Prostacyclin Analogues and Prostacyclin Receptor Agonists in the Treatment of Pulmonary Hypertension: A Systematic Review and Meta-Analysis
Meiling Zhang1,2, Kang Yang1,2, Hongyuan Lu2, Lingyun Lai1,2, Beining Zhang1,2, Yingshi Zhang3,*, Qingchun Zhao1,2,*
1Department of Pharmacy, General Hospital of Northern Theater Command, Shenyang 110840, People’s Republic of China
2Department of Pharmacy, China Medical University, China
3Shenyang Pharmaceutical University, Shenyang 110016, People’s Republic of China
Received Date: 02/08/2022; Published Date: 15/08/2022
*Corresponding author: Qingchun Zhao, Department of Pharmacy, General Hospital of Northern Theater Command, Shenyang 110840, People’s Republic of China, China Medical University, Shenyang 110016, China
Abstract
Background: Although selepag has been approved as a first-line drug in the treatment of pulmonary arterial hypertension, it is used to delay disease progression and reduce the risk of hospitalization for pulmonary arterial hypertension. Compared with prostacyclin analogs, its relative efficacy remains controversial. Therefore, we decided to use a meta-analysis to compare the efficacy of prostacyclin analogs and selexipag to evaluate the advantages and disadvantages of the two in the treatment of pulmonary arterial hypertension.
Methods: Relevant literature in PubMed, Embase and Cochrane libraries were searched for prostacyclin analogs, selepag and pulmonary arterial hypertension as search terms, and two authors independently performed literature screening, quality assessment and data extraction using RevMan5. 4. Make related icons. Odds Ratios (OR) and 95% Confidence Intervals (CI) were used to report dichotomous variables, while Mean Differences (MD) and 95% Confidence Intervals (CI) were used to report continuous variables.
Results: Among the 114 articles retrieved, four met the inclusion requirements with a total of 322 patients were included in the meta-analysis. Compared with prostacyclin analogs, Selexipag increased patients' 6-minute walk distance [MD=47.18, 95% CI (8.69-85.68), p=0.02] and improved cardiac index [MD=-0.13, 95% CI] (-0.2~-0.06), p<0.05], reduce mean pulmonary artery pressure [MD=-8.81, 95% (-10.06~-7.56), p<0.05], and decrease right atrial pressure. In improving pulmonary vascular resistance, Selexipag was not inferior to prostacyclin analogs.
Conclusion: The results of our meta-analysis showed that the prostacyclin receptor agonist improved patients' hemodynamic parameters better than prostacyclin analogues, and its efficacy was superior to prostacyclin analogues.
Keywords: Pulmonary hypertension; Prostacyclin analogs; Prostacyclin receptor agonist; Selexipag; Meta-analysis
Introduction
Pulmonary hypertension refers to the mean pulmonary arterial pressure at rest >25mmHg, and the pulmonary capillary pressure or left atrial pressure <15mmHg [1] -[3]. Pulmonary arterial hypertension is a chronic progressive and fatal disease[4]. The main reason is that primary lesions of small pulmonary arteries or other related diseases lead to increased pulmonary arterial resistance, manifested as increased pulmonary arterial pressure while normal pulmonary venous pressure and normal pulmonary capillary wedge pressure [5]. The patient developed right heart failure and even death [6].
In recent years, substantial progress has been made in the treatment of PAH, and many clinical studies have demonstrated prostacyclin analogs, prostacyclin receptor agonists, endothelin receptor antagonists, phosphodiesterase type 5 inhibitors and guanosine Efficacy and safety of acid cyclase inhibitors [7]. The first drug approved for the treatment of PAH is a synthetic prostacyclin, epoprostenol, which is a milestone. Its launch has greatly improved the quality of life and prognosis of PAH patients. Intravenous epoprostenol, subcutaneous treprostinil, intravenous treprostinil, and inhaled iloprost.It is currently approved for clinical PAH treatment. The efficacy of inhaled and oral treprostinil has also been confirmed by randomized clinical controlled studies [8-9]. Oral beraprost was marketed in Japan in 1995 for the treatment of PAH. Prostacyclin and its analogs are currently the first-line drugs for the clinical treatment of PAH.
In 2008, a new oral prostaglandin I2 receptor agonist Selexipag come into the market[10]. It has the characteristics of long-acting time, high selectivity and strong drug effect, which brings a new choice for the treatment of pulmonary hypertension. At present, many Randomized Controlled Trials (RCTs) have demonstrated the therapeutic effects of various prostacyclin analogs on PAH, but so far, prostacyclin analogs and prostacyclin receptor agonist drugs have been used in the treatment of pulmonary hypertension. There is a lack of evidence-based medical evidence for the comparison of efficacy and safety. Therefore, this study will compare the efficacy and safety of prostacyclin analogues and prostacyclin receptor agonists in the treatment of pulmonary hypertension by means of systematic evaluation and meta-analysis, in the hope of providing reference for clinical treatment of pulmonary hypertension.
Methods
Programs and Registration
This systematic review is pre-registered with Prospero (CRD42022314893) and is available at https://www.crd.york.
Search Strategy
Two investigators searched Chinese and English literature, including Cochrane Library, Embase, and PubMed databases, from inception to January 2022 for comparative prostacyclin analogs and prostacyclin receptor agonists in the treatment of patients with PAH prospectively and retrospective studies.
Selection Criteria
Included studies were eligible for retrospective or prospective trials comparing selepag and prostacyclin analogs in patients with established pulmonary arterial hypertension. In addition, the trial must report one of the outcome measures, such as 6MWD, cardiac index, mean pulmonary artery pressure, and right ventricular pressure.
Data Extraction and Synthesis
For each trial disclosed in the included article, basic information on the article, including author and year of publication; trial including blinding, PAH etiology, study duration, sex ratio; study group including mean age, sample size; interventions; further analysis. Endpoints were 6MWD, Cardiac Index (CI), mean Pulmonary Arterial Pressure (mPAP), mean Right Arterial Pressure (mRAP), pulmonary vascular resistance (PVR), NT-Pro BNP, hospitalization and drug discontinuation.
Research Quality Assessment
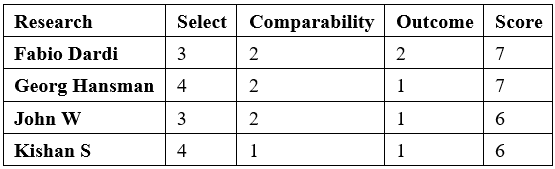
Risk of bias was assessed using the Newcastle-Ottawa Scale (NOS)20 to assess the quality of nonrandomized studies in the meta-analysis, which included the following three aspects: selection, comparability, and outcome. All included studies were prospective or retrospective single-arm studies. Quality assessment was performed independently by two authors (Z.M and Y.K.) and the quality evaluation of the included studies is shown in Table 1.
Table 1: Quality evaluation of the trials included in the meta-analysis.
Results
Description of Studies
A total of 114 articles were retrieved, including 12 articles from Cochrane Library, 34 articles from Emase, and 68 articles from PubMed. 19 duplicates were excluded using Note Express, and 67 were excluded after reading the title and abstract. The remaining 28 articles were read in full, and 24 articles were subsequently excluded. Among them, 19 were clinical trials and 5 were non-prostaglandin analog control experiments. Finally, 4 studies were included [11]-[14], and the literature screening flowchart is shown in Figure 1.
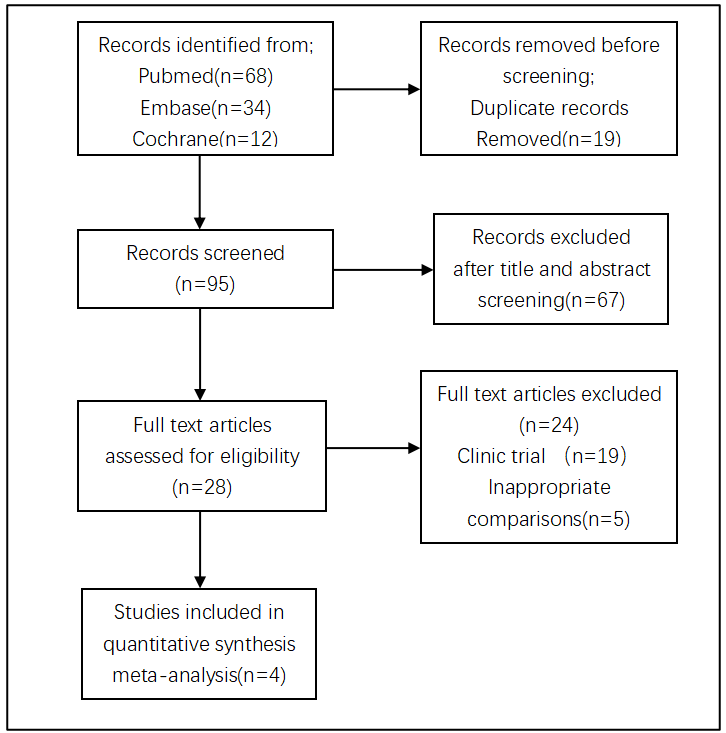
Figure 1: Flow chart of the meta-analysis selection process.
Eligible Studies
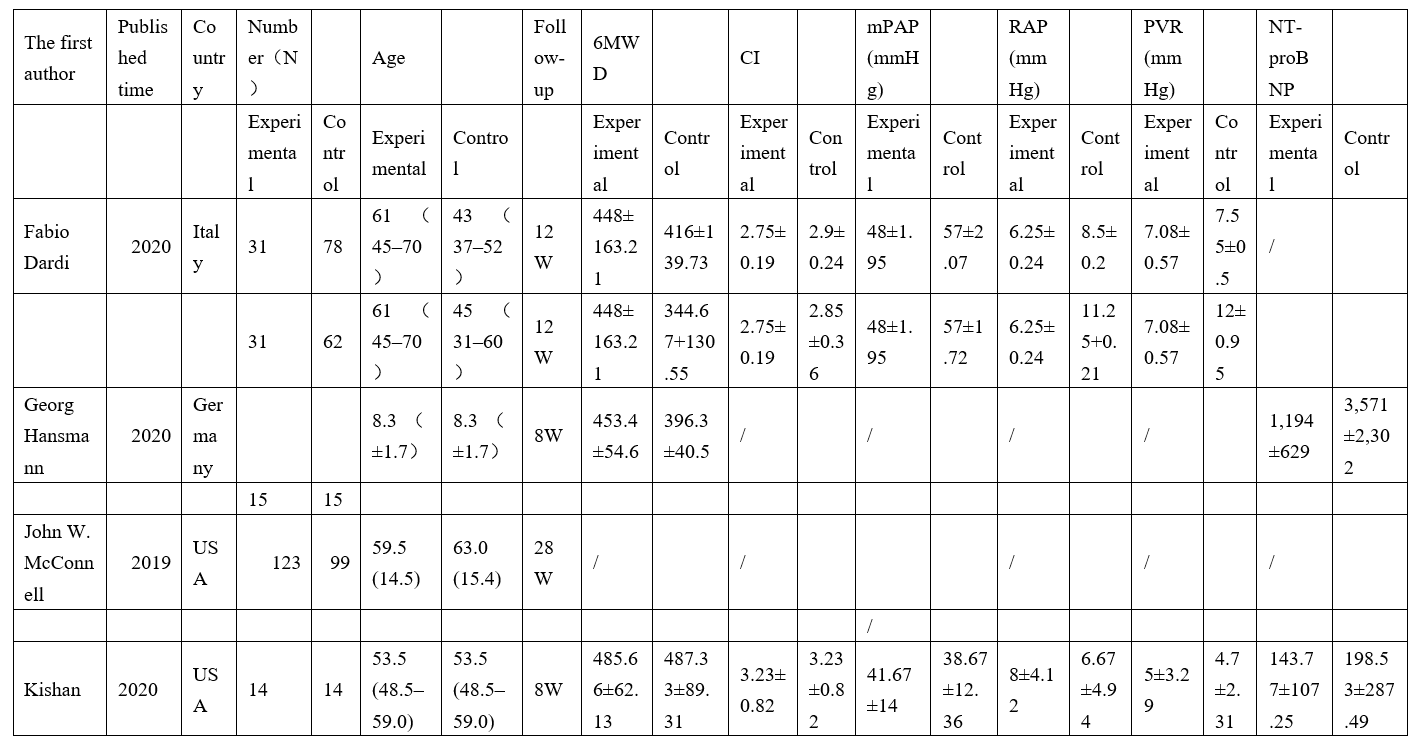
Table 2 summarizes the main characteristics of the four included studies. From 2019 to 2020, 451 patients were included, of whom 268 received prostacyclin analogs and 183 received prostacyclin receptor agonists.
Table 2: Characteristics of the trials included in the meta-analysis.
Three literatures and four original studies including 91 patients in the experimental group and 169 patients in the control group. Compared with prostacyclin analogs, the use of Selexipag significantly improved the patients' 6-minute walk distance, and the difference was statistically significant. (MD=47.18, 95% CI 8.69-85.68, p<0.05) The results are shown in Figure 2. 2 literatures and 3 original studies provided data on cardiac index. A total of 76 patients were included in the experimental group and 154 patients in the control group. patient. Compared with prostaglandin analogs, Selexipag reduced the cardiac index of patients, and the difference was statistically significant. (MD=-0.13, 95%CI-0.2~-0.06, p<0.05) The results are shown in Figure 2. In terms of improving mean pulmonary arterial pressure, a total of 2 literatures and 3 original studies were included, of which 76 patients in the experimental group and 154 patients in the control group were included. Compared with prostaglandin analogs, Selexipag reduced the mean pulmonary arterial pressure of patients, and the difference was statistically significant (MD=-8.81, 95% CI -10.06~-7.56, p<0.05). The results are shown in Figure 2 shown.
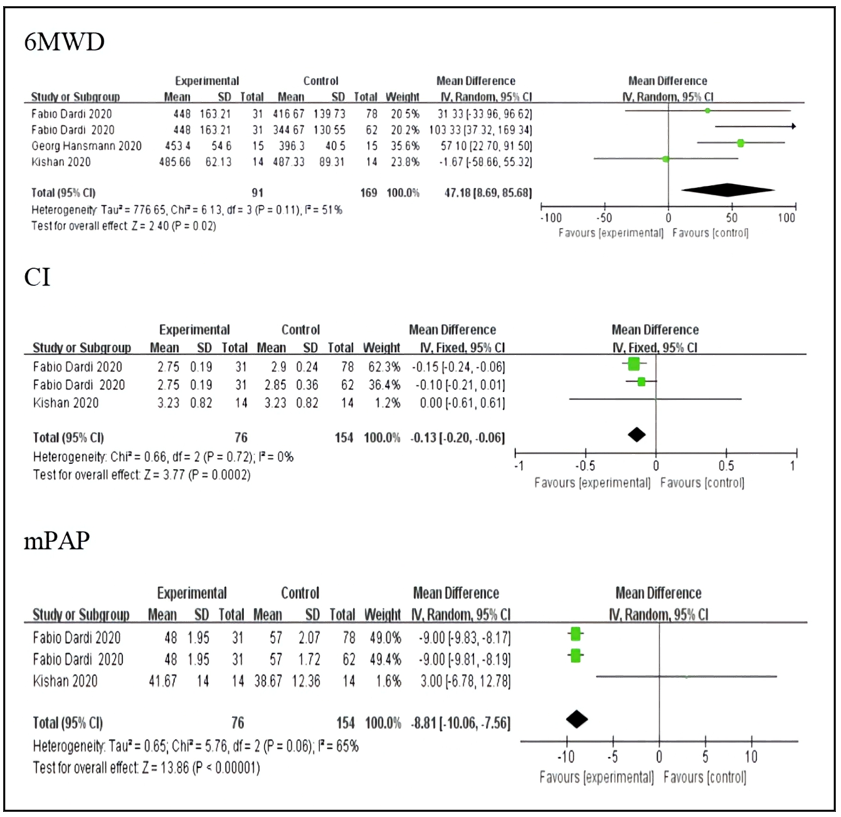
Figure 3: The effect of selexipag on RAP and PVR.
NT-pro BNP
In terms of improving NT-pro BNP levels, the prostacyclin receptor agonist Selexipag reduced circulating NT-pro BNP levels in patients compared with prostacyclin analogs, indirectly reflecting the use of the prostacyclin receptor agonist Selexipag in patients The improvement of cardiac function after cardiac arrest indicates that the prostacyclin receptor agonist Selexipag is more effective than prostaglandin analogs.
Discussion
A total of four studies were included in our meta-analysis, with a total of 183 patients in the experimental group and 263 in the control group. In 2 of the 4 included studies, a total of 29 patients switched to selepag for treatment with a prostacyclin analog. Our research result showed that compared to prostaglandin analogs, selexipag increased patient 6MWD and decreased patient CI, mPAP, RAP, PVR and NT-pro BNP. However, no safety-related events were reported in our study.
Pulmonary arterial hypertension is a type of malignant cardiovascular disease that progressively increases pulmonary arterial pressure and pulmonary vascular resistance, eventually leading to right heart failure and patient death, with a very poor prognosis [15]. Although the pathogenesis of pulmonary arterial hypertension is unclear, it is currently believed that pulmonary vasoconstriction and diastolic imbalance, in situ thrombosis, endothelial and smooth muscle dysplasia, inflammation, and pulmonary arteriolar remodeling play a role in pulmonary arterial hypertension played an important role in its development [16]. Selexipag as an oral non-prostacyclin-like prostacyclin receptor agonist targeting the prostacyclin pathway, the original drug and its metabolites can bind to the prostacyclin receptors with high selectivity, thereby exerting the effect of dilating blood vessels [17],[18]. Anti-vascular smooth muscle proliferation and anti-vascular fibrosis effect. In addition, clinical studies have also demonstrated that Selexipag has a good effect on idiopathic pulmonary hypertension, hereditary pulmonary hypertension, connective tissue-related pulmonary hypertension, congenital heart disease pulmonary hypertension and drug-induced pulmonary hypertension [19],[20].
Despite the small number included in the meta-analysis research and relevant index data, the report is not complete but prior to this research related meta-analysis comparing to pug and top ring element analogue between the relative efficacy of relevant reports, our study also in some way to justifying the division to pug and top ring between the relative efficacy of analogues. In the future, we will continue to pay attention to these studies to supplement relevant data and further verify the efficacy and safety of prostacyclin analogues and selpagate in the treatment of pulmonary hypertension.
Large heterogeneity was also found in our study, with the fourth original study identified by sensitivity analysis that led to heterogeneity in the meta-analysis. By carefully reading the original text and then analyzing the data processing of the original study, it may lead to the opposite result of the trial as a whole, that is, the data of this original study is completely opposite to the results of the other three studies, which leads to the large heterogeneity of the meta-analysis results.
Of course, there are still some shortcomings in our research. First, too few original studies were included in the meta-analysis, which made the results easily deviate from the actual results. Second, the included studies were not randomized controlled trials, so results from the original studies may have been biased and reported selectively. Furthermore, some patients included in the study had a short follow-up period. Finally, the two included studies were switching between drugs and included patients with different baseline characteristics. Therefore, further randomized controlled trials are needed to verify the pros and cons of the efficacy between prostacyclin analogs and Selexipag, so as to provide reference and basis for clinical rational drug use.
Overall, the results of our meta-analysis suggest that Selexipag is superior to prostacyclin analogs in terms of efficacy.
Conclusion
Our meta-analysis indicates that both prostacyclin analogues and prostacyclin receptor agonists improve pulmonary hemodynamic parameters and exercise tolerance. Overall, both prostacyclin analogues and prostacyclin receptor agonists were well tolerated. However, our findings need to be confirmed with further multicenter Randomized Control Trials (RCTs) and prospective observational studies.
Conflict of Interest: The authors declare no potential conflict of interests
References
- Abramson, Sandra V. Pulmonary Hypertension Predicts Mortality and Morbidity in Patients with Dilated Cardiomyopathy[J]. Annals of Internal Medicine, 1992; 116(11): 888-895.
- DB Badesch, Champion HC, Sanchez M, et al. Diagnosis and assessment of pulmonary arterial hypertension [J]. Journal of the American College of Cardiology, 2009; 54(1-supp-S).
- Gérald, Simonneau, David, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension [J]. The European Respiratory Journal, 2018.
- JL Vachiéry, Al E. Pulmonary Hypertension Due to Left Heart Diseases[J]. Journal of the American College of Cardiology, 2013; 62(25): D100-D108.
- Ali MK, Ichimura K, Spiekerkoetter E. Promising therapeutic approaches in pulmonary arterial hypertension. Curr Opin Pharmacol, 2021; 59: 127-139. doi: 10.1016/j.coph.2021.05.003. Epub 2021 Jun 30. PMID: 34217109.
- Luna-López R, Ruiz Martín A, Escribano Subías P. Pulmonary arterial hypertension. Med Clin (Barc), 2022; S0025-7753(22)00002-1. English, Spanish. doi: 10.1016/j.medcli.2022.01.003. Epub ahead of print. PMID: 35279313.
- Gorenflo M, Ziesenitz VC. Treatment of pulmonary arterial hypertension in children. Cardiovasc Diagn Ther, 2021; 11(4): 1144-1159. doi: 10.21037/cdt-20-912. PMID: 34527540; PMCID: PMC8410503.
- Jing ZC, Parikh K, Pulido T, et al. Efficacy and safety of oral treprostinil monotherapy for the treatment of pulmonary arterial hypertension: a randomized, controlled trial. [J]. Circulation, 2013; 127(5).
- Mclaughlin VV, Benza RL, Rubin LJ, et al. Addition of Inhaled Treprostinil to Oral Therapy for Pulmonary Arterial Hypertension a Randomized Controlled Clinical Trial[J]. Journal of the American College of Cardiology, 2010; 55(18): 1915-1922.
- Simonneau G, Torbicki A, Hoeper MM, et al. Selexipag: an oral, selective prostacyclin receptor agonist for the treatment of pulmonary arterial hypertension [J]. Journal of Medicinal Chemistry, 2012; 58(18): 7128-7137.
- Dardi F, Palazzini M, Zuffa E, et al. SHORT-TERM EFFECT OF SELEXIPAG IN COMPARISON TO PROSTACYCLIN ANALOGUES IN PULMONARY ARTERIAL HYPERTENSION PATIENTS STARTED ON DOUBLE-COMBINATION THERAPY WITH ERA AND PDE-5 INHIBITORS – ScienceDirec t[J]. Journal of the American College of Cardiology, 75(11).
- Parikh KS, Doerfler S, Shelburne N, et al. Experience in Transitioning from Parenteral Prostacyclins to Selexipag in Pulmonary Arterial Hypertension [J]. Journal of Cardiovascular Pharmacology, 2020; 75(4): 1.
- Mcconnell JW, Tsang Y, Pruett J, et al. Comparative effectiveness of oral prostacyclin pathway drugs on hospitalization in patients with pulmonary hypertension in the United States: a retrospective database analysis [J]. Pulmonary Circulation, 2020; 10(4).
- Hansmann G, Meinel K, Bukova M, et al. Selexipag for the treatment of children with pulmonary arterial hypertension: First multicenter experience in drug safety and efficacy [J]. The Journal of Heart and Lung Transplantation, 2020.
- Ali MK, Ichimura K, Spiekerkoetter E. Promising therapeutic approaches in pulmonary arterial hypertension[J]. Current Opinion in Pharmacology, 2021; 59: 127-139.
- Schermuly RT, Ghofrani HA, Wilkins MR, et al. Mechanisms of disease: pulmonary arterial hypertension[J]. Nature Reviews Cardiology, 2011; 8(8): 443-455.
- Kuwano K, Hashino A, Asaki T, et al. 2-{4-[(5,6-Diphenylpyrazin-2-yl) (isopropyl)amino] butoxy-N-(methylsulfonyl) acetamide (NS-304), an Orally Available and Long-Acting Prostacyclin Receptor Agonist Prodrug [J]. Journal of Pharmacology & Experimental Therapeutics, 2007; 322(3): 1181-1188.
- Provencher S, Granton JT. Current treatment approaches to pulmonary arterial hypertension. [J]. Canadian Journal of Cardiology, 2015; 31(4): 460-477.
- Simonneau G, Torbicki A, Hoeper MM, et al. Selexipag: an oral, selective prostacyclin receptor agonist for the treatment of pulmonary arterial hypertension[J]. Journal of Medicinal Chemistry, 2012; 58(18): 7128-7137.
- Olivier, Sitbon, Richard, et al. Selexipag for the Treatment of Pulmonary Arterial Hypertension. [J]. The New England journal of medicine, 2015.

