc Interv, 2019; 12(8): e007023. doi: 10.1161/CIRCINTERVENTIONS.118.007023. Erratum in: Circ Cardiovasc Interv. 2019; 12(10): e000044.
- Lin KH, Li JY, Chen RJ, Chen TY, Hsu SH, Wang HH, et al. Paclitaxel exerts antiplatelet and antithrombotic activities: Additional benefit from use of paclitaxel-coated balloons and -eluting stents in coronary revascularization and prevention of in-stent restenosis. Thromb Res, 2023; 225: 63-72. doi:10.1016/j.thromres.2023.03.017.
- Chen M, Ma F, Su B, Wang C, Zheng Q, Zhang Y, et al. Treatment effect of metformin combined with atorvastatin in reducing in-stent restenosis after percutaneous coronary intervention in coronary artery disease patients with type 2 diabetic patients. Medicine (Baltimore), 2022; 101(41): e31107. doi:10.1097/MD.0000000000031107.

