Familial Neurofibromatosis Type I (von Recklinghausen's Disease)
Sultan S Ahmed1,*, Syed A A Rizvi1,*, Jose Mendez DO2, Chloe Addison1, Nicole D Serrant3, Courtney Short3 and Eaman A Saleh4
1College of Biomedical Sciences, Larkin University, Miami, Florida, USA
2Fort Lauderdale, Florida, USA
3Ross University School of Medicine, Bridgetown, Barbados
4Department of Biological Sciences, Illinois State University, Normal, Illinois USA
Received Date: 13/03/2023; Published Date: 29/05/2023
*Corresponding author:
- Syed A A Rizvi, MD, PhD, MPH, MBA, College of Biomedical Sciences, Larkin University, 18301 N Miami Ave, Miami, FL 33169, USA
- Sultan S. Ahmed, MD, College of Biomedical Sciences, Larkin University, 18301 N Miami Ave, Miami, FL 33169, USA
Abstract
A 20-year-old female patient with no past medical history and no medication use, presented to our clinic complaining of "non -specific lower back pain”. Upon examining her back, multiple hyperpigmented cafe-au-lait spots with some pedunculated lesions were noted. The patient stated that she was born with multiple abnormal skin lesions all over her body. She is a nonsmoker without any organic symptomatology. The patient denied shortness of breath, chest pain, or abdominal pain at this time. Her mother, a 58-year-old female who accompanied the patient, also stated that she has a similar skin presentation.
Physical Examination
Vital signs: blood pressure, 118/75 mm Hg; pulse, 76 beats/min; respiratory rate, 16 breaths/min; height, 162.5 cm; weight, 52.7 kg; temperature, 37.9°C; and oxygen saturation, 98% on room air. During examinations of both eyes, the patient had multiple hypo-pigmented elevated lesions in her iris suggestive of Lisch nodules. Her visual acuity was 20/20 in both eyes. Examination of the face showed no visible craniofacial abnormalities (Figures 1 and 2 both of them provided verbal consent for using the figures). She had an oral neurofibroma with mild gingival hyperplasia and multiple dental caries in her molars. Dermatological examination showed multiple non-painful cutaneous lesions with smooth bordered café-au-lait macules on her back and lower extremities. She also presented with an elevated hairy rubber-like pigmented lesion suggesting a plexiform neurofibroma in her right lumbar and abdominal region. The patient had inguinal and axillary freckling consistent with Crowe sign. The patient’s mother had similar skin neurofibromas which we were able to compare with patient’s presentation during the examination. The patient had no dyspnea, no evidence of cyanosis, and no neurological focal signs. Furthermore, the patient's mother does not complain of any neurological symptoms. For our patient, due to non-specific back pain, she was advised to have an MRI of the lower lumbar region and anti-inflammatory medication was prescribed to address the pain.
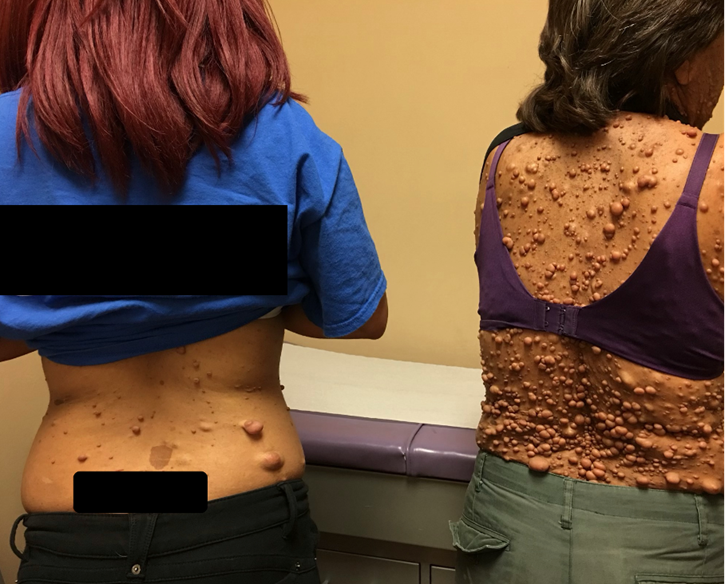
Figure 1: Patient on the left side with her mother on the right.
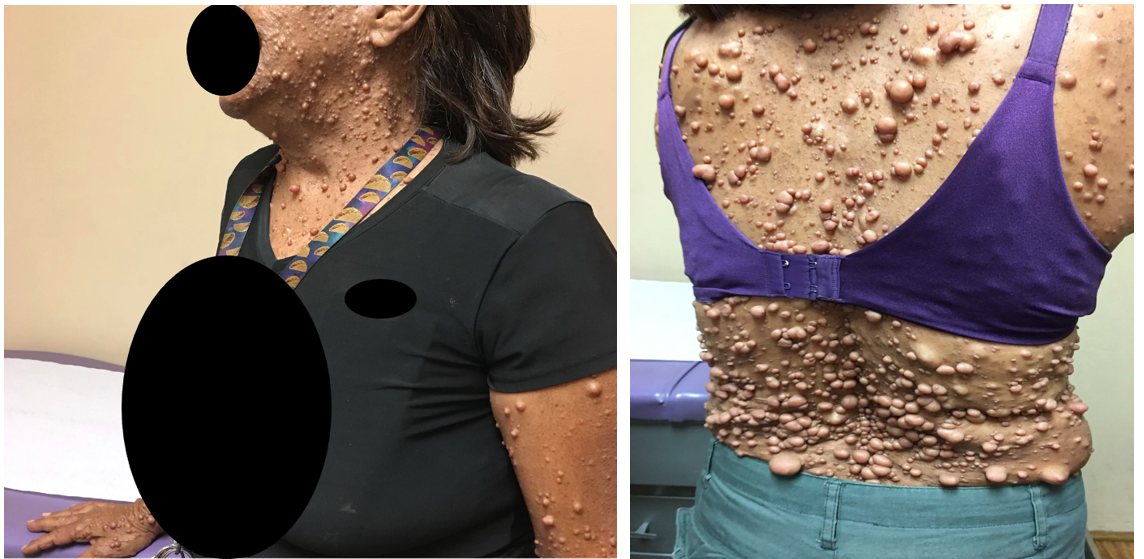
Figure 2: Skin presentation of the patient’s mother.
Discussion
In this case report, we described a familial presentation of a rare disorder Neurofibromatosis Type I also known as von Recklinghausen's disease. In 1768, a strange case was documented and published describing a boy with several skin neurofibromas, small nerve tumors that form soft bumps under the skin. It was found that the boy’s father presented with similar symptoms, leading scientists to the conclusion that this new disease may be genetically inherited. However, more evidence and cases were needed in order to study the condition in more detail [1]. The first scientific description of the disease was published by Dr. Mark Akenside. A German pathologist, Dr. Friedrich von Recklinghausen actually coined the name of the disease and was acknowledged as the one who discovered it later in 1882 [2,3]. Dr. Von Recklinghausen characterized the disease by a phenomenon of light brown spots on the skin that were likened to a warm cup of café-au-lait [1]. These spots tend to be concentrated in the groin and underarm areas of the body; this particular presentation is called Crowe sign. Recklinghausen, as well as scientists Riccardi and Kleiner, worked to better classify the disease by grouping symptoms and their intensities. Three forms of this disease, Neurofibromatosis (NF), were distinguished at the time: NF 1, NF 2, and Schwannomatosis.
Neurofibromatosis type 1 (NF1), also known as Von Recklinghausen's disease (NFvR), is an autosomal dominant genetic disorder. It is the most common type of NF and accounts for about 90% of all cases. NF1 is a rare disorder that affects males and females equally regardless of race and ethnicity. It has an incidence of approximately 1:2,600 to 1:3,000 individuals. Half of the NF1 cases are familial, while de novo mutations occur primarily in paternally derived chromosomes [5,6]. There is 100% penetrance with variable expressivity. Manifestations range from mild lesions to several complications and functional impairment. Interestingly in our case, both patient and her mother showed no signs of neurological deficit until now. Oral manifestations can be found in 72% of NF1 patients. The prevalence is estimated at 1:36,000 to 1:40,000 [7]. In the United States, the mean age at death for persons with NF1 is 54.4 years, and median of 59 years [8]. Lisch nodules are small pigmented lesions found in the iris (iris hamartomas) and mostly seen in adult patients, is a specific characteristic of NF1 that helps in diagnosis [9]. Neurofibromatosis type 2 (NF2) makes up about 3% of all cases and has a prevalence between 1:33,000 births and 1:87,410. Similar to NF1, there is no gender or race predilection. NF2 has variable presentations amongst different families. A more severe clinical presentation is associated with a frameshift or nonsense mutation that results in a truncated protein. Patients with NF2 are more likely to present with bilateral vestibular schwannomas, juvenile cataracts, meningiomas, or ependymomas. The third type of NF called Schwannomatosis is the rarest form of NF with its prevalence being mostly half that of the NF2 cases at 1 in 126,000 with unknown predilection of sex, race or ethnicity [10]. Its presentation develops mostly in adulthood with an average diagnosis age being 40 years old. Different from NF2 with vestibular schwannomas, Schanomatisis has multiple schwannomas without vestibular involvement [4].
NFvR occurs due to mutations found in Chromosome 17 (17qll.2). The mutations found in the NF gene contain insertions, deletions, splice mutations, amino acid changes, and chromosomic rearrangements. With alternative splicing, this gene can produce multiple mRNA outcomes. One of the more common transcripts is 13kb, which codes for a protein known as neurofibrin. Neurofibrin has many different functions. Arguably, one of the more important ones is inactivating the Ras protein that controls cellular growth and multiplication. If there is a mutation in the transcript 13kb that causes a decrease in the production of neurofibrin, cellular proliferation will not be controlled and can drive tumor development.1 This puts patients with NFvR at risk for developing cancer [11].
This is a similar situation to another tumor suppressor gene called MLL4 that helps regulate differentiation and tumor suppression through ferroptosis. Ferroptosis is a type of programmed cell death that is dependent on the presence of iron. A scientific study found that this gene’s role in tumor suppression contributes to the skin’s homeostasis. In fact, this gene is mutated very frequently across all human cancers [12]. Both the NF gene and MLL4 gene are tumor suppressor genes that help regulate cellular proliferation in the epidermis [13].
Solitary neurofibromas are benign and not associated with any systemic complications. The risk of transformation of solitary dermal neurofibromas into malignant peripheral nerve sheath tumors is exceedingly rare. Plexiform neurofibromas are most commonly seen in NF1. Plexiform neurofibromas can infiltrate surrounding tissues and impinge on vital structures. Neurological or functional impairment may occur. They also carry a risk for malignant transformation into a malignant peripheral nerve sheath tumor (MPNST). The exact incidence of malignancy is unknown but is estimated to be between 7 and 13%. Rapid growth or unrelenting pain in an otherwise stable plexiform neurofibroma are signs of malignant transformation. Malignant peripheral nerve sheath tumors (MPNSTs) frequently metastasize, and the outcome is poor [14].
Diagnosis of NFvR is based on the presence of at least 2 of the 7 following criteria: six or more cafe-au-lait macules over 5mm, freckling over the axillary and inguinal area; Lisch nodules over the iris; two or more neurofibroma or one plexiform neurofibroma; sphenoid dysplasia; optic glioma and a first-degree relative with NF-1 [15]. Skin biopsy and clinical signs help establish the diagnosis. If a skin nodule biopsy is performed, the expected findings would show the presence of hypocellular spindle-shaped cells, shredded carrot collagen, mast cells, and hypocellular myxoid areas without hypercellular areas [16]. Immunocytochemistry markers for the differential diagnosis of neurofibroma would show positivity for calretinin, S100 protein, and a stronger association for CD34, which is highly sensitive (p<0.001) [17].
Molecular genetic testing is often recommended to confirm NF1, especially in children displaying only pigmentary features. NF1 causes significantly increased malignancy risks in comparison to general population. Specifically, NF1 is associated with highly elevated risks of rhabdomyosarcoma, juvenile myelomonocytic leukemia, noninvasive pilocytic astrocytoma, malignant peripheral nerve sheath tumor, and optic pathway glioma (OPG). Clinical assessment for OPG is advised every 6 to 12 months up until 8 years of age. Routine MRI assessment is not currently advised in asymptomatic individuals with no signs of visual pathway disturbances. Surveillance for other malignancies is not recommended, however, clinicians and parents should be aware of the small risks of certain specific malignancies. Since tumors do contribute to both morbidity and mortality, a single whole-body MRI should be considered to assist in determining approaches to long-term follow-up [10].
The treatment for NF1 varies based on the patient's symptoms and presentation. Minimization of radiation treatment is important in patients with NF1 and central nervous system (CNS) tumors because of concerns about secondary CNS malignancies and vascular complications. Cutaneous and subcutaneous neurofibromas are not removed due to the increased risk of scarring or recurrence. If the patient experiences disfigurement or unmanageable bleeding and pain, removal is indicated. Various options for removal are surgery, laser, or electrodesiccation. Some patients experience pruritus that typically does not respond to antihistamine treatment but improves with medications used to treat neuropathic pain, such as gabapentin [18].
Plexiform Neurofibromas (PN) involve multiple nerve fascicles with serpiginous growth and significant vascularity. PNs can undergo malignant transformation to MPNSTs. Surgical treatment and pain management of PNs can be challenging depending on the location especially when there is progressive growth along the spinal column. For inoperable and symptomatic tumors, Selumetinib, an oral selective mitogen-activated protein kinase (MEK) inhibitor, can induce tumor regression [19]. Selumetinib was approved for the treatment of pediatric patients in April 2020. The clinical trial resulted in a ≥20 percent decrease in tumor volume from baseline for at least four weeks in 70% of the patients. The mean tumor-related pain intensity scores decreased by two points after one year of treatment. Approximately 50% of patients experienced improvements in quality of life [18]. In addition, genetic counseling should be provided for patients and families. Prenatal or preimplantation testing should include information on the inheritance of the disorder, prognosis, and psychosocial adjustment.
Disclosures: The authors report no relevant financial relationships.
References
- Boyd KP, Korf BR, Theos A. Neurofibromatosis type 1. J Am Acad Dermatol, 2009; 61(1): 1-16. doi:10.1016/j.jaad.2008.12.051.
- Brosius S. A history of von Recklinghausen's NF1. J Hist Neurosci, 2010; 19(4): 333-348. doi:10.1080/09647041003642885.
- Ruggieri M, Praticò AD, Caltabiano R, Polizzi A. Early history of the different forms of neurofibromatosis from ancient Egypt to the British Empire and beyond: First descriptions, medical curiosities, misconceptions, landmarks, and the persons behind the syndromes. Am J Med Genet A, 2018; 176(3): 515-550. doi:10.1002/ajmg.a.38486.
- Tamura R. Current Understanding of Neurofibromatosis Type 1, 2, and Schwannomatosis. Int J Mol Sci, 2021; 22(11): 5850. doi:10.3390/ijms22115850.
- Evans DG, Howard E, Giblin C, et al. Birth incidence and prevalence of tumor-prone syndromes: estimates from a UK family genetic register service. Am J Med Genet A, 2010; 152A(2): 327-332. doi:10.1002/ajmg.a.33139.
- Stephens K, Kayes L, Riccardi VM, Rising M, Sybert VP, Pagon RA. Preferential mutation of the neurofibromatosis type 1 gene in paternally derived chromosomes. Hum Genet, 1992; 88(3): 279-282. doi:10.1007/BF00197259.
- Ruggieri M, Huson SM. The clinical and diagnostic implications of mosaicism in the neurofibromatoses. Neurology, 2001; 56(11):1433-1443. doi:10.1212/wnl.56.11.1433.
- Rasmussen SA, Yang Q, Friedman JM. Mortality in neurofibromatosis 1: an analysis using U.S. death certificates. Am J Hum Genet, 2001; 68(5): 1110-1118. doi:10.1086/320121.
- Abaloun Y, Ajhoun Y. Nodules de lisch dans la neurofibromatose type 1 [Lisch nodule in neurofibromatosis type 1]. Pan Afr Med J, 2017; 27: 218. doi:10.11604/pamj.2017.27.218.11517.
- Korf BR, Lobbous M, Metrock LK. Neurofibromatosis type 1 (NF1): Pathogenesis, clinical features, and diagnosis, 2022.
- Landry JP, Schertz KL, Chiang YJ, et al. Comparison of Cancer Prevalence in Patients with Neurofibromatosis Type 1 at an Academic Cancer Center vs in the General Population From 1985 to 2020. JAMA Netw Open, 2021; 4(3): e210945. doi:10.1001/jamanetworkopen.2021.0945.
- Egolf S, Zou J, Anderson A, et al. MLL4 mediates differentiation and tumor suppression through ferroptosis. Sci Adv, 2021; 7(50): eabj9141. doi:10.1126/sciadv.abj9141.
- Bergoug M, Doudeau M, Godin F, Mosrin C, Vallée B, Bénédetti H. Neurofibromin Structure, Functions and Regulation. Cells, 2020; 9(11): 2365. doi:10.3390/cells9112365.
- Gnepp DR, Bishop JA. Gnepp's diagnostic surgical pathology of the head and neck. 3rd ed. Amsterdam: Elsevier Inc; 2020.
- Neurofibromatosis. Conference statement. National Institutes of Health Consensus Development Conference. Arch Neurol, 1988; 45(5): 575-578.
- Abdellatif E, Kamel D. Soft Tissue Neurofibroma. Pathology Outlines - Neurofibroma-general, 2022.
- Park JY, Park H, Park NJ, Park JS, Sung HJ, Lee SS. Use of calretinin, CD56, and CD34 for differential diagnosis of schwannoma and neurofibroma. Korean J Pathol, 2011; 45: 30-35. doi:10.4132/KoreanJPathol.2011.45.1.30.
- Gross AM, Wolters PL, Dombi E, et al. Selumetinib in Children with Inoperable Plexiform Neurofibromas [published correction appears in N Engl J Med, 24; 383(13): 1290]. N Engl J Med, 2020; 382(15): 1430-1442. doi:10.1056/NEJMoa1912735.
- Klesse LJ, Jordan JT, Radtke HB, et al. The Use of MEK Inhibitors in Neurofibromatosis Type 1-Associated Tumors and Management of Toxicities. Oncologist, 2020; 25(7): e1109-e1116. doi:10.1634/theoncologist.2020-0069.

