Effect of Raloxifene Venous Thromboembolism
Amir Shakibaei1, Hadis Monzavi2, Zahra Rabbani3, Bahar khaleghi fard4, MELIKA KHOSROSHAHI5, SEYED ALI TABATABAI MOGHADAM5 and Mehran Goodarzi6,*
1Medical student, Faculty of Medicine, Tehran Medical Sciences, Islamic Azad University, Iran
2A bachelor of accounting, Ragheb Isfahani Higher Education Institute, Iran
3Pharmacy student, Department of Pharmacology, Islamic azad University, Tehran Medical science, Iran
4Genetic student, Department of Medicine, Islamic Azad university, Islamic Azad University OF Tehran Varamin Pishva
5Department of Biomedical Engineering, Islamic Azad University, North Tehran Branch, Tehran, Iran
6Medical student, Faculty of Medicine, Tehran Medical Sciences University, Iran
Received Date: 20/09/2022; Published Date: 12/10/2022
*Corresponding author: Mehran Goodarzi, Medical student, Faculty of Medicine, Tehran Medical Sciences, Islamic Azad University, Iran
Abstract
Increasing evidence supporting a causal association between tamoxifen and VTE comes from several large, controlled, randomized clinical trials. Raloxifene treatment shifts the coagulation pattern toward prothrombosis, and the patients should be exhaustively informed about the risks associated with therapy. Also Tamoxifen reduces breast cancer risk, but can cause thromboembolic complications. Cerebral venous thrombosis is a rare form of stroke in which blood clots occlude the dural sinus or cerebral veins.
Keywords: Effects of Raloxifene; Raloxifene and venous thromboembolism; VTE; Tamoxifen
Introduction
Raloxifene is a nonsteroidal selective estrogen-receptor modulator (SERM) that binds to the estrogen receptor, leading to estrogen-agonist effects in some tissues and estrogen-antagonist effects in others [1]. Raloxifene therapy has been associated with improvement in the levels of serum lipoprotein cholesterol [2], fibrinogen [3] and homocysteine [4]. The favorable effect of raloxifene on markers of cardiovascular risk, coupled with evidence from observational studies that treatment with estrogen was associated with a reduced risk of coronary heart disease (CHD) in postmenopausal women [5,6].
An analysis of data from the Multiple Outcomes of Raloxifene Evaluation (MORE) trial (an osteoporosis treatment trial) showed no significant overall effect of raloxifene on cardiovascular events but suggested a reduced risk among women who were at increased risk for cardiovascular events [7].
Although the risk of venous thromboembolism (VTE) associated with menopausal hormone therapy and SERMs has been documented, subgroups of women at heightened risk for VTE when treated with raloxifene have not been identified [8-10].
Method of Search
Data were extracted and collected from PubMed, Google Scholar, Scopus, and Cochrane library from the overall term clinical and animal studies published in English between 1990 and April 2022.
Effect of Raloxifene on Stroke and Venous Thromboembolism
Lori Mosca and his colleagues conducted research on this subject, based on which the absolute increased risk for VTE associated with raloxifene was 0.12 per 100 woman-years. The incidence of pulmonary embolism, deep vein thrombosis, or retinal vein thrombosis individually did not differ significantly between treatment groups [11].
In accordance with findings from other trials, raloxifene did increase the risk for VTE in RUTH. The risk of VTE is increased 2- to 3-fold with estrogen plus progestin or tamoxifen [12-14]; in the STAR trial, the risk of VTE was lower in women assigned raloxifene versus tamoxifen [15].
The totality of evidence suggests that menopausal hormone therapy and SERMs are associated with increased risk of VTE, including pulmonary embolism and deep vein thrombosis, and that the absolute risk increase varies depending on the type of agent and the underlying risk of VTE [11].
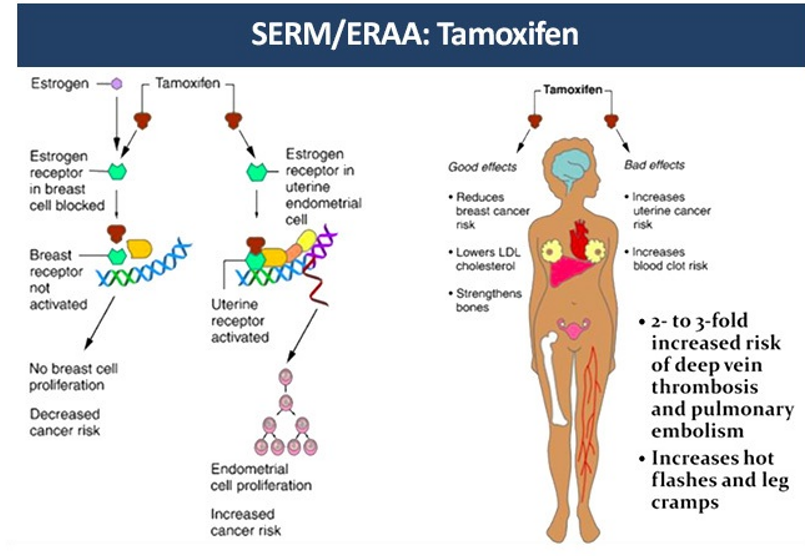
Tamoxifen is also effective in venous thromboembolism as the raloxifene
Tamoxifen reduces breast cancer incidence among healthy women, but is associated with an increased risk of venous thrombosis. studied the 6-month effects of tamoxifen on venous thrombosis risk factors in women without cancer. One hundred and eleven women at one center who were participants in a multicenter breast cancer prevention trial were randomized, in double-blind fashion, to receive 20 mg/d of tamoxifen or placebo. The activated protein C (APC) ratio and concentrations of antithrombin, protein C antigen, and total protein S were measured at baseline and 6 months of treatment. None of the factors changed over 6 months in placebo-treated women. Among tamoxifen-treated women, antithrombin and protein S, but not protein C or APC ratio were reduced. Sequential antithrombin concentrations with tamoxifen were 114% and 104% (P = 0·001 compared with placebo). Sequential protein S concentrations with tamoxifen were 18·42 and 17·30 µg/ml (P = 0·02 compared with placebo). Reductions in antithrombin and protein S were greater in postmenopausal women, but did not differ by other risk factors for venous thrombosis, such as body mass index. Reductions of antithrombin and protein S, but not protein C or APC resistance, might relate to the increased risk of venous thrombosis associated with tamoxifen treatment [16].
Despite such benefits rendered by the agent, there has been heightened concern that tamoxifen use increases the risk of thrombotic complications [17]. One meta-analysis showed that those treated with tamoxifen had an 82% increased risk of ischemic stroke [18]. In randomized trials, the association between the risk of thromboembolic events and the use of tamoxifen was also significant [19.20] The first two years after the initiation of tamoxifen therapy, which was also the case for our patient, was considered to be the most vulnerable time to developing venous thromboembolic complications [21] After diagnosis of CVT, the case patient took anastrozole in place of tamoxifen. The anastrozole is a new aromatase inhibitor and known to have fewer thromboembolic complications [22].
Even though the mechanisms by which tamoxifen increases the risk of thrombosis have yet to be elucidated, alterations in the concentrations of natural anticoagulants have been implicated [23].
A case illustrates concurrent CVT and DVT suspected to have arisen from the use of tamoxifen [24].
Raloxifene, Tamoxifen and vascular tone
Available evidence suggests that raloxifene and tamoxifen are capable of acting directly on both endothelial cells and the underlying vascular smooth muscle cells and cause a multitude of favourable modifications of the vascular wall, which jointly contribute to improved local blood flow [25].
Conclusion
In the RUTH study overall, the use of antiplatelet agents was not associated with a reduced incidence of VTE in either the placebo or raloxifene group. The increased risk of VTE with raloxifene compared with placebo was not influenced by the use of antiplatelet agents (aspirin or nonaspirin). Further studies of potential protective effects of antiplatelet agents against VTE are warranted in the secondary prevention sub-group of women.
A case illustrates concurrent CVT and DVT suspected to have arisen from the use of tamoxifen. Clinicians should warn about the possibility of thromboembolic complications with tamoxifen. Especially for those with an elevated risk of thromboembolism, a physician may need to consider new aromatase inhibitors without thromboembolic risk.
References
- Fuchs-Young R, Glasebrook AL, Short LL, et al. Raloxifene is a tissue-selective agonist/antagonist that functions through the estrogen receptor. Ann N Y Acad Sci 1995; 761: 355-360.
- Delmas PD, Bjarnason NH, Mitlak BH, et al. Effects of raloxifene on bone mineral density, serum cholesterol concentrations, and uterine endometrium in postmenopausal women. N Engl J Med, 1997; 337: 1641-1647.
- Walsh BW, Kuller LH, Wild RA, et al. Effects of raloxifene on serum lipids and coagulation factors in healthy postmenopausal women. JAMA, 1998; 279: 1445-1451.
- Walsh BW, Paul S, Wild RA, et al. The effects of hormone replacement therapy and raloxifene on C-reactive protein and homocysteine in healthy postmenopausal women: a randomized, controlled trial. J Clin Endocrinol Metab, 2000; 85: 214-218.
- Stampfer MJ, Colditz GA. Estrogen replacement therapy and coronary heart disease: a quantitative assessment of the epidemiologic evidence. Prev Med, 1991; 20: 47-63.
- Grady D, Rubin SM, Petitti DB, et al. Hormone therapy to prevent disease and prolong life in postmenopausal women. Ann Intern Med, 1992; 117: 1016-1037.
- Barrett-Connor E, Grady D, Sashegyi A, et al. Raloxifene and cardiovascular events in osteoporotic postmenopausal women: four-year results from the MORE (Multiple Outcomes of Raloxifene Evaluation) randomized trial. JAMA, 2002; 287: 847-857.
- Grady D, Wenger NK, Herrington D, Khan S, Furberg C, Hunninghake D, et al. Postmenopausal hormone therapy increases risk for venous thromboembolic disease: the Heart and Estrogen/progestin Replacement Study. Ann Intern Med. 2000; 132: 689–696.
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002; 288: 321–333.
- Grady D, Ettinger B, Moscarelli E, Plouffe L Jr, Sarkar S, Ciaccia A, et al. Multiple Outcomes of Raloxifene Evaluation Investigators. Safety and adverse effects associated with raloxifene: Multiple outcomes of raloxifene evaluation. Obstet Gynecol, 2004; 104: 837–844.
- Adomaityte Jurga, Maria Farooq, Rehan Qayyum. "Effect of raloxifene therapy on venous thromboembolism in postmenopausal women: a meta-analysis." Thrombosis and haemostasis, 2008; 99(2): 338-342.
- Grady D, Wenger NK, Herrington D, Khan S, Furberg C, Hunninghake D, et al. Postmenopausal hormone therapy increases risk for venous thromboembolic disease: the Heart and Estrogen/progestin Replacement Study. Ann Intern Med, 2000; 132: 689–696.
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA, 2002; 288: 321–333.
- Fisher B, Costantino JP, Wickerham DL, Redmon CK, Kavanah M, Cronin WM, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst, 1998; 90: 1371–1388.
- Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes. The NSABP study of tamoxifen and raloxifene (STAR) P-2 Trial. JAMA, 2006; 295: 2727–2741.
- Cushman, Mary, et al. "Effect of tamoxifen on venous thrombosis risk factors in women without cancer: the Breast Cancer Prevention Trial." British journal of haematology, 2003; 120(1): 109-116.
- Braithwaite RS, Chlebowski RT, Lau J, George S, Hess R, Col MF. Meta-analysis of vascular and neoplastic events associated with tamoxifenJ Gen Intern Med, 2003; 18: pp. 937-947.
- Bushnell CD, Goldstein LB. Risk of ischemic stroke with tamoxifen treatment for breast cancer: a meta-analysis Neurology, 2004; 63: pp. 1230-1233.
- Pritchard KI, Paterson AH, Paul AH, Zee B, Fine S, Pater J. Increased thromboembolic complications with concurrent tamoxifen and chemotherapy in a randomized trial of adjuvant therapy for women with breast cancer. National Cancer Institute of Canada Clinical Trials Group Breast Cancer Site Group J Clin Oncol, 1996; 14: pp. 2731-2737.
- Decensi A, Maisonneuve P, Rotmensz N, Bettega D, Costa A, Sacchini V, et al. Effect of tamoxifen on venous thromboembolic events in a breast cancer prevention trial Circulation, 2005; 111: pp. 650-656.
- Onitilo AA, Doi SA, Engel JM, Glurich I, Johnson J, Berg R. Clustering of venous thrombosis events at the start of tamoxifen therapy in breast cancer: a population-based experience Thromb Res., 2012; 130: pp. 27-31.
- Gibson LJ, Dawson C, Lawrence DH, Bliss JM. Aromatase inhibitors for treatment of advanced breast cancer in postmenopausal women Cochrane Database Syst Rev, 2009; 7: p. CD003370.
- Anderson JA, Weitz JI. Hypercoagulable states Clin Chest Med, 2010; 31: pp. 659-673.
- Kim, Yoon, Ok Joon Kim, Jinkwon Kim. "Cerebral venous thrombosis in a breast cancer patient taking tamoxifen: report of a case." International Journal of Surgery Case Reports, 2015; 6: 77-80.
- Leung, Fung Ping, et al. "Raloxifene, tamoxifen and vascular tone." Clinical and experimental pharmacology and physiology, 2007; 34(8): 809-813.

