Skin as Mechanical and Chemical Barrier against Microbial Infection
Ali M1,*, Nazifi Usman1, Ibrahim SI2, Lurwan Mu’azu3, Nas FS4 and Gambo BS5
1Department of Microbiology, Federal University Gusau, Nigeria
2Department of Pharmaceutical Technology, School of Technology, Kano State Polytechnics, Nigeria
3Department of Biological Sciences, Federal University Gusau, Nigeria
4Department of Biological Sciences, Bayero University Kano, Nigeria
5Department of Biological Sciences, Rabiu Musa Kwankwaso College of Advance and Remedial Studies, Nigeria
Received Date: 08/06/2023; Published Date: 24/10/2023
*Corresponding author: Muhammad Ali Department of Microbiology, Federal University Gusau, Nigeria
Abstract
Skin is an organ with a dynamic ecosystem that harbors pathogenic and commensal microbes, which constantly communicate amongst each other and with the host immune system. The skin acts as a physical barrier to prevent the invasion of foreign pathogens while providing a home to the commensal microbiota. The harsh physical landscape of skin, particularly the desiccated, nutrient-poor, acidic environment, also contributes to the adversity that pathogens face when colonizing human skin. The paper review how skin contributes to barrier immunity. The review also discusses specialized immune cells that are resident in steady-state skin including mononuclear phagocytes, such as Langerhans cells, dermal macrophages and dermal dendritic cells in addition to the resident memory T cells.
Keywords: Immune; Microbial infection; Skin; Mechanical barrier
Introduction
The body’s primary defenses against infection are mechanical barriers such as the skin and various mucous membranes. Like the skin, the mucous membranes of the airways, the digestive system and the urogenital system are in direct contact with the environment. Of these systems, the digestive tract has the largest area of contact with the microbial environment, approximately 200 square meters. The skin has a surface area of no more than 2 square meters. The mechanical barrier offered by the mucosal epithelial cells of the airways and the digestive tract is much less resistive than that of the skin, since the skin consists of several cell layers, with the topmost being a cellular horny layer. Therefore, there are only a few micro-organisms that are able to penetrate the intact skin, while it is relatively easy for them to pass through the more fragile mucous membranes. In addition to functioning as a physical barrier, the skin and mucous membranes have biochemical properties - e.g., low gastric pH, lysozyme in tears, fatty acids on the skin - that also make it difficult for microorganisms to survive at those places. The microbiota of the intestines and skin also constitute a biological barrier. This commensal microbiota uses a variety of mechanisms to make it harder for other - pathogenic or non-pathogenic - microorganisms to settle and then penetrate the body.
A healthy skin is not sterile but forms the niche for a complex and dynamic ecosystem consisting of approximately 1012 micro-organisms, mainly bacteria, but also including fungi, and viruses. The various skin sites can be classified into three microenvironments: sebaceous (glabella, alar crease, external auditory crease, retroarticular crease, occiput, manubrium, back), dry (volar forearm, hypothenar palm, buttock), and moist (nare, axillary vault, antecubital fossa, interdigital web space, inguinal crease, umbilicus, gluteal crease, popliteal fossa, toe web space, plantar heel) [2]. Overall, the skin microbiota is dominated by Staphylococcus spp (in particular S. aureus and S. epidermidis), S. epidermidis and Corynebacteria, which together form > 60% of the total population [2]. However, the differences in the microbiota occupying the various skin sites are enormous and have been described as “ecologically dissimilar as rain forests are to deserts” [3]. Skin microbiota composition thus is influenced by body region, by biological sex, age, health status, geographical location, ethnic background, depth of the skin, use of cosmetics and antibiotics, as well as life-style factors such as pet ownership and alcohol consumption [4,5].
The commensal skin microbiota contributes to the protection of the human host against infections in many different ways. Commensal skin microbiota competes with (potential) pathogens for an ecological niche. Commensal bacteria can also directly inhibit the growth of pathogens: lipoteichoic acid in the cell wall S. epidermidis inhibits the growth of Propionibacterium acnes [6]. The cutaneous fungus Malassezia produces azelaic acid by enzymatic degradation of external lipids and thus contributes to the low pH of the skin [7]. Via several mechanisms, skin microbiota stimulates the innate and acquired immune system of the skin.
Structure of Human Skin
The skin is the largest organ of the body, accounting for about 15% of the total adult body weight. It performs many vital functions, including protection against external physical, chemical, and biologic assailants, as well as prevention of excess water loss from the body and a role in thermoregulation. The skin is continuous, with the mucous membranes lining the body’s surface [8]. The integumentary system is formed by the skin and its derivative structures. The skin is composed of three layers (Figure 1): the epidermis, the dermis, and subcutaneous tissue [8]. The outermost level, the epidermis, consists of a specific constellation of cells known as keratinocytes, which function to synthesize keratin, a long, threadlike protein with a protective role. The middle layer, the dermis, is fundamentally made up of the fibrillar structural protein known as collagen. The dermis lies on the subcutaneous tissue, or panniculus, which contains small lobes of fat cells known as lipocytes. The thickness of these layers varies considerably, depending on the geographic location on the anatomy of the body. The eyelid, for example, has the thinnest layer of the epidermis, measuring less than 0.1 mm, whereas the palms and soles of the feet have the thickest epidermal layer, measuring approximately 1.5 mm. The dermis is thickest on the back, where it is 30–40 times as thick as the overlying epidermis [9].
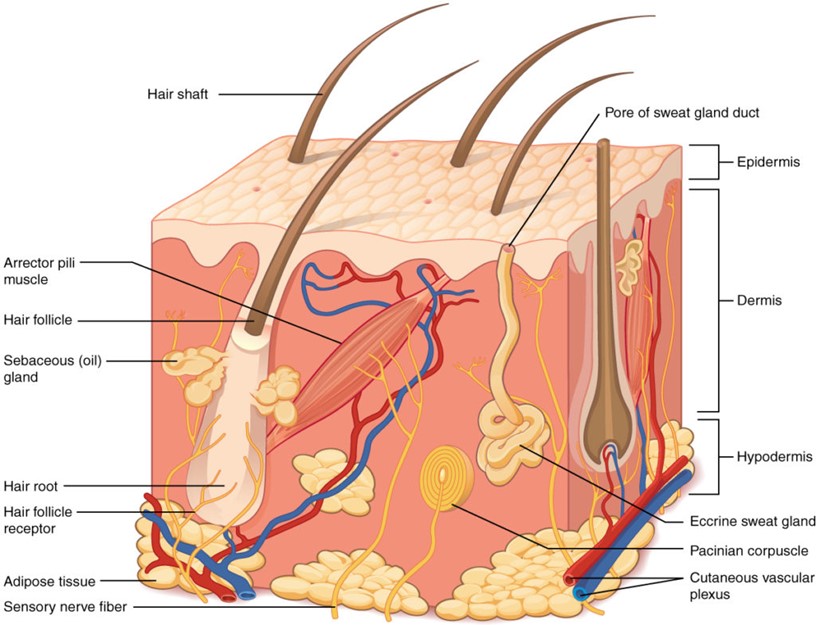
Figure 1: Structure of human skin.
Microbiota on Skin Surface
In sequencing surveys of healthy adults [10-12], the composition of microbial communities was found to be primarily dependent on the physiology of the skin site, with changes in the relative abundance of bacterial taxa associated with moist, dry and sebaceous microenvironments. Sebaceous sites were dominated by lipophilic Propionibacterium species, whereas bacteria that thrive in humid environments, such as Staphylococcus and Corynebacterium species, were preferentially abundant in moist areas, including the bends of the elbows and the feet. In contrast to bacterial communities, fungal community composition was similar across core body sites regardless of physiology [13,14]. Fungi of the genus Malassezia predominated at core body and arm sites, whereas foot sites were colonized by a more diverse combination of Malassezia spp., Aspergillus spp., Cryptococcus spp., Rhodotorula spp., Epicoccum spp. and others [13]. Bacteria were the most abundant kingdom across sites, and fungi were the least abundant [14]; however, there are many more bacterial reference genomes than fungal reference genomes available, which may partially contribute to this observed difference. Interestingly, the overall fungal abundance was low, even on the feet where fungal diversity was high.
In contrast to bacteria and fungi, colonization by eukaryotic DNA viruses was specific to the individual rather than anatomical site [15]. As no marker gene is universally shared among viruses, viral community diversity can be captured only with purified viral-like particles or shotgun metagenomics sequencing [15,16]. As an additional challenge, RNA viruses can be sequenced only with RNA sequencing, which has not been performed on skin samples from healthy individuals. Apart from bacteriophages, particularly those associated with Propionibacterium spp. and Staphylococcus spp., no core DNA virome has been found to be conserved across individuals [15,16]. This area of skin microbiome research requires further attention to understand the role of possible predator–prey or cooperative interactions between bacteriophages and bacteria in microbial community assembly. In addition to bacteriophages, eukaryotic viruses may also have a role in skin diseases, as highlighted by the discovery of Merkel cell polyomavirus, an oncovirus that causes a rare but aggressive form of skin cancer [17].
Skin Barrier Immunity
The skin is a complex organ that carries out numerous functions contributing to its barrier immunity function. Antimicrobial peptides and lipids are secreted onto the cell surface to control bacterial growth. These include dermcidin, which is secreted in human sweat and has broad antimicrobial activity against a range of pathogenic bacteria. Its antimicrobial activity is not affected by the low pH value and high salt concentrations of human sweat [18]. Sebum is made by sebaceous glands found independently of or near hair follicles. Within the sebum are antimicrobial lipids, such as lauric acid and sapienic acid, which play an important role in controlling pathogenic organisms [19].
However, the skin is not a sterile site, and there is extensive research showing the role that the skin microbiota plays in immunity by restricting the growth of pathogenic bacteria [20]. Commensal bacteria have been shown to produce an antimicrobial peptide that synergizes with the human antimicrobial peptide LL37, which together kill the pathogenic bacterium Staphylococcus aureus [21]. However, insult and pathogens are mostly controlled and prevented entry due to structure and barrier immunity in the skin. Skin-resident stromal cells Keratinocytes are the main component of the epidermis. They express Toll-like receptors (TLRs), which are crucial pathogen pattern recognition receptors that when triggered lead to the production of inflammatory cytokines and initiation of an immune response [22]. Keratinocytes have been shown to constitutively express TLR1, -2, -3, -5, -6 and -10 [23,24]. They also have the ability to sense wound damage and produce inflammatory cytokines and chemokines such as interleukin-1b (IL-1b), IL-8 and CCL20 to recruit leucocytes to the site of damage [25].
Keratinocytes express a raft of antimicrobial peptides that control bacterial growth, including adrenomedullin and b-defensins [26,27]. Defensin-1 is constitutively expressed by human keratinocytes and b-defensin-2 and -4 are up-regulated upon inflammatory challenge [2-29]. Keratinocytes can express the antimicrobial peptide Cathelicidin upon stimulation and can store Cathelicidin in cytoplasmic granules until needed [30,31]. Keratinocytes also constitutively express RNase [23], which is a very potent antimicrobial ribonuclease, and upon inflammatory or bacterial challenge there is further increased expression [32]. More recently, it has been proposed that keratinocytes have the ability to process and present antigen to CD4+ and CD8+ T cells, initiating an adaptive immune response [33]. In addition, keratinocytes are the key site for the first step in the vitamin D metabolism pathway, when pro-vitamin D3 (7-dehydro-cholesterol) is metabolized into vitamin D3, catalyzed by UVB. Vitamin D is an important component of a functioning immune system and its metabolism at the skin site contributes to barrier immunity [34].
Dermal fibroblasts are the structural cells of the dermis; their primary function is to secrete extracellular matrix components such as pro-collagen. Fibroblasts express the full range of TLRs, at a higher level than keratinocytes, demonstrating their important role in the detection of pathogens [35]. In vitro studies have shown that dermal fibroblasts can have differing roles in immunity, indeed TLR4 signalling results in the production of inflammatory cytokines such as IL-6, IL-8 and the monocyte chemo-attractant CCL2 [36]. Conversely fibroblasts have been shown to suppress T-cell proliferation via indoleamine 2,3-dioxygenase production, and to skew the T cells to produce immunoregulatory cytokines such as IL-10 [37]. The subcutaneous layer of the skin is predominantly composed of adipocytes – their primary function is to be a repository of energy that responds to hypothermia by producing heat. More recent work has identified the important role of adipocytes in barrier immunity as a significant source of antimicrobial peptides. In response to infection, for example with S. aureus, dermal fibroblasts can differentiate into adipocytes and produce the antimicrobial peptide cathelicidin [38].
Skin-resident immune cells mononuclear phagocytes within the epidermis there is a population of mononuclear phagocytes called Langerhans cells (LCs). These were believed to have been seeded at birth and maintained by local turnover to ensure a steady-state population [39]. However, a recent study demonstrated, in a murine model of immune injury, that repopulation of LCs from peripheral monocytes makes up for the slow repopulation from mature LCs [40]. Langerhans cells are located at the interface with the external environment and as such are multifunctional sentinels of the epidermis. They sample the environment by extension and retraction of their dendrites between the keratinocytes in an amoeba-like movement [41]. They present antigen to T cells within the epidermis to initiate a local immune response and also have the capacity to migrate to the lymph node and initiate immune responses [42]. Within the dermis, there is a more diverse population of mononuclear phagocytes including dermal dendritic cells (DCs) and dermal macrophage populations. Dendritic cells are the sentinels of the immune system, they sample the microenvironment and either present antigen to the resident T cells or migrate through the lymphatics to the lymph node to initiate an immune response [43]. Historical assessment of dermal DCs identified that they are more activated than their blood counterparts; dermal DCs had increased expression of co-stimulatory receptors and were strong stimulators of T-cell proliferation relative to their peripheral blood counterparts [44]. Two main populations of dermal myeloid DCs have been identified; the CD1c+ DCs and the CD141+ DCs. CD141+ DCs are the cells responsible for cross-presenting antigens to CD8+ T cells and have homology to the mouse CD103+ DCs [45]. Very few plasmacytoid DCs are observed in steady-state skin [46].
Macrophages are another type of antigen-presenting cell resident in the dermis and they sense pathogens and damage and initiate an appropriate immune response. In addition to the immune function, macrophages maintain tissue homeostasis through increasing appropriate anti-inflammatory mechanisms, contribute to wound healing, and heal nerves upon tissue injury [47,48]. Macrophages are thought to populate tissues early on but studies have also shown that they are replenished by circulating monocytes [49]. These data are supported by a study in humans showing that CD14+ cells were a transient population of monocyte-derived macrophages [50]. CD163 has been proposed to be a good marker for dermal macrophages, as it specifically identifies skin-specific macrophages that are not recently migrated monocytes [51]. Analysis of the location of these different mononuclear phagocyte populations in the dermis have shown that DCs can be found closer to the epidermis (around 0–20 μm beneath the dermo–epidermal junction) and macrophages are located deeper in the skin (around 40–60 μm beneath the dermo–epidermal junction) [52].
Conclusion
The innate immune system is integral to the prevention of skin infection and eradication of pathogenic bacteria and plays an essential role in skin healing. Recognition of bacteria and viruses initiates the inflammatory cascade involving the release of cytokines, recruitment of immune cells, and production of AMPs and ISGs. AMPs and ISGs represent one of the most important and robust immune mechanisms in the skin. However, pathogenic bacteria—such as S. aureus—and cutaneous viruses have evolved mechanisms to counteract innate immune mechanisms. Commensal skin bacteria assist the innate immune system with eradication of pathogens through production of AMPs and by enhancing the activity of innate immune cells. Finally, despite the importance of innate immunity, excess immune activation underlies some cutaneous diseases and is detrimental to wound healing.
Conflict of Interest: The authors declare no conflict of interest
References
- Abdallah F, Mijouin L, Pichon C. Skin Immune Landscape: Inside 1 and Outside the Organism. Mediators Inflammation, 2017: 5095293.
- Ross AA, Doxey AC, Neufeld JD. The Skin Microbiome of Co2. habiting Couples. mSystems, 2017: e00043-17.
- Grice EA, Kong HH, Conlan S, Deming CB, Davis J, et al. Topo3. graphical and temporal diversity of the human skin microbiome. Science, 2009; 324: 1190-1192.
- Bouslimani A, Porto C, Rath CM, Wang M, Guo Y, et al. Mo4. lecular cartography of the human skin surface in 3D. Proc Natl Acad Sci USA, 2015; 112: E2120-2129.
- Oh J, Byrd AL, Deming C, Conlan S, NISC Comparative Sequencing 5. Program, et al. Biogeography and individuality shape function in the human skin metagenome. Nature, 2014; 514: 59-64.
- Xia X, Li Z, Liu K, Wu Y, Jiang D, et al. Staphylococcal LTA-induced miR-143 inhibits Propionibacterium acnes-mediated inflammatory response in skin. J Invest Dermatol, 2016; 136: 621-630.
- Brasch J, Christophers E. Azelaic acid has antimycotic prop7. erties in vitro. Dermatology, 1993; 186: 55.
- Kanitakis J. Anatomy, histology and immunohistochemistry of normal human skin. European Journal of Dermatology, 2002; 12(4): 390–401.
- James WD, Berger TG, Elston DM. Andrews’ diseases of the skin: Clinical dermatology (10th ed.). Philadelphia: Elsevier Saunders, 2006,
- Costello EK, et al. Bacterial community variation in human body habitats across space and time. Science, 2009; 326: 1694–1697.
- Grice EA, et al. Topographical and temporal diversity of the human skin microbiome. Science, 2009; 324: 1190–1192.
- Grice EA, Segre JA. The skin microbiome. Nat. Rev. Microbiol, 2011; 9: 244–253.
- Oh J, et al. Biogeography and individuality shape function in the human skin metagenome. Nature, 2014; 514: 59–64.
- Findley K, et al. Topographic diversity of fungal and bacterial communities in human skin. Nature, 2013; 498: 367–370.
- Oh J, et al. Temporal stability of the human skin microbiome. Cell, 2016; 165: 854–866.
- Hannigan GD, et al. The human skin double-stranded DNA virome: topographical and temporal diversity, genetic enrichment, and dynamic associations with the host microbiome. mBio, 2015; 6: e01578‑15
- Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science, 2008; 319: 1096–1100.
- Schittek B, Hipfel R, Sauer B, Bauer J, Kalbacher H, Stevanovic S. Dermcidin: a novel human antibiotic peptide secreted by sweat glands. Nat Immunol, 2001; 2: 1133–1137.
- Wertz PW. Lipids and the permeability and antimicrobial barriers of the skin. J Lipids, 2018: 1–7.
- Belkaid Y, Segre JA. Dialogue between skin microbiota and immunity. Science, 2014; 346: 954–959.
- Nakatsuji T, Chen TH, Narala S, Chun KA, Two AM, Yun T. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci Transl Med, 2017; 9: eaah4680.
- Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol, 2011; 1: 135–145.
- Baker BS, Ovigne JM, Powles AV, Corcoran S, Fry L. Normal keratinocytes express Toll-like receptors (TLRs) 1, 2 and 5: modulation of TLR expression in chronic plaque psoriasis. Br J Dermatol, 2003; 148: 670–679.
- Kollisch G, Kalali BN, Voelcker V, Wallich R, Behrendt H, Ring J. Various members of the Toll-like receptor family contribute to the innate immune response of human epidermal keratinocytes. Immunology, 2005; 114: 531–541.
- Kennedy-Crispin M, Billick E, Mitsui H, Gulati N, Fujita H, Gilleaudeau P. Human keratinocytes’ response to injury upregulates CCL20 and other genes linking innate and adaptive immunity. J Invest Dermatol, 2012; 132: 105–113.
- Martinez A, Elsasser TH, Muro-Cacho C, Moody TW, Miller MJ, Macri CJ. Expression of adrenomedullin and its receptor in normal and malignant human skin: a potential pluripotent role in the integument. Endocrinology, 1997; 138: 5597–5604.
- Fulton C, Anderson GM, Zasloff M, Bull R, Quinn AG. Expression of natural peptide antibiotics in human skin. Lancet, 1997; 350: 1750–1751.
- Liu AY, Destoumieux D, Wong AV, Park CH, Valore EV, Liu L. Human b-defensin-2 production in keratinocytes is regulated by interleukin-1, bacteria, and the state of differentiation. J Invest Dermatol, 2002; 118: 275–281.
- Harder J, Meyer-Hoffert U, Wehkamp K, Schwichtenberg L, Schroder JM. Differential gene induction of human b-defensins (hBD-1, -2, -3, and -4) in keratinocytes is inhibited by retinoic acid. J Invest Dermatol, 2004; 123: 522–529.
- Braff MH, Di Nardo A, Gallo RL. Keratinocytes store the antimicrobial peptide cathelicidin in lamellar bodies. J Invest Dermatol, 2005; 124: 394–400.
- Sorensen OE, Cowland JB, Theilgaard-Monch K, Liu L, Ganz T, Borregaard N. Wound healing and expression of antimicrobial peptides/polypeptides in human keratinocytes, a consequence of common growth factors. J Immunol, 2003; 170: 5583–5589.
- Harder J, Schroder JM. RNase 7, a novel innate immune defense antimicrobial protein of healthy human skin. J Biol Chem, 2002; 277: 46779–46784.
- Black AP, Ardern-Jones MR, Kasprowicz V, Bowness P, Jones L, Bailey AS. Human keratinocyte induction of rapid effector function in antigen-specific memory CD4+ and CD8+ T cells. Eur J Immunol, 2007; 37: 1485–1493.
- Hewison M. Vitamin D and immune function: an overview. Proc Nutr Soc, 2012; 71: 50–61.
- Yao C, Oh JH, Lee DH, Bae JS, Jin CL, Park CH. Toll-like receptor family members in skin fibroblasts are functional and have a higher expression compared to skin keratinocytes. Int J Mol Med, 2015; 35: 1443–1450.
- Wang J, Hori K, Ding J, Huang Y, Kwan P, Ladak A. Toll-like receptors expressed by dermal fibroblasts contribute to hypertrophic scarring. J Cell Physiol, 2011; 226: 1265–1273.
- Haniffa MA, Wang XN, Holtick U, Rae M, Isaacs JD, Dickinson AM. Adult human fibroblasts are potent immunoregulatory cells and functionally equivalent to mesenchymal stem cells. J Immunol, 2007; 179: 1595–1604.
- Chen SX, Zhang LJ, Gallo RL. Dermal white adipose tissue: a newly recognized layer of skin innate defense. J Invest Dermatol, 2019; 139: 1002–1009.
- Merad M, Ginhoux F, Collin M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat Rev Immunol, 2008; 8: 935–947.
- Ferrer IR, West HC, Henderson S, Ushakov DS, Santos ESP, Strid J. A wave of monocytes is recruited to replenish the long-term Langerhans cell network after immune injury. Sci Immunol, 2019; 4: eaax8704.
- Nishibu A, Ward BR, Jester JV, Ploegh HL, Boes M, Takashima A. Behavioral responses of epidermal Langerhans cells in situ to local pathological stimuli. J Invest Dermatol, 2006; 126: 787–796.
- West HC, Bennett CL. Redefining the role of Langerhans cells as immune regulators within the skin. Front Immunol, 2017; 8: 1941.
- Collin M, Bigley V. Human dendritic cell subsets: an update. Immunology, 2018; 154: 3–20.
- McLellan AD, Heiser A, Sorg RV, Fearnley DB, Hart DN. Dermal dendritic cells associated with T lymphocytes in normal human skin display an activated phenotype. J Invest Dermatol, 1998; 111: 841–849.
- Haniffa M, Shin A, Bigley V, McGovern N, Teo P, See P. Human tissues contain CD141hi cross-presenting dendritic cells with functional homology to mouse CD103+ non-lymphoid dendritic cells. Immunity, 2012; 37: 60–73.
- Conrad C, Meller S, Gilliet M. Plasmacytoid dendritic cells in the skin: to sense or not to sense nucleic acids. Semin Immunol, 2009; 21: 101–109.
- Kolter J, Feuerstein R, Zeis P, Hagemeyer N, Paterson N, d’Errico P. A subset of skin macrophages contribute to the surveillance and regeneration of local nerves. Immunity, 2019; 50: 1482–1497 e7.
- Mowat AM, Scott CL, Bain CC. Barrier-tissue macrophages: functional adaptation to environmental challenges. Nat Med, 2017; 23: 1258–1270.
- Tamoutounour S, Guilliams M, Montanana Sanchis F, Liu H, Terhorst D, Malosse C. Origins and functional specialization of macrophages and of conventional and monocyte-derived dendritic cells in mouse skin. Immunity, 2013; 39: 925–938.
- McGovern N, Schlitzer A, Gunawan M, Jardine L, Shin A, Poyner E. Human dermal CD14+ cells are a transient population of monocyte-derived macrophages. Immunity, 2014; 41: 465–477.
- Zaba LC, Fuentes-Duculan J, Steinman RM, Krueger JG, Lowes MA. Normal human dermis contains distinct populations of CD11c+BDCA-1+ dendritic cells and CD163+FXIIIA+ macrophages. J Clin Invest, 2007; 117: 2517–1525.
- Wang XN, McGovern N, Gunawan M, Richardson C, Windebank M, Siah TW. A three-dimensional atlas of human dermal leukocytes, lymphatics, and blood vessels. J Invest Dermatol, 2014; 134: 965–974.

