Hepatorenal Syndrome- An Update
Lalita Gouri Mitra1,*, Udit Dhingra2 and Anil Yadav2
1Department of Anaesthesia, Critical Care and Pain, Homi Bhabha Cancer Hospital & Research Centre, India
2Department of Anaesthesia Critical Care, Institute of Liver and Biliary Sciences, India
Received Date: 11/10/2023; Published Date: 15/03/2024
*Corresponding author: Lalita Gouri Mitra, Department of Anaesthesia, Critical Care and Pain, Homi Bhabha Cancer Hospital & Research Centre, Medicity, Mullanpur, India
Abbreviations: HRS-Hepatorenal Syndrome; AKI-Acute Kidney Injury; CKD-Chronic Kidney Disease; GFR- Glomerular Filtration Rate; EABV-Effective Arterial Blood Volume; RAI-Relative Adrenal Insufficiency; DAMP-Damage Associated Molecular Pattern; PAMP-Pathogen Associated Molecular Pattern; TLR-Toll-Like Receptor; AKIN-Acute Kidney Injury Network; RIFLE-Risk, Injury, Failure, Loss, and End stage kidney disease; KDIGO-Kidney Disease Improving Global Outcome; ICA-International Club of Ascites; NAKI-Non-Acute Kidney Injury; ATN-Acute Tubular Necrosis; NGAL-Neutrophil Gelatinase Associated Lipocalin; FeNa-Fractional Excretion of Sodium; MAP-Mean Arterial Pressure; RRT-Renal Replacement Therapy; SBP-Spontaneous Bacterial Peritonitis; TIPS-Transjugular Intrahepatic Portosystemic Shunt; sCr- Serum Creatinine; SLK- Simultaneous Liver-kidney Transplant
Introduction
Advanced liver disease is characterized by portal hypertension, increased splanchnic blood flow, hyperdynamic circulation with increased cardiac output, deranged coagulation profile, and decreased central blood volume. Renal dysfunction is a common, life-threatening complication arising from the complex pathophysiological changes occurring in advanced liver disease [1,2], which could be prerenal, intrarenal, or post renal. Patients with cirrhosis develop a specific phenotype of renal dysfunction that has been termed Hepatorenal syndrome (HRS). HRS has been defined as renal dysfunction that occurs because of reduced renal perfusion, due to hemodynamic alterations in arterial circulation, as well as overactivity of the endogenous vasoactive systems [3]. Definition, terminology, and classification of HRS have evolved considerably over time due to various changes in staging and diagnosis of Acute Kidney Injury (AKI).
Pathophysiology
Pathophysiology of HRS is based upon observational studies in humans. Inducing liver injury in animal models with carbon tetrachloride and thioacetamide leads to kidney injury as well, hence similar reproducible studies in animal models are lacking. HRS has been termed as a functional dysfunction based on [4]:
- Absence of significant renal histological changes in post-mortem examinations.
- Reversibility of renal dysfunction by liver transplant.
- Classical images of HRS showing extreme but reversible renal vasoconstriction.
- Ability to use kidneys from patients with HRS as grafts for renal transplantation.
All clinical and histopathological observations point to uncompensated circulatory dysfunction as the hallmark of HRS. Other major factors include systemic inflammation, cirrhotic cardiomyopathy, and adrenal insufficiency.
Circulatory Dysfunction
Splanchnic vasodilatation occurs due to increased production of vasodilators like nitric oxide, carbon monoxide, prostacyclins, and endocannabinoids [5]. This leads to hemodynamic changes triggered by elevated intrahepatic vascular resistance causing the kidney functions to decline in cirrhosis.
Initial stages of the disease are characterized by moderate splanchnic vasodilation and slightly reduced Systemic Vascular Resistance (SVR), which is balanced by an increase in cardiac output. With advanced disease, vasodilation further worsens, and cannot be balanced by the increase in cardiac output, leading to decrease in Effective Arterial Blood Volume (EABV) and systemic arterial pressure [6].
Decrease in EABV and systemic arterial pressure results in activation of Systemic vasoconstrictor pathways, such as the Renin-Angiotensin-Aldosterone System (RAAS), sympathetic nervous system, and arginine vasopressin leading to sodium retention, impaired solute-free water excretion, and renal vasoconstriction, and, consequently, reduced renal blood flow [7].
As liver disease proceeds there is a degree of reduction of cardiac output due to development of cirrhotic cardiomyopathy, thus suggesting a role of cirrhotic cardiomyopathy in the pathogenesis of HRS [8].
In early stages of liver disease, the kidneys can maintain adequate glomerular filtration rate (GFR) by causing vasodilation of afferent arteriole by vasodilatory renal prostaglandins E2 and I2 despite reduced renal blood flow. As the disease progresses there is a disruption of balance leading to reduced vasodilatory prostaglandins and intense kidney vasoconstriction leading to compromised kidney perfusion thus decreasing the GFR, ultimately leading to the development of HRS (Figure 1).
Systemic inflammation
Recently, the concept of systemic inflammatory disease in cirrhosis has emerged, with growing evidence that inflammation plays a role in HRS [9].
Two diverse groups of molecules are responsible for the inflammatory response in cirrhotic patients: Pathogen-Associated Molecular Patterns (PAMPs) and damage-associated molecular patterns (DAMPs) [10]. PAMPs are bacterial products, such as lipopolysaccharide, flagellin, and nigericin, arising from translocation of gut bacteria, whereas DAMPs are released from injured hepatocytes, including high mobility group protein B1, heat shock protein, adenosine triphosphate, and double stranded genomic DNA. Without any apparent active bacterial infection, PAMPs and DAMPs drive inflammation [11] through release of pro-inflammatory cytokines by activating toll-like receptors (TLRs), leading to an increased arterial production of vasodilators (such as nitric oxide) and, consequently, further reducing the SVR and EABV (Figure 2).
Hepato-adrenal syndrome
Relative adrenal insufficiency (RAI) or hepatoadrenal syndrome is present in a subset of patients with decompensated cirrhosis [12]. RAI decreases arterial pressure, increases serum concentrations of renin and noradrenaline and increases risk of HRS [13].
Pathophysiology of RAI is not fully understood but may be due to exhaustion of substrates for synthesis of cortisol and impairment of the hypothalamus-pituitary axis by circulating PAMPs and pro-inflammatory cytokines [14].
Glucocorticoid replacement therapy has been shown to improve patients with RAI and septic shock but a similar effect for effective prevention and treatment of HRS with RAI has still not been proven [15].

Figure 1: Pathophysiology of HRS.
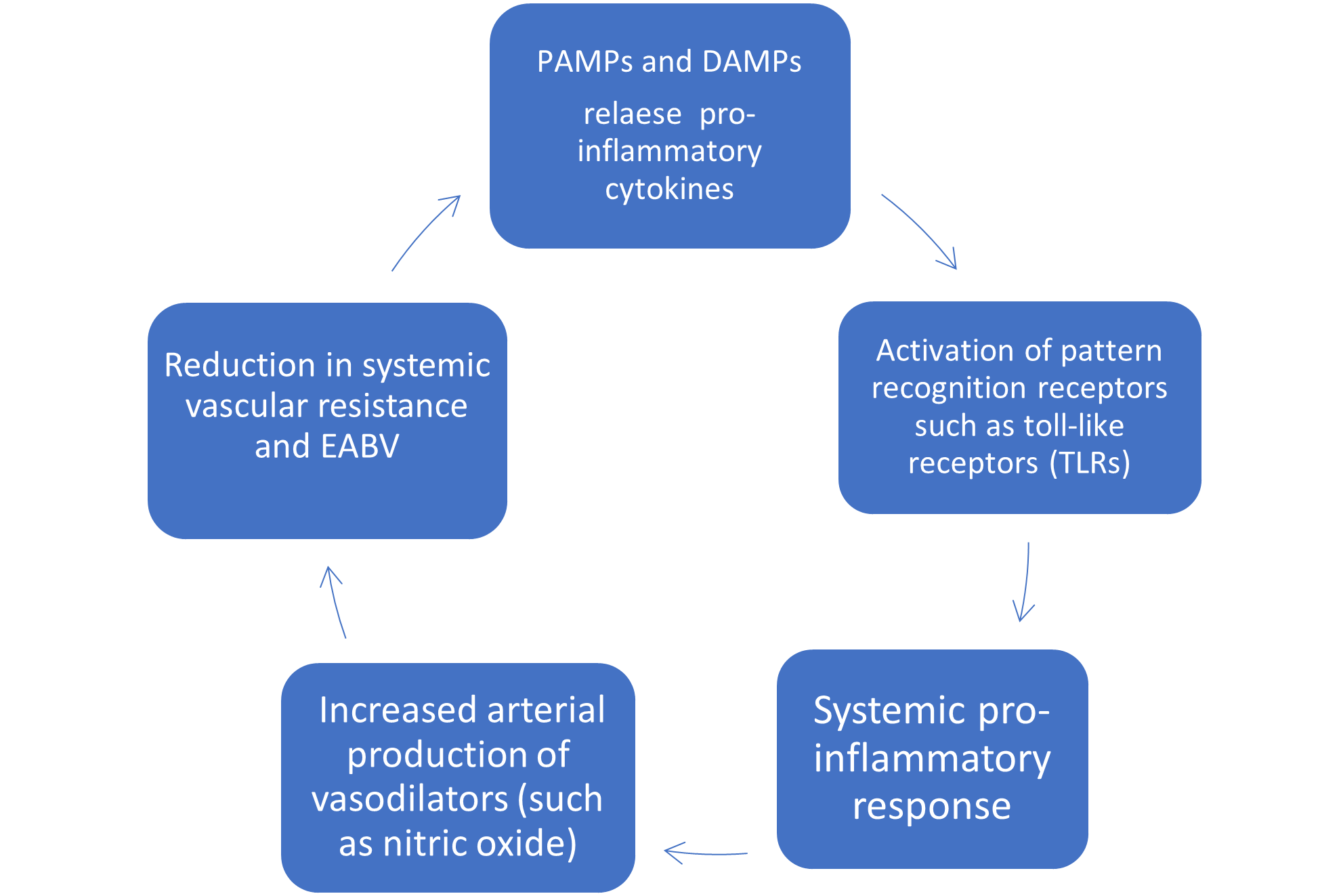
Figure 2: PAMP and DAMP’s driving systemic inflammation and peripheral vasodilatation.
Table 1: Comparison of old (1990) vs new definition (2015) of HRS.

Table 2: Staging of AKI according to international ascites club [23].

Table 3: Dosing regimens, side effects, goals of treatment of vasoconstrictors for HRS.
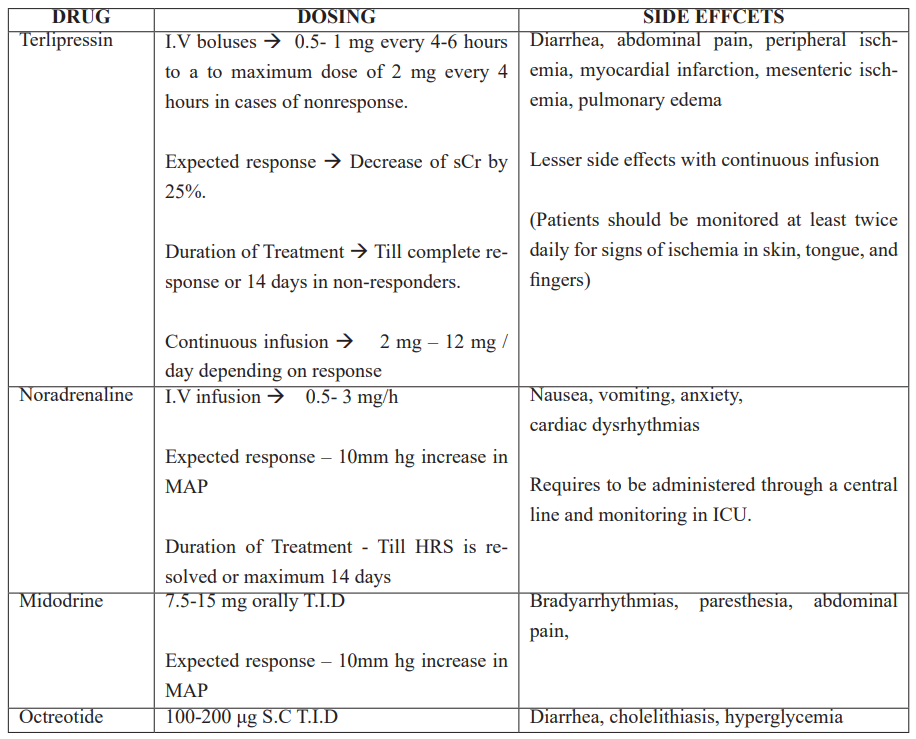
Table 4: Eligibility Criteria for Simultaneous Liver–Kidney Transplantation.

Definition and Classification
Impairment of renal function has been determined by an increase in serum creatinine (sCr). However, it is not the best marker for determining renal injury in patients with cirrhosis due to [16]:
- Impaired hepatic production of creatine (precursor of creatinine)
- Reduced muscle mass
- Tubular secretion of creatinine
- Inaccurate measurement of creatinine by calorimetric methods in the presence of elevated bilirubin
Despite the caveats, serum creatinine remains the most widely available and used assay for GFR estimation in patients with cirrhosis. Also, in dynamic conditions like acute renal failure, serial changes in serum creatinine and urine output are a better reflection of renal function.
The definition of acute renal failure has evolved over the past two decades with a better understanding of its pathophysiology and treatment outcomes. The RIFLE (risk, injury, failure, loss, and end stage kidney disease) classification was the earliest definition coined at the Consensus Conference of the Acute Dialysis Quality Initiative Group in 2002. It defined renal injury in term of percentage changes in serum creatinine or GFR, a decrease in urine output, or both [17]. In 2005 the term acute kidney injury (AKI) was proposed by the second multidisciplinary collaborative forum (AKIN) to include the entire spectrum of acute renal failure. Absolute increase in serum creatinine of 0.3 mg/dL within 48 hours of baseline was added as part of the definition of AKI [18].
Kidney Disease Improving Global Outcome (KDIGO) organization in 2012 defined AKI as [19]
- Increase in serum creatinine(sCr) by at least 0.3 mg/dL (26.5 μmol/L) within 48 hours.
- Increase in serum creatinine(sCr) to at least 1.5 times baseline within the previous week.
- Urine and or volume below 0.5 mL/kg/h for six hours.
The definition of HRS has similarly evolved over the past two decades aligning with changes made according to RIFLE, AKIN, and KIDIGO criteria.
Hepatorenal syndrome is a diagnosis of exclusion and other potential etiologies of acute or subacute kidney injury in patients with liver disease should be ruled out before a diagnosis of HRS is made.
In 1990 HRS was defined by the international ascites club as an increase in sCr of at least 50% from baseline to a final concentration of at least 1.5 mg/dL in cirrhotic patients. They further classified HRS into two clinical: Type 1 and Type 2 [20].
Studies demonstrated that diagnosis of AKI in patients with cirrhosis, based on an absolute
increase in sCr by at least 0.3 mg/dl or 50% from baseline, led to earlier identification of patients at increased risk of more severe disease, prolonged hospital stays, and ICU admissions [21,22]. This led the ICA to issue a revised set of consensus recommendations, without the cut-off value of sCr ≥1.5 mg/dl, thus ensuring early diagnosis and faster treatment. The lowest value of sCr in the past three months can be taken as baseline to avoid diagnostic delays. The ICA consensus updated the term hepatorenal syndrome (HRS) type 1 and renamed it HRS-AKI. HRS-AKI can be diagnosed even when the sCr is below 2.5 mg/dL. The terminology HRS type 2 was removed and replaced by HRS-NAKI (Table 1) [23].
Differential diagnosis
Patients with liver disease are also predisposed for development of acute tubular necrosis (ATN) and as the diagnostic criteria of HRS does not rule out the presence of tubular damage, ATN cannot be confidently excluded with the above criteria and may coexist along with HRS in patients with liver disease.
Urinary sodium (>40 mEq/L), fractional excretion of sodium (FeNa >2%), and low urine osmolality (<400 mOsm/L) are features suggestive of ATN. However, these parameters are not good predictors of ATN in patients with cirrhosis and ascites as [24]:
- Urinary sodium can be elevated secondary to diuretics, in patients with large-volume ascites.
- Low FeNa has also been observed in patients with biopsy-proven ATN.
Hence urinary sodium and FeNa have been excluded from diagnostic criteria of HRS.
Urine biomarkers of tubular injury have long been studied to differentiate between ATN and AKI-HRS in patients with cirrhosis. Biomarkers include [25,26]:
- Tubular proteins released during cell damageà N-acetyl-β-D-glucosaminidase, α-glutathione S transferase)
- Tubular proteins up-regulated by injury àkidney injury molecule-1, neutrophil gelatinase associated lipocalin (NGAL), liver-type fatty acid binding protein
- Plasma proteins with diminished tubular reabsorption à α-1-microglobulin, β-2-microglobulin, retinol binding protein
- Inflammatory markers à Interleukin-18
NGAL has shown the greatest diagnostic accuracy in differentiating ATN from AKI-HRS.
Urinary NGAL performs better than plasma NGAL when measured after a two-day volume challenge recommended in the management of AKI. The urinary NGAL cut-off value of 220 μg/g of creatinine obtained after the fluid challenge has the highest diagnostic accuracy for ATN [27].
Prevention
Acute impairment of renal function is a common, life threatening complication in patients with cirrhosis,1,2 so it is imperative to take appropriate steps to prevent development of hepatorenal syndrome (HRS) once a patient is diagnosed with cirrhosis.
Preventive strategies:
- Minimize hemodynamic and circulatory dysfunction,
- Avoid agents that precipitate AKI,
- Reverse acute decompensation,
- Delay progression of disease in compensated patients.
Hyponatremia, liver size, increased plasma renin activity, severity of ascites, as well as acute hemodynamic changes associated with large volume paracentesis, are important precipitant factors for development of HRS [28,29]. 30% of patients with spontaneous bacterial peritonitis (SBP) develop HRS emphasizing the need for antibiotic prophylaxis in such cases. Antibiotic prophylaxis in patients at risk of SBP not only prevents the development of HRS but also reduces overall mortality [30].
Albumin plays an important role in delaying the development and improving the overall survival in patients with HRS (1.5gm/kg on day 1 followed by 1gm/kg on day 3). Albumin along with antibiotics in patients with SBP improves the circulatory functions [31]. Along with its role as a volume expander albumin also has antioxidant and anti-inflammatory properties that help stabilize endothelial functions. It binds to endotoxins and inactivates them which improve circulation and kidney function [32]. Albumin administration following large volume paracentesis prevents hypotension and hyponatremia, both of which can precipitate HRS [33]. Long-term use of weekly Albumin has been shown to improve overall survival and reduce incidence of HRS [34]. However, it is expensive and may cause volume overload.
Management
Treatment of HRS should begin as soon as the diagnosis is confirmed as early treatment leads to higher reversal and better outcomes. Reversal of HRS is defined by at least 50% reduction in sCr to a value below 1.5gm/dl. The updated diagnostic criteria with removal of minimum serum creatinine criteria aids in earlier diagnosis and treatment, rather than waiting for sCr to reach 2.5 g/dl. Treatment of HRS depends on the stage of AKI (Table 2). Initial management of HRS starts with a fluid challenge of 20-25% albumin 1m/kg/day for 2 days regardless of the stage of AKI and withdrawal of diuretics, beta-blockers and other drugs causing AKI.
Mainstay of treatment includes vasoconstrictors, Albumin, and reversal of precipitating factors, and antibiotics for SBP. Stage 1A AKI is mostly secondary to hypovolemia and resolves in more than 90% of patients with fluid challenge and diuretic withdrawal as compared to 40% patients with stage 1B disease. Hence use of vasoconstrictors is recommended for patients with AKI-HRS stage 1B or greater [35].
Vasoconstrictors: These drugs act by causing splanchnic vasoconstriction which results in reduction in the portal pressure and increases the EABV. These effects are more pronounced when combined with Albumin. Ascites decreases the mean arterial pressure (MAP) which further decreases renal blood flow. Vasoconstrictors also increase the MAP which results in increased likelihood of reversal of HRS [36]. Terlipressin, Noradrenaline, and combination of Octreotide and Midodrine are the available options (Table 3).
Terlipressin
Terlipressin is a Synthetic vasopressin analog with predominant vasopressin 1A action causing splanchnic vasoconstriction [37]. It also acts on vasopressin 1B receptor causing release of adrenocorticotropic and cortisol hormones, which helps in counteracting the relative adrenal insufficiency seen in patients with decompensated cirrhosis. It also has indirect vasopressin mediated anti-inflammatory effects [38]. Terlipressin also acts on vasopressin 2 receptors, worsening hyponatremia and may cause volume overload [39]. Studies have demonstrated that combination of Terlipressin and Albumin is more effective than Albumin alone in treating AKI [40].
Other agents
Noradrenaline (intravenous) and Midodrine (oral) act via activation of alpha1 adrenergic receptors on vascular smooth muscle cells. Another somatostatin analog, Octreotide acts via inhibiting secretion of glucagon, a splanchnic vasodilator, and is a direct mesenteric vasoconstrictor [41].
Efficacy of vasoconstrictors
Studies evaluating Terlipressin, Norepinephrine, and/or Octreotide/ Midodrine have found Terlipressin, in combination with intravenous Albumin, to be the most effective drug treatment for AKI-HRS [42]. Combination of Terlipressin plus Albumin have an efficacy ranging from 19% to 56% compared to 3-14% of Albumin alone in reversal of HRS. Terlipressin alone was found markedly inferior to a combination of Terlipressin and Albumin [43-46].
Norepinephrine has been found to be an effective alternate for Terlipressin in AKI-HRS, with similar rates of HRS reversal in some randomized studies [46,47]. Norepinephrine is cost effective compared to Terlipressin, but requires central line placement, intensive care unit admission, which may offset the cost benefit. Terlipressin in comparison can be given through a peripheral line in wards.
Both Octreotide and Midodrine monotherapy and combination of Midodrine/Octreotide have limited benefits in treating HRS [48,49].
Albumin: Albumin’s multifaceted mode of action makes it the colloid of choice in treatment of HRS. Apart from being a volume expander and consequently increasing the EABV, Albumin has several other benefits. It is an antioxidant with a positive inotropic effect [50] and immunomodulatory properties. As compared to hydroxyethyl starch, Albumin causes reduced endothelial activation as lower plasma levels of von Willebrand related antigen and factor VII are seen in patients who were administered Albumin. Its ability to bind with variety of substances like bile acids, hormones cytokines, endotoxins, and bacterial products results in significant reduction in serum creatinine. Albumin (20%, 25%) should be used at a dose of 20-40g/day till HRS reverses. Serial central venous pressure measurements can be used to assess central blood volume and avoid circulatory overload [44].
Renal replacement therapy: Renal replacement therapy is indicated in patients unresponsive to drug treatment, worsening acidosis, electrolyte disturbances, or circulatory overload. RRT is usually a bridge to transplantation and does not provide any survival benefit [51]. Patients with cirrhosis and AKI who are not candidates for transplant have a mortality of 90%, making RRT futile in these settings [52].
Transjugular intrahepatic portosystemic shunt (TIPS): TIPS aims to reduce the portal pressures by creating an intrahepatic shunt in patients who have refractory ascites, diuretic intolerant, or uncontrolled variceal bleeding. Significant reduction in plasma renin activity, aldosterone, and noradrenaline levels has been reported [53]. Role of TIPS to reverse or limit the progress of HRS is limited as patients with markedly elevated bilirubin, overt encephalopathy or active infection are not good candidates for the procedure.
Liver transplantation: Liver Transplant (LT) remains the best optional treatment for HRS. The functional nature of HRS means that renal function is expected to improve after LT. However, accurately predicting renal recovery after transplant remains a mystery. About 10% of patients with AKI or chronic kidney disease (CKD) may continue to have renal failure post LT alone [54]. Renal recovery after LT depends on factors like duration of kidney disease, presence, or absence of ATN, age, etiology of AKI. In such cases, simultaneous liver-kidney transplant (SLK) should be considered. The Organ procurement and transplantation network policy has developed listing criteria for SLK transplant and includes elements like duration of AKI, need for dialysis, and evidence of CKD. It includes patients with sustained AKI, defined as those who need dialysis or calculated creatinine clearance or GFR of 25ml/min (Table 4) [55].
Newer Modalities
The efficacy of albumin and vasoconstrictors is limited to less than half of the patients with HRS, which leads to search for newer novel agents. One such agent Serelaxin (recombinant human relaxin 2) acts on renal vasculature and results in increased renal blood flow, decreased renal vascular resistance, and reversal of endothelial dysfunction. It has also shown to reduce intrahepatic vascular resistance in animal models [56,57].
Treatments targeting systemic inflammation like DAMPs and PAMPs need further research. Assessment of arterial kidney resistive indexes by doppler ultrasonography, contrast enhanced ultrasonography and magnetic resonance elastography need to be further explored for better diagnosis and management of HRS [58].
References
- Wu CC, Yeung LK, Tsai WS, et al. Incidence and factors predictive of acute renal failure in patients with advanced liver cirrhosis. Clin Nephrol, 2006; 65: 28-33. doi:10.5414/CNP65028
- Piano S, Rosi S, Maresio G, et al. Evaluation of the Acute Kidney Injury Network criteria in hospitalized patients with cirrhosis and ascites. JHepatol, 2013; 59: 482-489. doi:10.1016/j.jhep.2013.03.039
- Salerno F, Gerbes A, Gines P, et al. Diagnosis, prevention and treatment of the hepatorenal syndrome in cirrhosis a consensus workshop of the International Ascites Club. Gut, 2007; 56: 1310-1318.
- Arroyo V, Gines P, Gerbes AL, Dudley FJ, Gentilini P, Laffi G, et al. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. Hepatology, 1996; 23: 164-176.
- Schrier RW, Arroyo V, Bernardi M, Epstein M, Henriksen JH, Rode´s J. Peripheral arterial vasodilation hypothesis: A proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology, 1988; 8: 1151–1157.
- Ruiz-del-Arbol L, Monescillo A, Arocena C, et al. Circulatory function and hepatorenal syndrome in cirrhosis. Hepatology, 2005; 42: 439-447. doi:10.1002/hep.20766
- Varga ZV, Erdelyi K, Paloczi J, et al. Disruption of Renal Arginine Metabolism Promotes Kidney Injury in Hepatorenal Syndrome in Mice. Hepatology, 2018; 68: 1519-1533. doi:10.1002/hep.29915.
- Krag A, Bendtsen F, Henriksen JH, Møller S. Low cardiac output predicts development of hepatorenal syndrome and survival in patients with cirrhosis and ascites. Gut, 2010; 59: 105–110.
- Bernardi M, Moreau R, Angeli P, Schnabl B, Arroyo V. Mechanisms of decompensation and organ failure in cirrhosis: From peripheral arterial vasodilation to systemic inflammation hypothesis. J Hepatol, 2015; 63: 1272–1284.
- Thabut D, Massard J, Gangloff A, et al. Model for end-stage liver disease score and systemic inflammatory response are major prognostic factors in patients with cirrhosis and acute functional renal failure. Hepatology, 2007; 46: 1872-1882. doi:10.1002/ hep.21920.
- Sole C, Sola E, Huelin P, et al. Characterization of inflammatory response in hepatorenal syndrome: Relationship with kidney outcome and survival. Liver Int, 2019; 39: 1246-1255. doi:10.1111/ liv.14037
- Jang JY, Kim TY, Sohn JH, et al. Relative adrenal insufficiency in chronic liver disease: its prevalence and effects on long-term mortality. Aliment Pharmacol Ther, 2014; 40: 819-826. doi:10.1111/apt.12891
- Kim G, Huh JH, Lee KJ, Kim MY, Shim KY, Baik SK. Relative Adrenal Insufficiency in Patients with Cirrhosis: A Systematic Review and Meta-Analysis. Dig Dis Sci, 2017; 62: 1067-1079. doi:10.1007/s10620- 017-4471-4478.
- Bornstein SR. Predisposing factors for adrenal insufficiency. N Engl J Med, 2009; 360: 2328-2339. doi:10.1056/NEJMra0804635.
- Fernandez J, Escorsell A, Zabalza M, et al. Adrenal insufficiency in patients with cirrhosis and septic shock: Effect of treatment with hydrocortisone on survival. Hepatology, 2006; 44: 1288-1295. doi:10.1002/hep.21352.
- Sherman DS, Fish DN, Teitelbaum I. Assessing renal function in cirrhotic patients: problems and pitfalls. Am J Kidney Dis, 2003; 41: 269-278. doi:10.1053/ajkd.2003.50035
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, Acute Dialysis Quality Initiative workgroup. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care, 2004; 8: R204-212.doi:10.1186/cc2872.
- Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care, 2007; 11: R31. doi:10.1186/cc5713.
- Summary of Recommendation Statements. Kidney Int Suppl 2011, 2012; 2: 8-12. doi:10.1038/kisup.2012.7.
- Arroyo V, Gines P, Gerbes AL, et al. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. International Ascites Club. Hepatology, 1996; 23: 164-176. doi:10.1002/hep.510230122
- Belcher JM, Garcia-Tsao G, Sanyal AJ, et al, TRIBE-AKI Consortium. Association of AKI with mortality and complications in hospitalized patients with cirrhosis. Hepatology, 2013; 57: 753-762. doi:10.1002/hep.25735.
- de Carvalho JR, Villela-Nogueira CA, Luiz RR, et al. Acute kidney injury network criteria as a predictor of hospital mortality in cirrhotic patients with ascites. J Clin Gastroenterol, 2012; 46: e21-26. doi:10.1097/MCG.0b013e31822e8e12.
- Angeli P, Gines P, Wong F, et al. International Club of Ascites. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. Gut, 2015; 64: 531-537. doi:10.1136/gutjnl-2014-308874
- Dudley FJ, Kanel GC, Wood LJ, Reynolds TB. Hepatorenal syndrome without avid sodium retention. Hepatology, 1986; 6: 248-251. doi:10.1002/hep.1840060216
- Francoz C, Nadim MK, Durand F. Kidney biomarkers in cirrhosis. J Hepatol, 2016; 65: 809-824. doi: 10.1016/j.jhep.2016.05.025
- Allegretti AS, Sola E, Gines P. Clinical application of kidney biomarkers in cirrhosis. Am J Kidney Dis, 2020; S0272- 6386(20)30691-0.
- Huelin P, Sola E, Elia C, et al. Neutrophil Gelatinase-Associated Lipocalin for Assessment of Acute Kidney Injury in Cirrhosis: A Prospective Study. Hepatology, 2019; 70: 319-333. doi:10.1002/ hep.30592.
- Ginès A, Escorsell A, Ginès P, et al. Incidence, predictive factors, and prognosis of the hepatorenal syndrome in cirrhosis with ascites. Gastroenterology, 1993; 105: 229-236. doi:10.1016/0016- 5085(93)90031-7
- Wong F, Jepsen P, Watson H, Vilstrup H. Un-precipitated acute kidney injury is uncommon among stable patients with cirrhosis and ascites. Liver Int, 2018; 38: 1785-1792. doi:10.1111/liv.13738
- Follo A, Llovet JM, Navasa M, et al. Renal impairment after spontaneous bacterial peritonitis in cirrhosis: incidence, clinical course, predictive factors, and prognosis. Hepatology, 1994; 20: 1495-1501. doi:10.1002/hep.1840200619.
- Fernández J, Angeli P, Trebicka J, et al. Efficacy of Albumin Treatment for Patients with Cirrhosis and Infections Unrelated to Spontaneous Bacterial Peritonitis. Clin Gastroenterol Hepatol, 2020; 18: 963-973. e14. doi:10.1016/j.cgh.2019.07.055
- Chen TA, Tsao YC, Chen A, et al. Effect of intravenous albumin on endotoxin removal, cytokines, and nitric oxide production in patients with cirrhosis and spontaneous bacterial peritonitis. Scand J Gastroenterol, 2009; 44: 619-625. doi:10.1080/00365520902719273
- Ruiz-del-Arbol L, Monescillo A, Jimenéz W, Garcia-Plaza A, Arroyo V, Rodés J. Paracentesis-induced circulatory dysfunction: mechanism and effect on hepatic hemodynamics in cirrhosis. Gastroenterology, 1997; 113: 579-586. doi:10.1053/gast.1997.v113. pm9247479
- Caraceni P, Riggio O, Angeli P, et al. ANSWER Study Investigators. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet, 2018; 391: 2417-2429. doi:10.1016/S0140-6736(18)30840-7.
- European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu, European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol, 2018; 69: 406-460. doi: 10.1016/j.jhep.2018.03.024.
- Facciorusso A, Chandar AK, Murad MH, et al. Comparative efficacy of pharmacological strategies for management of type 1 hepatorenal syndrome: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol, 2017; 2: 94-102. doi:10.1016/S2468- 1253(16)30157-1.
- Jamil K, Pappas SC, Devarakonda KR. In vitro binding and receptor-mediated activity of terlipressin at vasopressin receptors V1 and V2. J Exp Pharmacol, 2017; 10: 1-7. doi:10.2147/JEP.S146034.
- Tanoue A, Ito S, Honda K, et al. The vasopressin V1b receptor critically regulates hypothalamic-pituitary-adrenal axis activity under both stress and resting conditions. J Clin Invest, 2004; 113: 302-309. doi:10.1172/JCI200419656.
- Krag A, Bendtsen F, Pedersen EB, Holstein-Rathlou NH, MøllerS. Effects of terlipressin on the aquaretic system: evidence of antidiuretic effects. Am J Physiol Renal Physiol, 2008; 295: F1295-300. doi:10.1152/ajprenal.90407.2008.
- Sanyal AJ, Boyer TD, Frederick RT, Wong F, Rossaro L, Araya V, et al. Reversal of hepatorenal syndrome type 1 with terlipressin plus albumin vs. placebo plus albumin in a pooled analysis of the OT‐0401 and REVERSE randomised clinical studies. Aliment Pharmacol Ther, 45: 1390-1402. https://doi.org/10.1111/apt.14052.
- Cavallin M, Piano S, Romano A, Fasolato S, Frigo AC, Benetti G, et al. Terlipressin given by continuous intravenous infusion versus intravenous boluses in the treatment of hepatorenal syndrome: A randomized controlled study. Hepatology, 2016; 63: 983-992.
- Martin-Llahi M, Pepin MN, Guevara M, et al, TAHRS Investigators. Terlipressin and albumin vs albumin in patients with cirrhosis and hepatorenal syndrome: a randomized study. Gastroenterology, 2008; 134: 1352-1359. doi: 10.1053/j.gastro.2008.02.024
- Solanki P, Chawla A, Garg R, Gupta R, Jain M, Sarin SK. Beneficial effects of terlipressin in hepatorenal syndrome: a prospective, randomized placebo-controlled clinical trial. J Gastroenterol Hepatol, 2003; 18: 152-156. doi: 10.1046/j.1440-1746.2003.02934.x
- Neri S, Pulvirenti D, Malaguarnera M, et al. Terlipressin and albumin in patients with cirrhosis and type I hepatorenal syndrome. Dig Dis Sci, 2008; 53: 830-835. doi:10.1007/s10620-007-9919-9
- Boyer TD, Sanyal AJ, Wong F, et al. REVERSE Study Investigators. Terlipressin Plus Albumin Is More Effective Than Albumin Alone in Improving Renal Function in Patients with Cirrhosis and Hepatorenal Syndrome Type 1. Gastroenterology, 2016; 150: 1579-1589.e2. doi: 10.1053/j.gastro.2016.02.026
- Wong F, Curry M, Reddy R, et al, CONFIRM Study Investigators. LO5 The CONFIRM study: A North American randomized controlled trial (RCT) of terlipressin plus albumin for the treatment of hepatorenal syndrome type 1 (HRS-1). Hepatology, 2019; 70(Suppl 1): 10.
- Sharma P, Kumar A, Sharma BC, Sarin SK. An open label, pilot, randomized controlled trial of noradrenaline versus terlipressin in the treatment of type 1 hepatorenal syndrome and predictors of response. Am J Gastroenterol, 2008; 103: 1689-1697. doi: 10.1111/ j.1572-0241.2008.01828.x
- Pomier-Layrargues G, Paquin SC, Hassoun Z, Lafortune M, Tran A. Octreotide in hepatorenal syndrome: a randomized, double-blind, placebo-controlled, crossover study. Hepatology, 2003; 38: 238-243. doi:10.1053/jhep.2003.50276.
- Angeli P, Volpin R, Piovan D, et al. Acute effects of the oral administration of midodrine, an alpha-adrenergic agonist, on renal hemodynamics and renal function in cirrhotic patients with ascites. Hepatology, 1998; 28: 937-943. doi:10.1002/hep.510280407
- Bortoluzzi A, Ceolotto G, Gola E, et al. Positive cardiac inotropic effect of albumin infusion in rodents with cirrhosis and ascites: molecular mechanisms. Hepatology, 2013; 57: 266-276. doi:10.1002/ hep.26021.
- Lenhart A, Hussain S, Salgia R. Chances of Renal Recovery or Liver Transplantation After Hospitalization for Alcoholic Liver Disease Requiring Dialysis. Dig Dis Sci, 2018; 63: 2800-2809. doi:10.1007/ s10620-018-5170-9.
- Allegretti AS, Parada XV, Eneanya ND, et al. Prognosis of Patients with Cirrhosis and AKI Who Initiate RRT. Clin J Am Soc Nephrol, 2018; 13: 16-25. doi:10.2215/CJN.03610417
- Guevara M, Ginès P, Bandi JC, et al. Transjugular intrahepatic portosystemic shunt in hepatorenal syndrome: effects on renal function and vasoactive systems. Hepatology, 1998; 28: 416-422. doi:10.1002/hep.510280219.
- Chauhan K, Azzi Y, Faddoul G, et al. Pre-liver transplant renal dysfunction and association with post-transplant end-stage renal disease: A single-center examination of updated UNOS recommendations. Clin Transplant, 2018; 32: e13428. doi:10.1111/ ctr.13428.
- Organ Procurement and Transplantation Network. Policies, 2020.
- Conrad KP. Unveiling the vasodilatory actions and mechanisms of relaxin. Hypertension, 2010; 56: 2-9. doi:10.1161/HYPERTENSIONAHA.109.133926.
- Fallowfield JA, Hayden AL, Snowdon VK, et al. Relaxin modulates human and rat hepatic myofibroblast function and ameliorates portal hypertension in vivo. Hepatology, 2014; 59: 1492-1504. doi:10.1002/ hep.26627.
- Mindikoglu AL, Dowling TC, Wong-You-Cheong JJ, Christenson RH, Magder LS, Hutson WR, et al. A pilot study to evaluate renal hemodynamics in cirrhosis by simultaneous glomerular filtration rate, renal plasma flow, renal resistive indices and biomarkers measurements. Am J Nephrol, 2014; 39: 543–552.

