Role of Developing Resistance to Combined Ceftriaxone - Azithromycin Regimen for Treatment of Gonococcal Infection and An Updated Gonococcal Treatment Guidelines by Centers of Disease Control and Prevention (CDC): A Systematic Review
Jaimin Patel*
Consultant Biochemist & Lab Administrator, Zydus Hospital, India
Received Date: 30/06/2023; Published Date: 16/11/2023
*Corresponding author: Jaimin Patel, Consultant Biochemist & Lab Administrator, Zydus Hospital, India
Abstract
Gonorrhea is a sexually transmitted disease caused by Neisseria gonorrhoeae that is also considered a human pathogen. Neisseria gonorrhoeae has developed resistance overtime to all preliminary and first-line antimicrobial monotherapies. Hence, it is now known as a superbug that will transmit the world into a pandemic stage. This disease has a high burden rate of morbidity with more than new cases of 106 million and is increasing day by day. Since the bacteria are constantly evolving and developing their resistance against all sorts of antimicrobial treatments available, CDC declared that the treatment of gonorrhea would be untreatable in the long term and currently, there is no vaccine-related to gonorrhea; the only treatment options available to prevent this disease are diagnosis, which includes mainly antibiotic treatment and educating people about it. This systematic review focused on explaining the evaluation of gonorrhea and finding alternatives to its molecular dosages and testing. The task is to make gonorrhea a treatable infection ultimately.
Introduction
Gonorrhea is the oldest infection humankind has been suffering from, known as one of the STIs – sexually transmitted infections. Gonorrhea infection has been widely studied since 2014 because the bacteria known by the scientific name Neisseria gonorrhoeae, a strain of drug-resistant bacteria, is causing inflammation in the pelvic area for women [1,2]. The most common site of Neisseria gonorrhoeae infection in the urogenital tract, causing dysuria with penile discharge in men and women may experience mild vaginal mucopurulent discharge, severe pelvic pain, or no symptoms [2,3]. If not treated, N. gonorrhoeae can disseminate to other parts of the body, most commonly present as papules that progress into petechiae, bullae, and necrotic lesions [3]. In earlier days, those suffering from the problem had frequent urination urges along with a clapping sensation while urinating. These mild symptoms could be prevented by giving a high dose of glucose at intervals [3,4]. For medical terms, six infusions of 1% mercurochrome solution were injected intravenously, at an increasing dose, a few days apart". When countries started to cure this disease, especially in the industrial settings, rates of the disease declined considerably, as observed in the late 1980s to 1990s, since after that, STIs increased largely [3,4]. These issues were diagnosed easily, but nowadays, with the new kind of Neisseria gonorrhoeae bacteria, men and women are largely becoming asymptomatic [3].
If gonorrhea spread cannot be stopped, which is a major concern now due to its capabilities of reducing the effectiveness of antimicrobial monotherapy or ceftriaxone even. Currently, there are 106 million cases throughout the world which is common among people of the ages between 15 to 49, multi-drug resistance (MDR strains whose resistance =1) and extensive drug resistance (XDR strains whose resistance ≥2) combinations are to be tested in the absence of a gonococcal vaccine [4,5]. When there is no vaccine, the main focus should be adequate prevention. diagnostic testing and screening [3].
Treatment should be given empirically, without test results since it is also responsible for causing infertility and creating an easy transmission of the Human Immunodeficiency Virus [4-6]. Over the last 80 years, treatment of N. gonorrhoeae has been complicated by the fact that N. gonorrhoeae has acquired or developed resistance mechanisms for most of the antibiotics, including sulfonamides, penicillin, ciprofloxacin, tetracycline, and now recently azithromycin and ceftriaxone, making this microorganism a candidate to cause an untreatable disease [6].
In 2010, CDC recommended a single 250 mg intramuscular (IM) dose of ceftriaxone and a single 1 g oral dose of azithromycin for treatment of uncomplicated gonococcal infections of the cervix, urethra, and rectum as a strategy for preventing ceftriaxone resistance and treating possible coinfection with Chlamydia trachomatis. However, low incidence of ceftriaxone resistance and the increased incidence of azithromycin resistance, has led to a reevaluation of this recommendation. This report, which updates previous guidelines, recommends a single 500 mg IM dose of ceftriaxone to treat uncomplicated urogenital, anorectal, and pharyngeal gonorrhea [4].
Methods
This systematic review follows the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines [Prisma reference here]. We searched multiple electronic databases for data collection, such as PubMed, Google Scholar, Medline, PubMed Central, and the Cochrane Library.
Data was collected in May 2021. We used keywords such as "Antimicrobial Resistance," "Gonococcal infection," "Azithromycin," "Neisseria Gonorrhoeae Infection," "update to treatment" separately and in combination with each other. We found 3590 articles' screening was done by going through the topic and abstracts and keeping the ones relevant to our research question. Inclusion and exclusion criteria were applied, and articles were further narrowed down to relevant ones. We did the quality appraisal of all the reference articles by following guidelines, and good quality 26 articles were kept.
Inclusion Criteria
Study selection included the following criteria: studies conducted in English, on humans over 19 years of age, in the last ten years, that were relevant to our topic and research
question, peer- reviewed, full texts, including these study types – a systematic review, literature review, and RCT. [1-21]
Exclusion Criteria
Grey literature, books, letters to the editor, editorials, duplicate and overlapping studies, animal studies, and papers discussing pediatric populations [1-21].
Results
A total number of 3595 studies were identified from the databases. Filters were applied based on inclusion criteria (full-text studies in English, last ten years, on humans, all types of reviews, and RCT), and studies were filtered and reduced to 47. Screening of the articles was done, and relevant studies were kept- 27. Quality appraisal was done for all studies, and the number of studies included was reduced to 21. These included three RCTs, one in vitro study, 15 literature reviews. Two articles from the website of CDC were included. A total of 21 articles were included for review [1-21].
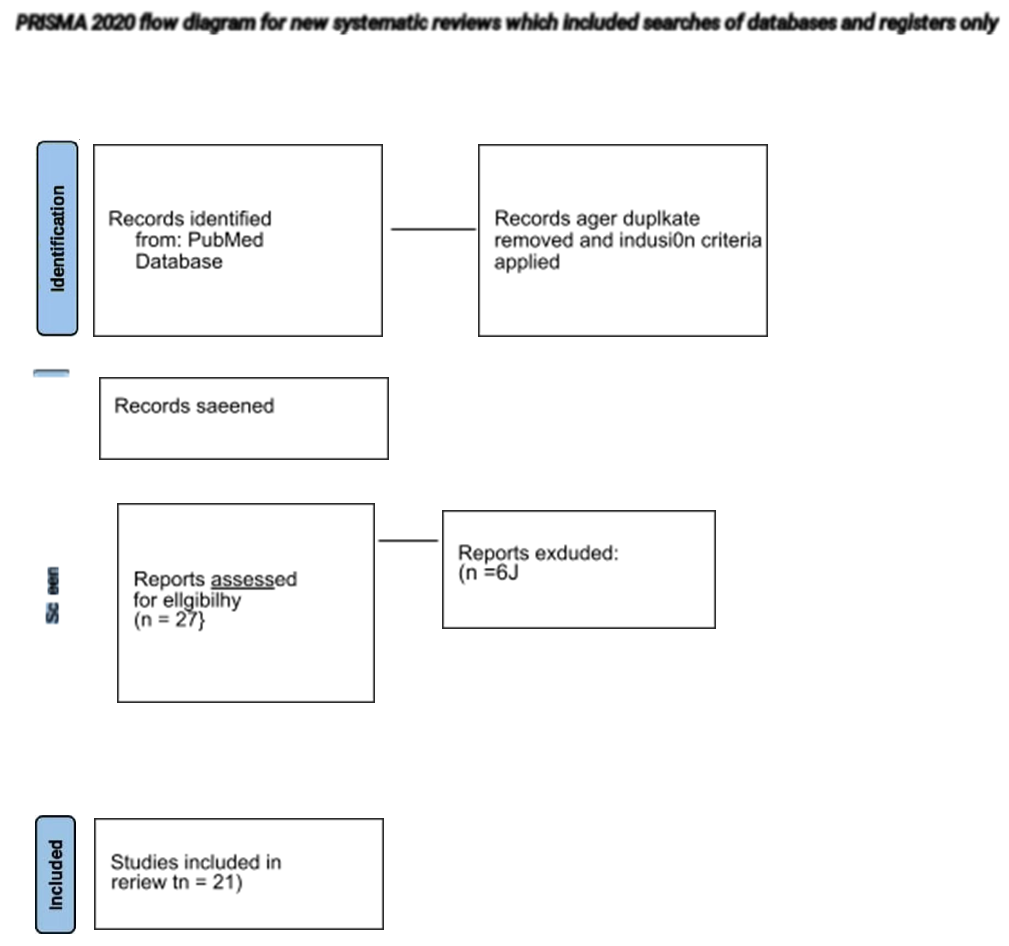
Consider, if feasible to do so, reporting the number of records indemnified from each database or register searched (father 0Ian 0›e tatal number across dl databases/reglstam).” If automation were used, indicate how many records were excluded by a human and how many were excluded by Fa' Page MJ, McKenzie JE, Bossuyt PM, Boulton I. Haffmann TC, MuIro\v CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systerra0c iedews. BMJ 2021;372:n71. doi: 10.u36/bmj.n71 Not more information, visit: httoJ/ .prisma-statementor
Discussion
Factors that lead to understanding the high rates of Sexually transmitted Infections, both among men and women [1,2], are.
- Ethnic background
- History of having STIs
- Sexuality
- Sexual preferences
- Sexual mixing patterns
- Gender
- Disparities observed in economic status or who has good access to service or
- Intrinsic features of underlying
N. gonorrhoeae is efficient, particularly at acquiring resistance to antimicrobial agents, which have undoubtedly helped this genome persist in the human population. N. gonorrhoeae can take up DNA from all other gonococci and bacteria from other genera at all of their life cycles, which makes N. gonorrhoeae resistant [1]. In the United States, rates of reported gonorrhea are rising every year, making gonorrhea 2nd most commonly occurring sexually transmitted infection. In 2019, the US center for disease control and prevention (CDC) received 616,392 case reports [2]. Data on evolution show that this antimicrobial resistance in N. gonorrhea is caused by several factors, including widespread access to antimicrobials, inappropriate selection, and misuse of antibiotics [3]. To monitor antimicrobial susceptibilities in countries worldwide, WHO implements the "Global Action Plan to Control the Spread and Impact of Antimicrobial Resistance in N. gonorrhoeae" to make smooth, effective actions against the spread of multi-drug resistant N. Gonorrhoeae. Also, WHO is strengthening surveillance systems for antimicrobial resistance in countries with high infection rates [4].
Gonococcal Antimicrobial Surveillance Program (GASP)
There are many efforts to monitor antimicrobial susceptibility worldwide. The extraordinary ability of N. Gonorrhoeae to develop resistance to most antimicrobials made it essential to monitor the susceptibility patterns, track newly appearing resistance, and modify the treatment recommendations of N. gonorrhoeae [5]. Therefore, in 1986 CDC established Gonococcal Isolate Surveillance Program (GISP). Country-specific surveillance programs for N. gonorrhoeae include the Gonococcal Isolate Surveillance Program (GISP) in the USA, the Gonococcal Resistance to Antimicrobial Surveillance (GRASP), the enhanced Surveillance of Antimicrobial-Resistant Gonorrhea (ESAG) in Canada, the Australian Gonococcal Surveillance Program (AGSP) in Australia and Europe the European Gonococcal Antimicrobial Surveillance Program (EURO-GASP). GISP data were used to modify and inform US transmitted disease treatment guidelines [6]. In 2018, 66 countries in six regions reported data on antimicrobial resistance in N. gonorrhoeae to WHO Gonococcal Antimicrobial Resistance Surveillance Program (WHO-GASP) [4]. Since 1992, WHO-GASP has monitored the longitudinal trends in AMR and provided data to inform treatment guidelines [5].
The table shows the number of countries reporting susceptibility testing of at least one drug to GASP, 2016-2018. [4,5].

Enhanced Gonorrhea Antimicrobial Surveillance Project Implementation
Based on the 2013 GISP report, CDC characterized antimicrobial drug resistance gonorrhea as an urgent threat that required aggressive action [1,6]. To enhance this global response, CDC collaborates with countries to strengthen antibiotic resistance. Recognizing the known successes of GISP and the need to enhance global health security, WHO and CDC came up with the Enhanced Gonococcal Antimicrobial Surveillance Program (EGASP) at the end of 2015. The main objective of EGASP is to observe antimicrobial susceptibilities in gonorrhea and to improve the quality and timeline of gonococcal antimicrobial resistance data across multiple countries [6].
Current activities of the CDC also include The STD Surveillance Network (SSuN), which conducts sentinel and enhanced STD surveillance activities to improve the capacity of national, state, or local STD programs to detect, observe, and respond [7].
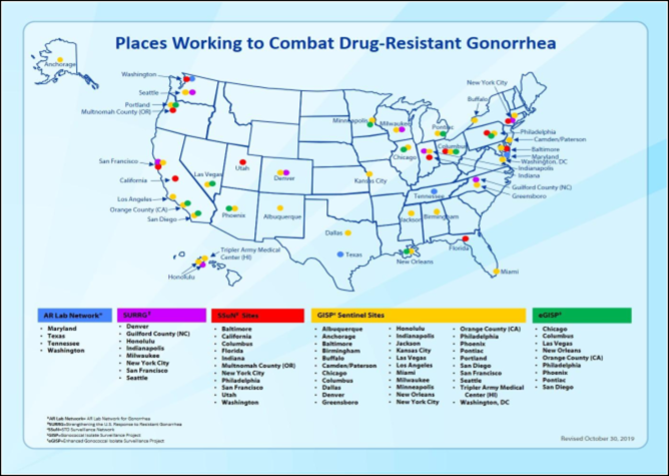
Strengthening the United State Response to Resistance Gonorrhea is one more program began in 2016, to enhance domestic gonorrhea surveillance [8]. CDC's Antibiotic Resistance Laboratory Network also supports multi-site Activity, rapid detection, and the response of gonorrhea, which will develop and strengthen local and state health departments [6,8].
(This map shows CDC's key activities to combat antimicrobial resistance gonorrhea, across the nation) [8].
Growth and colonization: N. Gonorrhea can grow in all aerobic, microaerobic, anaerobic conditions within the host [14]. Mucosal colonization requires Gonococcal pili, which helps initial adherence to host mucosal cells and tissues, self-adherence, and adherence to other N. gonorrhoeae cells. The outer membrane protein of N. gonorrhoeae increases attachment, and it translocates to the host cell mitochondria and impairs the ability of phagocytes to kill bacteria [14].
Diagnosis and screening: N. gonorrhoeae is gram-negative diplococcus that contains its virulence factors: pili, por proteins, Opa proteins, lipooligosaccharides, and IgA protease, making them highly ineffective [9]. Many studies show that asymptomatic infections are prevalent in both sexes; it is more common in men with presenting symptoms, usually dysuria with penile discharge. Women may experience mild vaginal mucopurulent discharge, severe pelvic pain, or present Asymptomatic [3,9]. If left untreated or not appropriately treated, it can result in various serious complications and sequelae, including upper genital tracts such as epididymitis and salpingitis; in women, it includes pelvic inflammatory disease (PID), chronic pelvic pain, ectopic pregnancy, and infertility [10].
As mentioned above, gonorrhea presents with nonspecific symptoms or asymptomatic, therefore confirmed diagnosis is important. Since the updated cure of gonorrhea was first recommended by the CDC. This recommendation states that women above the age of 25 who are sexually active must go for screening annually to avoid this disease accumulating and building over time and causing failures in the body. It also states that all pregnant women must also go for screening in the first three months by NAAT (nucleic acid tests of amplification) with the help of urine. In women and men, screening is essentially especially for MSM (Men with Men sex) because their exposure of pharynx and rectum to gonorrhea is the highest than women's. [13,14].
NAAT tests have been criticized recently because while antimicrobial testing goes on, widespread susceptibility testing is lost. Vaginal swabs or GC testing have been adopted as alternatives to testing. However, the NAAT tests are under study because their specimen collection techniques are easy and flexible with situations and the culture of people. [10,3 of intro,7] The CDC also recommended the dosage was Ceftriaxone 250mg with 100mg of doxycycline instead of azithromycin [19].
Molecular Mechanism of Resistance
In the past, antibiotics that were effective on N. gonorrhoeae were not given as the medication for the first-line monotherapy because the resistance powers of gonococci have spread throughout the world. These medications include tetracyclines, macrolides, quinolones, and penicillin. N. Gonorrhoeae has been classified as a "Priority 2" microorganism in WHO Global Priority List of Antibiotic-Resistance Bacteria to Guide Research, Discovery, and Development of New Antibiotics [9,12]. N. gonorrhoeae can collect DNA of other Neisseria species and incorporate other DNA into its genome, which makes N. Gonorrhoeae antimicrobial-resistant [13]. Other mechanisms by which it acquires resistance include a change in penicillin-binding protein 2(PBP2), increased efflux of antibiotics by overexpressing an efflux pump, or preventing cephalosporins from entering the cell [12,13].
The table illustrates the evolution of first-line antibiotic therapy for treating uncomplicated gonorrhea since the 1930s [12]:
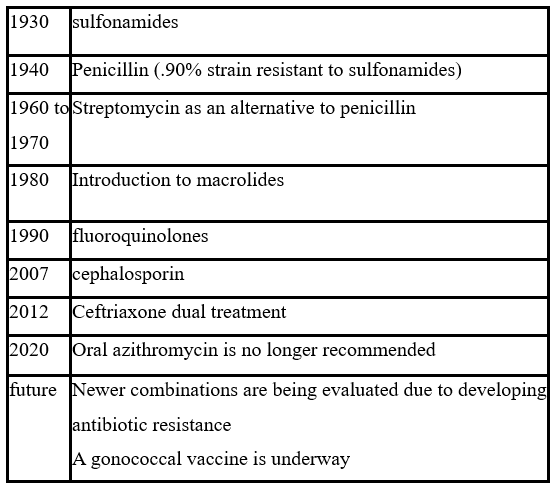
The WHO recommends removing an antibiotic from first-line therapy when >5% of individuals in a community are resistant to the antibiotic. Since 2006, the only class of antimicrobials that the CDC has recommended is cephalosporins [13].
Since 2010, CDC recommended dual therapy with a cephalosporin plus either azithromycin or doxycycline. Due to gonorrhea treatment failure in many countries, in 2015 CDC modified the recommendation to only the combination of ceftriaxone plus azithromycin [2,13]. However, cases of ceftriaxone, high-level azithromycin-resistant gonorrhea isolates, have been reported in 2018 [15].
Currently Available treatment
Now, it has already been discussed that gonorrhea can transform its genetic material via various mutations; it survives by testing itself against a different human host, hostile environments and hence is classified as "survival of the fittest." The treatment of gonorrhea must be regularly updated according to direct guidelines following surveillance data. Ideally, recommended empirical therapy should be affordable and highly curative without any toxic effects. However, criteria to update empiric therapy include antimicrobial resistance development, treatment cost and strategies, epidemiology, and disease prevalence [10,11]. All the combinations of antibiotics can only provide limited options for the treatment of gonorrhea. Still, no current drugs have been developed to control the infections of N. gonorrhoeae which is also resistant to multi-drug [3,9,10].
Table showing recommended first-line and alternative therapy for Anogenital Gonococcal infection in the USA, CANADA, AUSTRALIA, UNITED KINGDOM, EUROPE [11,21].
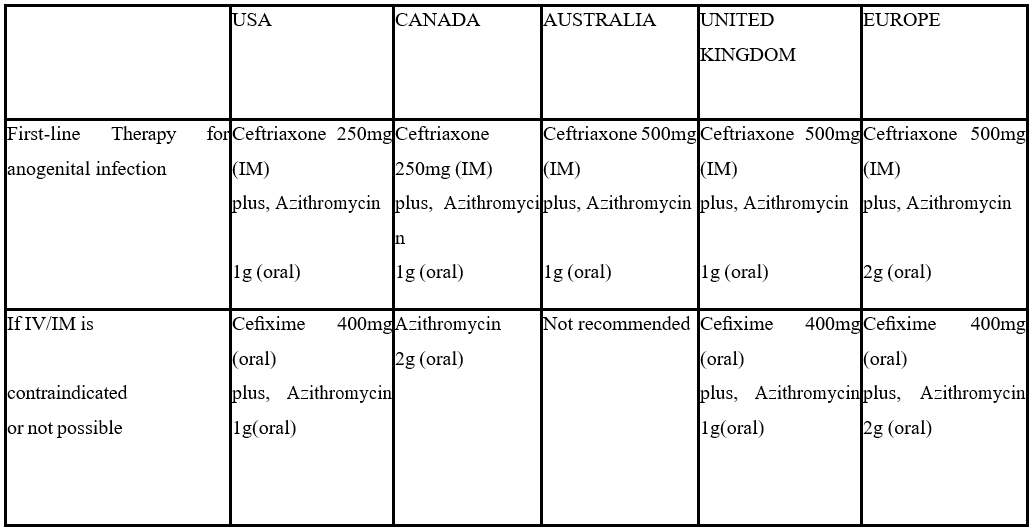
It is ideal to follow up with a test of cure, two to three weeks post-treatment in all infected patients. Treatment failure is considered when you find intracellular gram-negative diplococci on microscopy at least 72 hours post-treatment or positive culture results for gonorrhea taken at least 72 hours after treatment or positive NAAT results taken at least two to three weeks after completion of treatment [13,14].
The disease has already started to resist the current implementation and the expansion of the cephalosporin-based treatments. Gentamicin cure was not more painful than that of ceftriaxone. Later, it was administered to cure all three types of infection of gonorrhea, with the dosage of 240 mg and a 2g oral dose of azithromycin. Hence, this was one treatment where Ceftriaxone-resistant bacteria was seen. [15,16]. Although scientific research may evolve the medication of gonorrhea with the pathogen's genetic plasticity, which can be combined with the proficiency with it at collecting the genes encrypting the resistance powers of the disease, which can be determined according to the environment. It can develop a new medication that will be effective on the disease [17].
The actual long-term treatment strategy designed to label the burden of gonorrhea, which has become a global burden, includes vaccination [18,19]. It has been estimated that 90% of gonorrhea can be reduced universally in 20 years. It can be achieved if a non-disappearing gonococcal vaccine which has the effectiveness of 50%, is provided to all children of 13 years old. Currently, there is no available vaccination for the disease of gonorrhea. The gonorrhea vaccines, which have proper potential, have not even developed across the stages of discovering the development of clinical aspects. The evaluation of preclinical aspects to recognize the immunity power and substitutes of protection. Scientific publications have their predominance on gonorrhea, but there are few gonococcal vaccines [18- 20].
Conclusion
As it has been discussed before that the resistance power of the disease is much more, new options of treatment are important to prevent the disease. There are currently three approaches that have been accompanied to improve various new therapies against the resistance power of gonococci towards the drugs that have been used in the treatments. These three approaches include- (a) various combinations of the antibiotics which already exist, (b) developing a new combination of antibiotics, (c) creating an alternative option for therapies that might help in decreasing the resistance power of the disease.
The most common treatment guideline that the CDC has implemented against gonorrhea is a single 500mg dose of intramuscular ceftriaxone. If a chlamydia infection has not been excluded, concurrent treatment with 100mg dose of oral doxycycline for seven days.
References
- Dillon J-AR, Parti RP, Thakur SD. Antibiotic resistance in Neisseria gonorrhoeae: will infections be untreatable in the future? Culture, 2015; 35: 0965-0989.
- https://www.cdc.gov/std/statistics/prevalence-incidence-cost-2020.htm.
- Unemo M, Seifert HS, Hook EW 3rd, Hawkes S, Ndowa F, Dillon JR. Gonorrhoea, 2019; 5(1): 79. doi:10.1038/s41572-019-0128-6.
- St Cyr S, Barbee L, Workowski KA, et al. Update to CDC's Treatment Guidelines for Gonococcal Infection,2020. 2020;69(50):1911-1916. doi:10.15585/mmwr.mm6950a6.
- Suay-García B, Pérez-Gracia M-T. Neisseria gonorrhoeae Pathogens, 2020; 9(8): 647. https://doi.org/10.3390/pathogens9080647.
- Epidemiology, Treatments, and Vaccine Development for Antimicrobial- Resistant Neisseria gonorrhoeae: Current Strategies and Future Directions, DOi: 10.1007/s40265-021-01530-0.
- https://who.int/news-room/fact-sheets/detail/multi-drug-resis tant-gonorrhoea.
- Gonococcal antimicrobial susceptibility 4 pages, extract from report on global sexually transmitted infection surveillance,
- Weston EJ, Wi T, Papp J. Strengthening Global Surveillance for Antimicrobial Drug–Resistant Neisseria gonorrhoeae through the Enhanced Gonococcal Antimicrobial Surveillance Emerging Infectious Diseases, 2017; 23(13). https://doi.org/10.3201/eid2313.170443.
- https://cdc.gov/std/ssun/default.htm, or https://www.cdc.gov/std/gonorrhea/stdfact-gonorrhea.htm.
- https://cdc.gov/std/gonorrhea/arg/carb.htm.
- Suay-García B, Pérez-Gracia M-T. Neisseria gonorrhoeae Infections, 2020; 9(8): 647. https://doi.org/10.3390/pathogens9080647.
- Unemo M, Shafer WM. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future, 2014; 27(3): 587-613. doi: 1128/CMR.00010-14.
- Unemo Current and future antimicrobial treatment of gonorrhoea – the rapidly evolving Neisseria gonorrhoeae continues to challenge. BMC Infect Dis, 2015; 15: 364. https://doi.org/10.1186/s12879-015-1029-2.
- Jefferson A, Smith A, Fasinu PS, Thompson DK. Sexually Transmitted Neisseria gonorrhoeae Infections—Update on Drug Treatment and Vaccine Medicines, 2021; 8(2): 11. https://doi.org/10.3390/medicines8020011.
- Barbee LA, Dombrowski JC. Control of Neisseria gonorrhoeae in the era of evolving antimicrobial Infect Dis Clin North Am., 2013; 27(4): 723-737. doi: 10.1016/j.idc.2013.08.001.
- Unemo M, Seifert HS, Hook EW, et Gonorrhoea. Nat Rev Dis Primers, 2019; 5: 79. https://doi.org/10.1038/s41572-019-0128-6.
- Jennison AV, Whiley D, Lahra MM, et Genetic relatedness of ceftriaxone-resistant and high-level azithromycin resistant Neisseria gonorrhoeae cases, United Kingdom and Australia, February to April 2018. Euro Surveill, 2019; 24(8): 1900118. doi:10.2807/1560- 7917.ES.2019.24.8.1900118.
- Pogany L, Romanowski B, Robinson J, et al. Management of gonococcal infection among adults and youth: new key Can Fam Physician, 2015; 61(10): 869-e456.
- Jefferson A, Smith A, Fasinu PS, Thompson DK. Sexually Transmitted Neisseria gonorrhoeae Infections-Update on Drug Treatment and Vaccine Development. Medicines (Basel), 2021; 8(2): 11. doi:10.3390/medicines8020011.
- St Cyr S, Barbee L, Workowski KA, et al. Update to CDC's Treatment Guidelines for Gonococcal Infection, MMWR Morb Mortal Wkly Rep, 2020; 69(50): 1911-1916. doi:10.15585/mmwr.mm6950a6.

