An Overview of Non-Allopathic Management on COVID-19
Prajakta A Mahale, Sunita S Deore, Sumit R Deore, Anuja S Muley, Sushil P Narkhede, Pravin K Jha, Makrand S. Gambhire and Vishal S Gulecha
School of Pharmaceutical Sciences, Sandip University, India
Received Date: 12/11/2022; Published Date: 30/11/2022
*Corresponding author: Prajakta A Mahale, School of Pharmaceutical Sciences, Sandip University, India
Abstract
Coronavirus 2019-nCoV-2 infection, also known as COVID-19, has a pandemic outbreak in city Wuhan in China. Corona virus transmitted human to human to animal via air bone droplets. It is a viral disease due to the Several Acute Respiratory Syndrome. In this various guideline for management of COVID-19 was described. The aim of the study is to analyze various clinical trials registered in Clinical Trial registry of India (CTIR) during this pandemic period of COVID-19 and it also describes non allopathic treatment, prevention and management for disease SARS-CoV-2.
Keywords: COVID-19; Trials; Ayush; Homeopathy; Unani; Siddha
Introduction
Covid-19
History and origin
First case of corona virus was notified as cold in 1960. According to the Canadian study 2001, approximately 500 patients were identified as flu-like system. 17-18 cases of them were confirmed as infected with corona virus strain by polymerase chain reaction. Corona was treated as simple non-fatal virus till 2002. In 2003, various reports published with the proofs of spreading the corona to many countries such as United States America, Hong Kong, Singapore, Thailand, Vietnam and in Taiwan. Several cases of severe acute respiratory syndrome caused by corona and their mortally more than 1000 patient was reported in 2003. This was started focus to understand these problems. After a deep exercise they conclude and understand the pathogenesis of disease and discovered as corona virus. But till total 8096 patient was confirmed as infected with corona virus. So, in 2004, world health organization and centres for disease control and prevention declared as “state emergency”. Another study report of Hong Kong was confirmed 50 patients of severe acute respiratory syndrome while 30 of them were confirmed as corona virus infected. In 2012, Saudi Arabian reports were presented several infected patient and deaths.
After 2012, the outbreak of a febrile respiratory illness due to a coronavirus 2019-nCoV, the city of Wuhan in China, is under global attention. In December 2019, several occurrences of pneumonia noticed in Wuhan, Hubei province in China. However, in response to a notification on 31 December 2019, by the Chinese Health Authorities, the World Health Organization (WHO) issued warnings about a possible emergence of a novel and a major threat to public health. This novel coronavirus is named as 2019-nCoV-2 by WHO, which matches 70% in genetic sequence to previous severe acute respiratory syndrome coronavirus or SARS-CoV. The researchers proposed SARS-CoV-2 had a bat-human transmission, although unconfirmed. The recent occurrence of clusters of pneumonia due to 2019-nCoV poses substantial threats to global health. Suffered patients were presenting with fever, malaise, dry cough, shortness of breath, respiratory distress, leucopenia, and lymphopenia. Considering the clinical features, several diseases, including severe pneumonia, acute respiratory distress syndrome, septic shock, etc. were listed in the differential diagnosis. However, many questions about the new coronavirus remain unanswered, and with limited clinical information of the 2019-nCoV infection and data, WHO acknowledged this current infection as a ‘Pandemic Infection’ [1].
Microbiology of COVID-19
Corona virus is spherical or pleomorphic, single stranded, enveloped RNA and covered with club shaped glycoprotein. Corona viruses are four sub types such as alpha, beta, gamma and delta corona virus. Each of sub type corona viruses has many serotypes. Some of them were affect human of other affected animals such as pigs, birds, cats, mice and dogs.
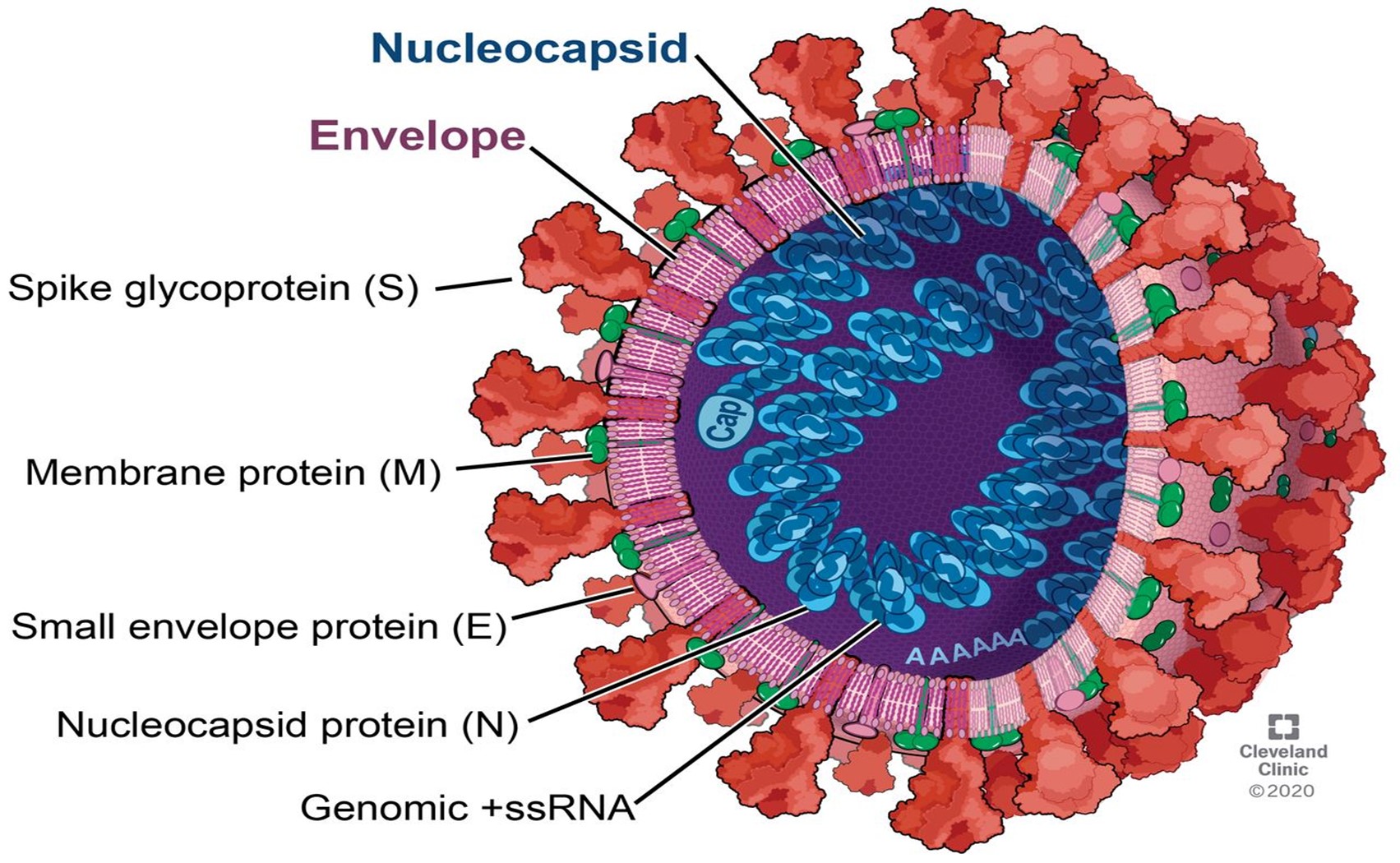
Figure 1: Structure of Coronavirus [2].
Mode of spreading of covid-19

Peoples can get the infection through close contact with a person who has symptoms from the virus includes cough and sneezing. Generally, corona virus was spread via air-borne zoonotic droplets. Virus was replicated in ciliated epithelium that caused cellular damage and infection at infection site. According to a study published in 2019, angiotensin converting enzyme 2 (ACE.2), a membrane exo-peptidase in the receptor used by corona virus in entry to human cells.
Prevention of COVID-19
There is nothing to provide complete guidance to prevent from corona virus but some guidelines were presented by WHO and ECDC. Basically, these guidelines are for health profession to set during the caring of infected patient. Because many evidence was presented by studies about human-to-human transmission of corona from Wuhan, china. Another study reported about air bone transmission of virus while no one was presenting the solid evidence. As the lack of transmission evidence health professionals were not able to prevention guidelines. According to WHO, some general guidelines were published such as separate the infected patient from other family member to single room, implementation of contact and droplet precaution, airborne precaution etc. European Centre for Disease Prevention and Control (ECDC) also published the information leaflet to peoples i.e., Avoid contact with sick people, in particular those with a cough. Avoid visiting markets and places where live or dead animals are handled, wash your hands with soap and water or use an alcohol based disinfectant solution before eating, after using the toilet and after any contact with animals, avoid contact with animals, their excretions or dropping [3,4].
Clinical Management of COVID-19

Triage: Early recognition of patients with COVID-19 – The purpose of triage is to recognize and sort all patients with COVID- 19 at first point of contact with health care system.
Definition:
SARI: An ARI with history of fever or measured temperature > 38C and cough, onset within the last – 10 days; and requiring hospitalization.
Surveillance case: SARI in a person, with history of fever and cough requiring admission to hospital, with no other etiology that fully explains the clinical presentation (Clinicians should also be alert to the possibility of atypical presentations in patients who are immune compromised.
Close Contact:
- Health care associated exposure
- Working together in close proximity
- Travelling together with COVID 19 patient
Living in the same household as a COVID- 19 patients.

Figure 1: Clinical syndromes associated with COVID 19 infection.
Immediate implementation of appropriate IPC measures. (Typically, the Emergency Department)
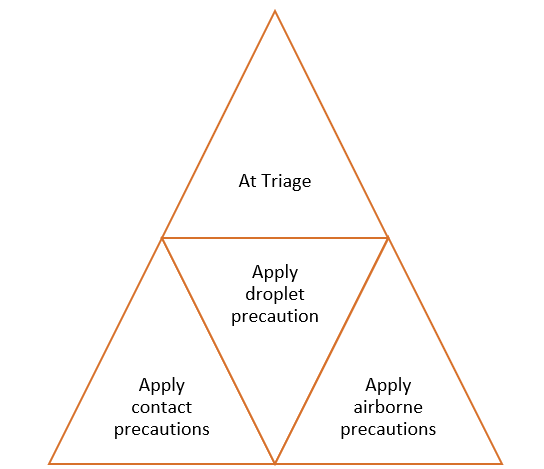
Figure 2: How to implement infection prevention and control measure for patients with suspected or confirmed COVID- 19 infection.
Early supportive therapy and monitoring:
- Give supplemental oxygen therapy
- Use conservative fluid management in patients with SARI
- Give empiric antimicrobials to treat like pathogens
- Do not routinely give systemic corticosteroids for treatment of viral pneumonia or ARDS.
- Closely monitor patients
- Understand the patients’s co-morbid conditions.
- Communication
Collection of specimens for laboratory diagnosis:
- Collect blood samples for bacteria.
- Collect specimen of nasopharyngeal swab
- Use appropriate PPE for specimen collection
- Dual infections
- Hospitalized patients.
Management of hypoxemic respiratory failure and ARDS:
- To recognize severe hypoxemic respiratory failure
- High- flow nasal catheter oxygenation
- NIV guidelines
- Recent publications
- Endotracheal intubation
- Implement mechanical ventilation
- Severe ARDS
- Conservative fluid management
- Expertise in extracorporeal
- Avoid disconnecting the patient
Management of Septic shock:
- Recognize septic shock
- Absence of lactate measurement
- Resuscitation from septic shock
- Fluid resuscitation
- Crystalloids
- Administer vasopressors
Other therapeutic Measures:
For patients with progressive deterioration of oxygenation indicators, rapid worsening on imaging and excessive activation of the body’s inflammatory response, glucocorticoids can be used for a short period of time (3 to 5 days). It is recommended that dose should not exceed the equivalent of methylprednisolone 1 – 2mg/kg/day. Note that a larger dose of glucocorticoid will delay the removal of coronavirus due to immunosuppressive effects. For pregnant severe and critical cases, pregnancy should be preferably terminated. Consultations with obstetric, neonatal, and intensive care specialists (depending on the condition of the mother) are essential. Patients often suffer from anxiety and fear and they should be supported by psychological counselling.
Prevention of complication:
Specific COVID 19 treatment and clinical research:
There is no current evidence from RCTs to recommend any specific treatment for suspected or confirmed patients with COVID - 19. No specific anti–virals are recommended for treatment of COVID – 19 due to lack of adequate evidence from literature. The use of Lopinavir/ Ritonavir in PEP regimens for HIV (4 weeks) is also associated with significant adverse events which many a times leads to discontinuation of therapy. In light of the above, Lopinavir/ Ritonavir should ONLY be used with proper informed expressed consent on acase to case basis for severe cases, within the under-mentioned framework along with supportive treatment as per need.
Administration of Lopinavir/ Ritonavir:
Administration of Lopinavir/ Ritonavir to be considered in Laboratory confirmed cases of COVID – 19 when the following criteria are met:
Symptomatic patients with any of the following:
i. hypoxia,
ii. hypotension,
iii. new onset organ dysfunction (one or more)
Increase in creatinine by 50% from baseline, GFR reduction by >25% from baseline or urine output of <0.5 ml/kg for 6 hours.
- Reduction of GCS by 2 or more
- Any other organ dysfunction
High Risk Groups:
- Age> 60 yrs.
- Diabetes Mellitus, Renal Failure, Chronic Lung disease
- Immune – compromised persons
- Dosage:
i. Lopinavir/ Ritonavir (200 mg/ 50 mg) – 2 tablets twice daily
ii. For patients unable to take medications by mouth: Lopinavir 400mg/ Ritonavir 100 mg – 5ml suspension twice daily
- Duration: 14 days or for 7 days after becoming asymptomatic [5].
ICMR Guidelines for covid-19:
Statement of General Principles:
Four basic principles – respect for persons, beneficence, non-maleficence and justice must guide research in order to protect the dignity, rights, safety and well-being of research participants while conducting biomedical and health research.
General Ethical Issues:
- Benefit-risk assessment
- Privacy Confidentiality
- Distributive Justice
- Payment for participation
- Compensation for research-related harm
- Conflict of interest
- Community Engagement
- Post research access and benefit sharing
- Storage of Biological material/datasets
- Collaboration in research
- Public health and socio behavioral research
- Role of Agencies/ sponsors and Governance of Research
- Biosafety in laboratories and hospitals
Ethical Review Procedures:
- Categories of Research
- Ethics Committee (EC)
- Special situations
- Ethics Review
- Review of Multicentre Research
- Continuing Review and Monitoring
- Decisions Regarding Ongoing Studies
- Review of new non-COVID Research
Informed Consent:

Vulnerability:
- Vulnerable Person
- Additional Safeguards
- Safety of health care workers (HCW) involved in research
- Psychological needs and mental health [6]
CDSCO Guidelines for covid-19:
Introduction: The main objective of development of vaccine is to generate adequate data on quality, safety, immunogenicity and efficacy to support application for marketing authorization. This guidance provided in these documents will be applicable in general for CMC, nonclinical and clinical development of any vaccine including COVID-19 vaccines.
Background: In general, all vaccines including the vaccines againt CORONA virus infection manufactured/imported into the country are required to comply with the requirements and guidelines specified in the Drugs and Cosmetics Rules, 1945 and New Drugs and Clinical Trials Rules, 2019 and other applicable guidelines published by CDSCO form time to time in case of manufacturer r-DNA derived vaccines the requirements and guidelines prescribed by Department of Biotechnology are also required to be complied with.
Chemistry, manufacturing and controls:
- General consideration
- Manufacturing
- Potency
- Stability
- Batch release and independent laboratory evaluation
Toxicity assessments:
- Study design
- Special consideration for COVID-19 vaccine
- Animal species, sex, age and size of groups
- Dose, route of administration and control groups
- Parameters Assessed
- Local tolerance
Additional toxicity assessments:
- Special immunological investigations
- Developmental and reproductivetoxicity studies
- Genotoxicity and carcinogenicity studies
Safety pharmacology:
- Pharmacokinetics studies
- Adjuvants
- Additives
- Vaccine formulation and delivery device
- Alternative routes of administration
- Animal models
- Dose
- End-points
- Immunogenicity assessment
Clinical development Programme:
- General consideration
- Special consideration for COVID-19 Vaccine
- Immunogenicity
Efficacy trials:
- Efficacy trial design
- Clinical End Points
- Primary End Point
- Secondary End Point
- Special consideration for efficacy of COVID-19 Vaccine
- Statistical Considerations
Safety Consideration: The size of the pre-approval safety database should be decided on a case-by-case basis.
Post Marketing clinical evaluation- After approval of vaccine, it is essential to monitor vaccine safety in routine use. Studies can be designed to address specific safety issues that were identified as potential concerns from pre-approval trials may need to be conducted [7].
Ayush Guidelines for COVID-19:
Clinical Trial Protocol- It is a document that describes that how the clinical trial will be conducted (The objective, design, methodology, statistical considerations and organization of a clinical trial) and ensure the safety of the trial subjects and integrity of the data collected. It provides a structured framework to individuals conducting the study, serves as the basis for trial registration.
Protocol development- Researchers should refer SPIRIT Statement (Standard Protocol Items: Recommendations for Interventional Trials) to improve the completeness and quality of trial protocols.
National Ethical Guidelines:
The principles of Ethics are as follow-
- Essentiality
- Voluntariness
- Non-exploitation
- Social responsibility
- Ensuring privacy and confidentiality
- Risk minimization
- Professional competence
- Maximization of benefit
- Institutional arrangements
- Transparency and accountability
- Totality of responsibility
- Environmental protection
Clinical trial Registry: In this research there is involving human participants to undergo independent and unbiased review by the Institutional EC and then register with Clinical Trial Registry of India before enrolment of first study participant.
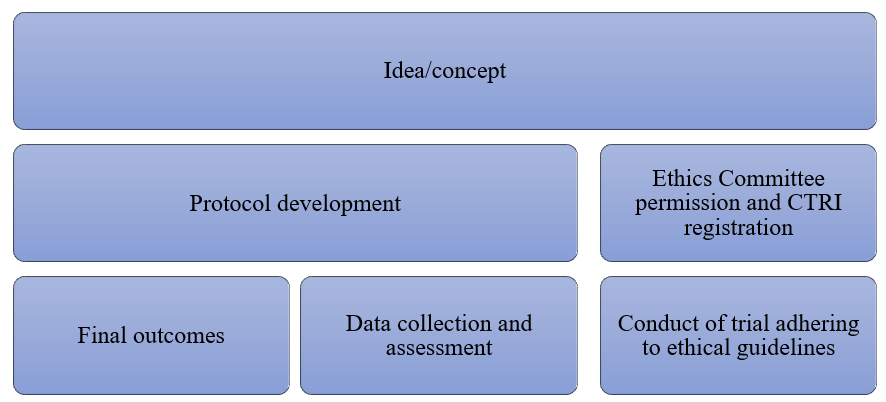
Protocol for Trials on Ayush Interventions:
The following are the components of clinical trial protocol:
Title: In the title section, Investigator should provide a succinct description that conveys the topic, acronym, and basic study design- including the method of intervention allocation in the title.
Introduction- The introduction consists of free-flowing text, in which investigator explains the scientific background and rationale for the trial.
The introduction has following components:
- Background
- Rationale
Objectives- Objectives reflect the scientific questions to be answered by the trials, and define its purpose and scope.
The examples of objective are:
- Prophylactic trial
- Treatment trial
- Recovery trial
Trial design- The word ‘design’ is often used to refer to all aspects of how a trial is set up that includes details of articulation of various methods such as randomization and blinding. The preferred type of trials for COVID-19 is parallel group over other types such as crossover, factorial, single group.
Methods:
- Study setting
- Eligibility criteria
- Inclusion criteria
- Exclusion criteria
- Interventions
- Criteria for discontinuing allocated interventions/withdrawal of a trial participant
- Prophylaxis trial
- Treatment trial
- Recovery trial
- Outcomes
- Time schedule of enrolment, interventions, and assessment
- Allocation of participants to study groups
- Blinding
- Data collection methods
- Retention
- Statistical methods
- Data monitoring
- Harms
- Auditing
Ethics and dissemination:
- Research ethics approval
- Consent or assent
- Confidentiality
- Access to data
- Ancillary and Post-trial care
- Dissemination policy
Indemnity issues- The COVID-19 trials have put forth many indemnity issues for both, the trial participants and the Investigator. Each participant recruited in the trial should be covered under clinical trial liability policy. The investigator safety should be also considered with importance.
Appendices:
- Informed consent material
- Biological specimens [8]
Clinical Trials on COVID-19
A clinical trial is a research study that finds new ways to prevent, diagnose or treat disease. For any new drug to enter in clinical trial, it must pass preclinical studies. Research may include pharmacodynamics, pharmacokinetics, absorption, distribution, metabolism and excretion studies, and toxicity testing. During preclinical studies, in vitro and in vivo testing is performed. If preclinical studies show that the therapy is safe and effective, then the clinical trials can be defined as “scientifically controlled studies of the safety and effectiveness of a therapeutic agent using consenting human subjects”, are started.
The four possible outcomes of trials are:
- The new treatment has a large beneficial effect and is superior to standard treatment.
- The new treatment is equivalent to standard treatment.
- The new treatment is neither clearly superior nor clearly inferior to standard treatment.
- A new treatment is inferior to standard treatment.
Clinical trial phases are steps in the research to determine if an intervention would be beneficial or detrimental to humans and include phases O, I, II, III, IV, and V clinical studies. In phase O, pharmacodynamics and pharmacokinetics are determined. Safety studies are evaluated during Phase I trials. Efficacy during phase II trial can be determined. In phase III trial confirmation of safety and efficacy. Sentry studies are done in phase IV and comparative effectiveness research and community-based research in Phase V [9,10].
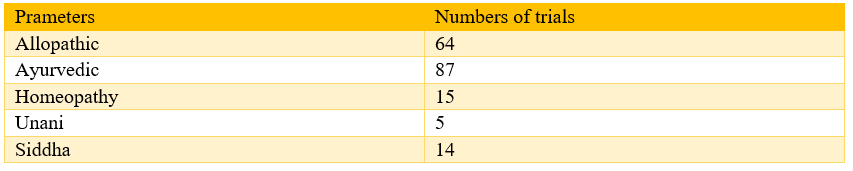
There were around 60 COVID-19 related clinical trials in January 2020 which increased to around 4000 in first week of July. ICMR National task force for COVID 19 constituted groups related to clinical research, research on diagnostic and biomarkers, epidemiology and surveillance, operational research, vaccine and drug research to set up the research priorities for COVID-19 in India. It is done at the government level; it is expected that high-quality research should be undertaken in India and the synthesized results should be available to give further guidance. India is one of the countries amongst the top 10 countries where the registered clinical trials is available. Availability of such information is required for the better understanding of this not only researchers and academicians but also to policy makers and government. From this objective, we have designed this study to explore the Clinical Trial Registry of India (CTIR) data to observe that what kind of clinical trials are being conducted for COVID 19 in India. In total 331 studies were registered in CTRI as of 11th July. Out of these, 203 are trials, and 128 were observational studies. Out of 203 trials, 125 are the Ayush trials, and 64 were allopathic trials. Amongst the Ayush 87 were trial exploring ayurvedic interventions followed by homeopathy and siddha. The majority of trials were national, and only 3 were global. Single centre trials were predominant as compared to the multicentric trials. Sponsoring was almost equal form public and private sector, and amongst the public funding, the significant portion goes to Ayush trials 73 and same in the case of private funding, where around 50% trials were Ayush trials. Majority of the trials were of phase 2 and phase 3. Around 45% of trials has a duration of less than 6 months. The majority of the trials were associated with the treatment of COVID 19 as compared to prophylaxis of COVID 19. Leading states where these trials, are being conducted are Maharashtra and Delhi. In the case of Ayush trials, most frequent interventions which were explored in the trials were Arsenicum Album, Ashwagandha, Ayush-64 and Guduchi Ghan Vati. Twelve trials related to the chloroquine and Hydroxychloroquine as monotherapy or with other drugs are registered on CTRI. There were ten trials related to the convalescent plasma therapy, 6 trials were related to the Itolizumab and 2 trials were related to the Favipavir.
Table 1: COVID 19 related clinical trials in different states of India.

Table 2: Characteristics of COVID 19 related trails registered in CTRI [11].
Material and Methods
Ayush:
The central government has released the “National clinical management protocol based on Ayurveda and Yoga for the management of covid-19”. The protocol, which was released by Union Health Minister Harsh Vardhan, suggests the use of medicines such as Ashwagandha, Guduchi Ghana Vati, or Chyawanaprasha as prophylactic care for high-risk population and primary contacts of patients.
The protocol mentions the dose of these medicines that is to be taken. The guidelines stated that in addition to these medicines, general and dietary measures have to be followed. Individuals with moderate to severe coronavirus infection may make an informed choice of treatment options and all severe cases will be referred, the protocol said.
For Prophylactic care (high-risk population, primary contacts), Ashwagandha (Aqueous extract of withania somnifera IP) or its powder can be given. One needs to intake 500mg extract or 1-3gram powder twice daily with warm water for 15 days or one month or as directed by Ayurveda physician. For Prophylactic care, Guduchi Ghana vati (Samshamani vati or Giloy Ghana vati having Aqueous extract of Tinospora cordifolia IP) or the powder of Tinospora cordifolia can be given. One needs to intake 500mg extract or 1-3gram powder twice daily with warm water for 15 days or one month or as directed by Ayurveda physician. For Prophylactic care, Chyawanaprasha can be consumed after mixing with 10-gram warm water or milk once a day. The protocol has stated that in addition to these aforementioned medicines, general and dietary measures should be followed.
For Asymptomatic COVID-19 positive patients, Guduchi Ghana vati can be given. One needs to intake 500mg extract or 1–3-gram powder twice daily with warm water for 15 days or one month or as directed by Ayurveda physician. One can also take Guduchi and Pippali (Aqueous extracts Tinospora cordifolia IF and Piper longum IF). One needs to intake 375mg twice daily with warm water for 15 days or as directed by an Ayurveda physician. One can also take AYUSH 64. One needs to intake 500 mg twice daily with warm water for 15 days or as directed by an Ayurveda physician. However, these medicines must be taken for the prevention of disease progression to symptomatic and severe form and to improve the recovery rate.
For mild Covid 19 positive patients, who have fever, headache, tiredness, dry cough, sore throat, nasal congestion but do not have evidence of breathlessness or hypoxia, can be given Guduchi and Pippali. One can intake 31mg twice daily with warm water for 15 days or as directed by Ayurveda physician. One can also take AYUSH 64. One needs to intake 500mg twice daily with warm water for 15 days or as directed by Ayurveda physician.
For Post COVID-Management, one can take 500mg extract or 1–3-gram powder of Ashwagandha twice daily with warm water for 15 days or one month or as directed by Ayurveda physician. One can intake 10 gram of Chyawanprasha with warm water or milk once a day [12].
Unani Medicines:
Generally, the unani medicines (plant-based medicines) are non-toxic and without any side effects. Unani and Ayurvedic methods of the treatment are based on the plant materials.
Unani medicines useful in symptomatic management of corona virus infection:
- Sharbatunnab 10-20ml twice a day
- Tiryaqarba 3-5g twice a day
- Tiryaqnazla 5g twice a day
- Khamiramarwareed 3-5g once a day
- Massage on scalp and chest with RoghanBaboona/ Roghan Mom/ Kafoori Balm
- Apply Roghan Banafsha gently in the nostrils
- Take ArqAjeeb 4-8 drops in fresh water and use four times a day
- In case of fever, take Habb e IkseerBukhar 2 pills with lukewarm water twice daily.
- Sharbat Nazla 10ml mixed in 100ml of lukewarm water twice daily.
- Qurs e Suaal 2 tablets to be chewed twice daily
- Arq extracted from following single unani drugs along with SharbatKhaksi is very useful:
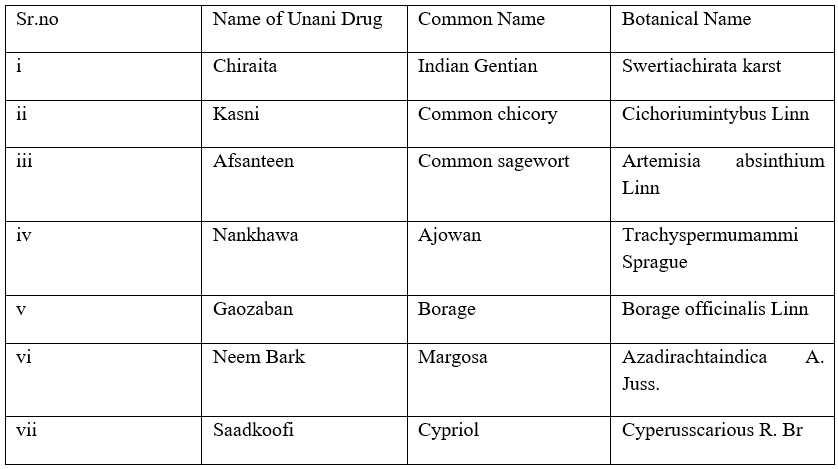
- Decoction of the following single Unani drugs can be used:

- In case of sore throat use decoction of the following Unani drugs:

- Dietary Recommendations:
As per recommendation of Unani physicians, easily digestible, light and soft diets are advised.
Homeopathy:
In homeopathy, arsenic at very low concentration is considered beneficial for several diseases including viral infections. Recently, Directorate of AYUSH, New Delhi, India issued an order dater on January 30, 2020, to take prophylactic medicine to avoid coronavirus infection. The directorate suggested taking 4 pills of Arsenic Album-30 medicine once daily in empty stomach for 3 days. Arsenic Album-30 is highly diluted arsenic trioxide and work as homeopathic prophylaxis. It is important to mention here that there is no clinical evidence for Arsenic Album-30 medicine as an effective medicine. After that, a criticism for homeopathy came into existence and it was called as pseudoscience. Some people criticized homeopathy to manage COVID 19 infection. The persons who criticized are Dr. David Robert Grimes (Irish science write) and Dr. Edzard Ernst (an emeritus professor, University of Ernst (an emeritus professor, University of Exeter, UK and a critic of homeopathy). However, Dr. Mitchell Fleisher (second vice president, American Institute of Homeopathy) advised to carry out a comparative clinical study on the acute coronavirus infection by giving to homeopathic medicines to an individual and experimental group, and allopathic medicines to another, for 250 patients in each group. It was stated to confirm the scientific truth. But after this statement again Dr. David Robert Grimes criticized it as completely unethical and according to him, Homeopathy has no reasonable mechanism of action. Furthermore, he added that it is irresponsible to propose a trial for a serious pandemic. He also mentioned that many studies on homeopathy have indicated that it does not work. Also, the new director of Thailand Medical New Jakkapong watcharachaijunta criticized the use of homeopathic medicine in controlling COVID-19. At this point, it is very important to mention the work of Dr. Robert T. whose research work described that Arsenicum album medicine as effective to reduce fever, runny nose, headache, sore throat in the patients with swine flu symptoms. During writing this article under this section, it was realized that the subject matter is debatable and needs the scientific study to support the working of homeopathic medicine for COVID-19. It is suggested that some research work should be funded by the government and the research work should be funded by the government and the research should be carried out to make the situation clear. It is significant to add here that personally I (Prof. Imran Ali) used some homeopathic medicine when living in india for some diseases and found them effective. Besides, I also observed that some homeopathic medicines are effective to treat a variety of diseases.[13]
Siddha:
Traditional medicine is playing a key role in meeting global healthcare needs. Siddha is a unique system of medicine, which is originated from Tamil Nadu and has its origin in the Tamil language. Literally, the word “Siddha” means “established truth”. Siddha medicine is claimed to alleviate the root cause of the disease by maintaining the equilibrium among vatham, pitham, and kapam. There are many Siddha formulations, such as Kudineer, mattirai, chooranam, parpam, chendurum, karuppu, and mezhugu. The aim is to explore some of the herbal and herbomineral formulations used to prevent or treat COVID-19.
Siddha formulations:
The siddha drugs selected for the management of COVID-19 depends on its pharmacological action. The herbal formulations and its documented pharmacological activity are shown in Table.
Some of the Siddha medicine used for the management of COVID-19

Adathodai Manapagu
This preparation is based on the Adhatoda Vasica (AV) leaf juice.Adhathoda vasica belongs to the Acanthaceae family. The plants consist of “quinazoline alkaloids (vasicine, 7-hydroxy vasicine, vasicinolone, 3-deoxy vasicine, vasicol, vasicoline, vasicolinone, triterpenes, anisotine), betaine, steroids carbohydrate, and alkanes. In the flowers, triterpenes (a-amirine) and flavonoids (apigenin, astragalin, kaempferol, quercetin, vitexin) have been” found. The crude extract of justiciar adhatoda acts against the influenza virus by Hemagglutination (HA) decrease. In two unique layouts of simultaneous and posttreatment, assay shows antiviral activity in the non-cytotoxic range. Methanolic extract of AV revealed a 100% rebate “in HA in the simultaneous and posttreatment at the concentration of 10mg/ml. the aqueous extract of J.adhatoda at 10 and 5 mg/ml concentrations shows reduced HA to 33% and 16.67%, respectively, in the simultaneous assay. These results showed that aqueous and methanolic extracts of J. adhatoda have strong antivirus activity that can inhibit viral attachment and/or viral replication, and may be used for viral” prevention. The compound vasicine shows the excellent antiviral property in Dock assay.
Kabasura Kudineer
The Siddha classical formulation kabasura kudineer chooranam consists of 15 ingredients of herbs “(Zingiber officinale, Piper longum, Syzygium aromaticum, Tragia involucrate, Anacyclus pyrethrum, Hygrophila auriculata, Terminalia chebula, AV, Coleus amboinicus, Saussurea lappa, Tinospora cordifolia, Clerodendrum serratum, Andrographis paniculate, sida acuta, and Cyperus rotundus)”. The mechanism of action of the phytocompounds present in the kabasura kudineer Siddha formulation attracting/binding multiple amino acid at different sites of viral proteins which corroborated with the well-known antimalarial drug, artemisinin. This showed the synergistic activity of phytocompounds not only against the viral proteins but also modulate the immune system for fighting against viral replication. The active molecules of the respective medicinal plants, Trichosanthes cucumerina, T. cordifolia, H. auriculata, A.pyrethrum, A. paniculate, AV, S. lappa, C. serratum, S. aromaticum, and Z. officinale, might inhibit the viral pathogenesis at various levels spanning from prevention to cure. It revealed that the functionally significant formulations against corona viral protein showed a more efficient inhibitory effect against viral replication.
Thontha Sura Kudineer
Thontha sura kudineer chooranam consist of 10 ingredients of herbs (Z. Officinale, AV, A. paniculate, T. cordifolia, Elettaria cardamomum, Solanum xanthocarpum, T. cucumerina, Tephrosia purpurea, Mollugo cerviana, and Vitis vinifera) was studied for antiviral activity by in silico docking analysis. The phytocompounds in thontha sura kudineer had promising activity against the viral spike glycoprotein which prevents the spike proteins binding with host cell receptor.
Nilavembu Kudineer (NVK)
“Nilavembu kudineer is a polyherbal formulation with A. paniculata as the main ingredient that controls all types of fever related to body pain. Other components include Vetiveria zizanioides, V. zizanioides, Santalum album, T. cucumerina, C. rotundus, Zingiber officinale, Piper nigrum, and M. cerviana. All these plants are utilized traditionally in the treatment of pyretic, “inflammation, arthralgia, arthritis, gastric ulcer, jaundice, and general debility conditions”. Nilavembu kudineer extensively controls fever through its managing consequences effects on temperature, inflammation control, body pain, and it also acts in a way to improve immunity. All the components in this formulation have the bioactive molecules that shows excellent activity against dengue, chikungunya, herpes simplex virus (HSV), and influenza virus.
Vajra Kandi Chenduram
It is a herbomineral preparation broadly utilized particularly in Siddha practitioners regards the management of several acute and chronic illnesses ranging from fever to chronic inflammatory disorders and immune-mediated diseases. This formulation is made of purified lingam, veeram, pooram, and rasa sindhuram. This component shows antipyretic, anti-inflammatory, and antioxidant activity. Vajra kandi chenduram through its antipyretic and anti-inflammatory activity can be the potential to prevent the release of the inflammatory mediators and cytokine storm of COVID-19 which is a major cause for severe lung complication. Therefore, this formulation can be advised as a safe and effective supportive therapy in the absence of any specific target treatment measures. “In targeting key molecules within the inflammatory cytokine network such as Interleukin-6 (IL-6) is a novel strategy for COVID-19 induced” Cytokine Release Syndrome (CRS). “Interleukin-6 inhibitors may ameliorate severe damage to bronchial tissue caused by cytokine release in patients with serious COVID-19 infections. Several damages to bronchial tissue caused by cytokine release in patients with serious COVID-19 infections are being reported. Several studies have indicated a “cytokine storm” with the release of IL-6, IL-2, and IL-8 along with ‘Tumor Necrosis Factor’ α (THF α) and other inflammatory mediators”.
Visha Sura Kudineer
Visha Sura Kudineer (VSK) is a polyherbal formulation from Siddha literature “Kaaviya Sura Nool”. The components were Azadirachta indica, Indigofera tinctoria, Z. officinale, Hemidesmusw indicus, Aristolochia bracteate, V. zizanioides, Glycyrrhiza glabra, E. cardamomum, and Santalum album. Each of the component shows antiviral activity against wide range of viruses. “Aqueous leaf extract of A. indica offers antiviral activity against vaccinia virus, chikungunya measles virus, dengue virus type-2, and HSV” type-1, it also has immune stimulant, anticomplement activity. Indigofera tinctoria shows an inhibitory effect of HIV-1 (III B) and HIV-2. “Zingiber officinale has antiviral activity against the human respiratory syncytial virus in human respiratory tract cell lines”. Hemidesmus indicus antiviral activity was studied against the Ranikhet virus. “A component of licorice root glycyrrhizic acid has antiviral activity by inhibiting the growth and cytopathic effect of several DNA AND RNA viruses, such as vaccinia, HSV-1, Newcastle disease, and vesicular stomatitis viruses”. It also shows antiviral activity against flaviviruses, such as dengue, Japanese encephalitis, yellow fever, mammalian tick-borne encephalitis, influenza, and hepatitis A, B, C viruses. Sandalwood oil, the essential oil of Santalum album L., indicated antiviral activity against HSV-1 and HSV-2 [14].
Conclusion
This SARS-CoV-2 infection is causing countless deaths worldwide and globle health members are struggling in finding an effective solution to control this infection. The rapid widening of infection permits exceptional surveillance and involvement in an activity protocol to prevent further transmission. Through this work all the ingredients of the Ayush, Homeopathy, Unani and Siddha formulations have been explored scientifically for its pharmacological actions, and their drugs information is safe in human. Global health authorities and researchers may consider to defeat this pandemic COVID-19.
References
- Nazmul Hasan, Mesbah Uddin Md Rezwanun Nayem, Mohammad Mohshinuzzaman, Anwar H Biswas, Md Emrul Kayes, Md Abdul Hakim, et al. Homeopathic Approach to COVID-19: A Review,Malaysian Journal of Medical and Biological Research, 2020; 7(1).
- Comella C Bergmann, Robert H Silverman. COVID-19: Coronavirus replication, pathogenesis, and therapeutic strategies, Cleveland Clinic Journal of Medicine, 2021.
- Kumar D, Malviya R, Kumar Sharma P. Corona Virus: A Review of COVID-19. Department of Pharmacy, Faculty of Medicine and Allied Sciences, Galgotias University, Gautam Buddha Nagar, Uttar Pradesh, India. EJMO, 2020; 4(1): 8-25.
- https://www.healthline.com
- Guidelines on Clinical Management of COVID-19, Government of India Ministry of Health and Family Welfare Directorate General of Health Services (EMR Division), 2020.
- Draft Regulatory Guidelines for Development of Vaccines with special consideration for COVID-19 Vaccine, Central Drugs Standard Control Organization Directorate General of Health Services Ministry of Health and Family Welfare Government of India, 2020.
- National Guidelines for Ethics Committees Reviewing Biomedical and Health Research During COVID-19 Pandemic, Indian Council of Medical Research, 2020.
- Clinical trials on AYUSH Interventions for COVID-19: Methodology and Protocol Development, A Publication by Interdisciplinary AYUSH Research and Development Task Force Ministry of AYUSH, Govt. of India, 2020.
- Chris Frampton, Professor Shaun Holt, Clinical Trials- An Overview, Research Review Educational Series, 2012.
- Thorat SB, Banarjee SK, Gaikwad DD, Jadhav SL, Thorat RM. Clinical Trial: A Review, Vishal Institute of Pharmaceutical Education and Research, Ale, Pune, 2010; 1(2): 019
- https://www.natureindex.com/news-blog/the-top-coronavirus-research-articles-by-metrics.
- Chaturvedi S, Kumar N, Tillu G, Deshpande S, Patwardhan B. Ayush modern medicine and the Covid-19 pandemic, Indian Journal of Medical Ethics, 2020.
- Imran Ali, Omar ML. Alharbi, COVID-19: Disease, management, treatment, and social impact, The science of the total environment.
- JV Sabarianandh, Lazer Bernaitis, Kumarappan Manimekalal. COVID-19 in Siddha Medicine: A Review, SBV Journal of Basic, Clinical and Applied Health Science, 2020; 3(2).

