A Brief Review of Multiple Sclerosis Treatments
Parand Danafar, Mohammadmatin Nourikhani, Hasti Beigverdi, Mahan Fazel, Mohammad Mehdi Fazeli,Fatemeh Sadat Shariat Mostafavi, Amir Shakibaei, Leila Khalili, Samaneh Vafaei, Alex avakian, Mahdokht Yazdani Shokouh and Seyed Ali Hosseini Zavareh*
Department of Medicine, Islamic Azad University Kashan Branch, Iran
Medical Laboratory Science Student, Faculty of Allied Medicine, Tehran Medical Sciences, Islamic Azad University,Iran
D.V.M Student, Faculty of Veterinary Medicine, Razi University, Iran
Medical laboratory sciences student, Department of Laboratory Sciences, Chalus Branch, Islamic Azad university,Iran
Medical Student, Faculty of Medicine, Tehran Medical Sciences, Islamic Azad University, Iran
Molecular cell bachelor graduated, Department of Faculty of Modern Sciences and Technologies, Islamic Azad University Tehran Medical Siences, Iran
Department of Medicine, Islamic Azad University Sari branch, Iran
Medical Student, Department of Medicine, Islamic Azad University Tehran Medical Sciences, Iran
Received Date: 21/04/2022; Published Date: 06/05/2022
*Corresponding author: Sayed Ali Hosseini Zavareh, Department of Medicine, Islamic Azad University Tehran Medical Sciences, Iran
Abstract
MS is a well-known disease and chronic inflammation in the central nervous system is one of the diseases whose etiology, despite its well-known autoimmune nature, is still debated, although according to recent studies, the role of viruses such as EBV approved. Based on the different phases that are defined for MS and the degree of progression of the disease, a specific protocol and treatment process is defined for the patient. New and safe treatments for autoimmune diseases have been a constant challenge, although side effects, albeit on a small scale and with few reports, are inevitable; In this study, we tried to classify based on how it is used on drugs used in the treatment of this disease, and at the same time the progress and achievement of new cases such as the use of monoclonal antibodies, despite all the progress made. We still do not fully meet the treatment needs of MS based on studies, in other words, in the classification system and review study, in the group of injectable drugs, we reviewed drugs such as Daclizumab, Alemtuzumab, Natalizumab, Copaxone and Mitoxanthron. Oral drugs Fingolimod, Teriflunomide, and Siponimod were also discussed. In addition to discussing MS medication, we reviewed other treatments such as the use of bone marrow stem cells that were previously discussed in preventing bone marrow suppression during severe therapies such as chemotherapy in the treatment of MS.
Keywords: Multiple Sclerosis; MS Treatments
Introduction
Multiple Sclerosis (MS) is an autoimmune nerve disease that causes demyelination and axonal degeneration as inflammatory immune responses (chronic inflammation) in the Central Nervous System (CNS), including the brain and spinal cord. MS is recognized as the most common non-traumatic neurological cause worldwide. The primary course of the disease in most cases includes Relapsing-Remitting MS (RRMS) with recurrent periods followed by recovery periods. More than 50% of these patients develop secondary progressive MS (SPMS) over a period of approximately two decades. [1-6] On the other hand, about 15% of patients have undergone the phase of primary progressive MS (PPMS), which is a continuous and slow deterioration without recurrence of the disease [7,8]. Regarding the etiology of this disease There has also been a lot of discussion, and for example, viruses have always been the underlying etiology of MS. Recent research has shown that antibodies to the virus's ashtray load on glial cells in the brain, leading to disease that the event confirms the research conducted in this field. [9] Contemporary classification guidelines focus on the inflammatory picture of inflammation, which has the ability to appear at all stages of the disease and can be treated with DMTS [10]. We now have access to a number of DMTs for treatment (RRMS) that straw the level of recurrence and severity of inflammation in the CNS is their main target [11]. Over the past decades, there have been several promising advances in the treatment of MS. To date, after years of experimenting with DMTs, such as interferon beta (IFNB) and Glatiramer Acetate (GA) (the main treatment options), new highly effective treatments for MS have become available [12]. In 2010, Fingolimod was the first approved DMT oral drug to be an agonist of sphingosine-1-phosphate (S1P) receptors, and a number of other oral drugs have either been approved or are in Phase III testing [13,14]. Monoclonal antibodies are currently being proposed as new therapies, and good progress has been made in the treatment of MS. There is promising progress in the treatment of MS and according to the mentioned cases, the treatment of MS in different phases of this disease is under investigation; But existing drugs are not enough to fully meet future needs due to the complex nature of MS. [15,16] Therefore, in this review article, we have tried to summarize the current existing treatments for MS and review the progress of new MS treatments.
Drugs in use and approved
Injectable drugs
The three main IFNB products are available for administration as first-line DMTs for the treatment of recurrent MS. Stages of double-blind, placebo-controlled clinical trials, phase ||| were approved [17].
Of these three products, two are injected subcutaneously and the other intramuscularly.
Copaxone
Copaxone, which is a synthetic copolymer of four amino acids and is a synthetic analogue of myelin basic protein; After a randomized clinical trial phase ||| Was confirmed and shown to be effective in the treatment of RRMS. [20-18] Copaxone and IFNB have different immunomodulatory effects but have almost the same function in reducing recurrence rates by up to 30% [21], a large observational cohort study showed that treatment with IFNB and Copaxone improved the progression of disability as assessed by the Extensive Disability Status Scale (EDSS) of 6 years of drug use [22], treatment with IFNB and Copaxone is generally considered safe and tolerable. However, both IFNB and Copaxone require periodic and long-term self-injection. [23] Side effects of IFNB include elevated liver enzymes, flu-like symptoms, and adverse reactions at the injection site. On the other hand, Copaxone side effects are adverse reactions at the injection site as well as post-injection reactions that occur in approximately 15% of patients [24,25]. Of the drugs approved for the treatment of MS, human antibodies such as Alemtuzumab, natalizumab, Daclizumab, and Mitoxantrone have been shown to have promising effects, but have side effects and should be injected under controlled conditions [26]. Medications are so common that alemtuzumab, for example, may have severe side effects and therefore require regular and accurate follow-up; Other drugs include multifocal progressive leukoencephalopathy (PML) caused by natalizumab, liver damage, skin reactions and colitis caused by daclizumab, and finally systolic dysfunction and acute leukemia caused by mitoxantrone. The last drug was not reported [30-27].
Natalizumab
Natalizumab, a recombinant human monoclonal antibody, targets the -a4 integrin. This biologic drug inhibits the migration of leukocytes from the peripheral blood to the CNS by inhibiting the binding of leukocytes by -a4 integrin to the vascular cell adhesion molecule (VCAM) located in the endothelial cell [26], interfering with blocking the binding and subsequent diaphysis of lymphocytes Blood-brain barrier (BBB) has a beneficial effect on CNS inflammation [31] In a placebo-controlled phase III trial that confirmed Natalizumab, intravenous injection of 300 mg monthly increased RR by 68% Reduced the progression of disability to 42% for 2 years [32] and reduced MRI activity by 92% [33]. Later, Natalizumab was re-introduced in 2006 with a description of risk management programs. [34] The risk of PML classification in patients with MS on Natalizumab underlies treatment duration is due to previous use of immunosuppressants, and the JCV antibody condition indicates JVC infection [35,36]. Studies have shown that after 3 years of using this drug, people who were positive for two factors of previous use of immunosuppressants and anti-JCV antibody were at greater risk [37].
This increases the risk classification in treatment with Natalizumab [38]. However, hypotheses have been proposed to change the dose intervals of the drug to reduce the incidence of side effects, which shows that increasing the dose interval to 8 weeks reduces the saturation of a4-integrin receptors without affecting the clinical effectiveness while the level of safety Properly created in the CNS to prevent PML; Therefore, this change has no negative effect on the effectiveness of the drug [39,40]. Natalizumab treatment may result in the production of stable neutralizing antibodies (NABs) in 4 to 6% of cases, which usually occurs within the first 12 months [41]. NABs have also been shown to be associated with increased infusion-related adverse response rates and may reduce treatment efficacy [42].
Alemtuzumab
Alemtuzumab, a human monoclonal antibody, targets CD52 expressed on natural killer cells (NK), lymphocytes, monocytes, and some other granulocytes [43,44]. Alemtuzumab, through antibody-dependent cytotoxicity (ADCC), causes rapid lymphopenia that lasts for years (average half-life is 22 days) [45]. A course of taking almetozumab has long-term effects on the immune system, and the prescription for taking almetozumab is currently two courses with an interval of 12 months [46]. Subcutaneous administration of Alemtuzumab was compared with IFNB-1 injection three times a week in two phase III RRMS trials. According to the results, Alemtuzumab increased the annual recurrence rate (ARR) to 55-49%, the rate of progression to 42% to 30%, and lesions. Gadolinium booster reduced MRI by 63-61% [47-49], risks of almetozumab treatment include hyper / hypo thyroidism, kidney disease, thrombocytopenia; Secondary autoimmune disease after almetozumab treatment also has a long latent period before onset [50]. Secondary autoimmunity after the treatment period, it is prescribed as a second-line drug [42].
Daclizumab
Daclizumab, a human monoclonal antibody, targets IL--2 CD25) receptor subunit expressed on T cells. Although the effect of Daclizumab on the reduction of CD25 * T cells is short and low, but it causes the proliferation of CD56 bright NK cells, which is related to the clinical efficacy of the drug [51,52]. Double-blind randomized trials (Phase II and III trials) showed that daclizumab had a promising effect in both forms of adjunctive therapy for FNB-B1a or placebo (demonstration recorded by MRI) [55-53]. In addition, Daclizumab showed no signs of relapse after stopping treatment [56]. Unique side effects of Daclizumab include skin side effects. Most skin problems are patches of eczema that usually do not require medication, although mild to severe rashes require discontinuation in 19% of cases. Skin lesions show non-specific features of Athos eczema dermatitis. Infiltration of CD56 + lymphocytes, which were not associated with clinical manifestations [57,58]. Because Daclizumab is approved by the FDA for the treatment of RRMS, it should be prescribed to patients who have an inadequate response to two or more conventional treatments for MS, and due to side effects, evaluate patients' liver function before starting treatment with Daclizumab is required for patients as well as monthly before each dose, and thereafter, up to 6 months after the last dose [59].
Mitoxanthron
Mitoxanthron by inhibiting topoisomerase type || And disrupts DNA synthesis. Mitoxanthron is transported through a disrupted blood-brain barrier (BBB) and may induce microglial death [60,61], as approved by the FDA for rapid recovery of SPMS and RRMS after a number of clinical trials [62,63] Mitoxanthron is administered as a monthly infusion at a dose of 12 mg / m2, although its cumulative dose is limited due to blood and cardiac side effects [64], Mitoxanthron is administered due to severe complications such as acute leukemia and also due to the appearance More effective and less toxic alternative drugs decreased rapidly, which we mentioned at the beginning of the discussion [65].
Oral Medications
Teriflunomide
Teriflunomide has been approved for the treatment of mild to moderate rheumatoid arthritis (RA) [66]. The mechanism of action of this drug is that it interrupts the mitochondrial enzyme involved in the new synthesis of pyrimidine dihydrorutate dehydrogenase (DHODH) [67] Studies in two phase III trials in RRMS showed that Teriflunomide ARR The placebo reduced the level of progression of disability by 31-36% to 26.27% and showed gadolinium-enhancing lesions by 80% MRI. Studies have shown that Teriflunomide has the same effects on ARR and discontinuation of treatment as IFNB-1a subcutaneously, and that both antiproliferative and anti-inflammatory activities are performed [68,69]. Teriflunomide has been evaluated in a double-blind, randomized, placebo-controlled trial of patients with clinically isolated syndrome (CIS) with silent MRI lesions, leading to recurrence progression and improvement in recent MRI lesions [70 ]. Including the side effects of Teriflunomide. Increased alanine aminotransferase (ALT), diarrhea, headache, nausea and thinning hair [71,72]. More precisely, according to studies, in at least 10% of the teriflunomide group included inflammation of the nasopharyngeal duct, injection site reactions, alopecia areata, upper respiratory tract infection, headache, diarrhea, serious destructive events in 7.9%. The teriflunomide-treated group was observed [73], the most common reason for stopping triflunomide treatment being increased ALT. Therefore, periodic evaluation of ALT in the first 6 months of treatment and every second thereafter is recommended [71].
The recently approved oral DMT for the treatment of RRMS is delayed dimethyl fumarate (DMF) administered in a 240 mg capsule twice daily. Although its mechanism of action has not yet been fully elucidated, according to paraclinical studies, DMF has immunomodulatory and antioxidant properties similar to other DMTs such as IFNBs, and it has been suggested that DMF activates nuclear factor (2 erythroid derivatives) such as 2 (Nrf2) [74 , 75). DMF was evaluated in two phase III trials in RRMS, which showed a reduction in ARR of up to 53-44%, a progression of disability of up to 32-32%, and an MRI of gadolinium-enhancing MRI of up to about 94-75% [76, 77]. In addition, phase III trials showed that DMF treatment reduced clinical disease and MRI activity [78]. Common side effects of DMF include nausea, diarrhea, hot flashes, and abdominal pain. [77] In addition, DMF may cause leukopenia and elevated hepatic transaminases [79].
Fingolimod
was approved by the FDA in 2010 and was the first oral treatment line for recurrent forms of MS. It is administered as a 0.5 mg capsule once daily. Fingolimod is a sphingosine-1-phosphate (S1P) receptor antagonist and acts selectively on lymphocytes by degrading the S1P1 receptor [80,81]. It absorbs T lymphocytes into secondary lymphoid tissues, which is to counteract the invasion of native tissue and thus improves inflammation in MS. [82,83] Fingolimod in two phase III trials in RRMS was evaluated and showed a reduction in ARR of 55-48%, a rate of progression of disability of up to 25-30%, and gadolinium-enhancing MRI lesions of more than 80%. [84] Compared to IFNB-1a, intramuscular injection of Fingolimod once a week reduces ARR by 52%, progression of disability by up to 25%, and MRI of gadolinium-enhancing lesions by more than 50% [85]. A phase Fingolimod III trial in patients with PPMS resulted in no delay in progression of disability [86]. The most common side effects of fingolimod are cough, diarrhea, headache, back pain and upper respiratory tract infection [87]. Due to the possibility of bradycardia and atrial block at the first administration, it is recommended that electrocardiogram monitoring be performed for 6 hours after the first dose of fingolimod. Then in cases treated with fingolimod, examination of varicella zoster infection is recommended [88,89]
Siponimod
is a new selective S1P: / S1Ps agonist and a cost-effective treatment for RRMS and SPMS. Wenckebach shows that they are well tolerated. [90,91] Peak plasma levels of oral Siponimod max (10 mg) and total radioisotope components at 4 and 6 hours after ingestion and time of maximum radioactivity (Tmax) for single-dose Siponimod 3 to 6 hours and for multi-dose 2: Up to 8 hours after consumption. Unchanged Siponimod accounts for 57% of total plasma radioactivity, indicating significant exposure to metabolites. The main metabolite of Siponimod is circulating plasma M3 and is the most important systemic metabolite in M17 mice. During 9 days after consumption, the mean total recovery of radioactivity in urine was 0.4 + 3.6% with the predominance of M3 metabolite and in feces 43.5% with 84.1 with the dominance of M5 metabolite and on the 13th day the radioactivity recovery is nearing completion (2.7 + 90.4%). The predominant factor in the biotransformation is the CYP 2C9 (P450 2C9) and the small contribution of CYP 3A4 and other cytochrome P450 enzymes. [92,93] To evaluate the safety and efficacy of Siponimod, it was designed in an experimental study in which those who received Siponimod continued to receive the initial dose of Siponimod and those who received placebo received one of 5 doses of 10.2, They received 1.25,0.5,0.25 randomly and the initial treatment was titrated within 10 days. In people receiving Siponimod 1.25, 2, 10 0.5, the estimated mean number of T1 lesions decreased and with increasing dose, the number of T1 enhancing lesions decreased. In patients who switched from placebo to Siponimod, the number of Gd-enhancing T1 lesions was lower than the baseline extension. Doses of 10,2,1.25 Siponimod showed less recurrence and doses 2 and 1.25 showed less Tz lesion enlargement than other groups; lymphopenia was also highest in the 10 mg group. [94,95] Cardiovascular findings after titration, slight reductions in HR and secondary ventricular atrial blocks shortly after ingestion (days 1 and 7) and AVB and Mobitz type 1 in the long term (12 months) after showed of consumption. Reduction of lymphocyte count to less than 200 during dose blinded extension phase at 10mg Siponimod in 54.5% of patients, at 2mg dose at 87.2% of patients, at 1.25mg dose at 9.3% patients and none of patients at 0.5mg and 0.25mg doses Occurred. Sip onium at 2 mg and 10 mg doses had stable effects on MRI and clinical procedures, low disease activity and low ARR. In general, higher doses reduced overall recurrences [94,96]
Compared with Siponimod and placebo, 26% of patients receiving Siponimod and 32% of patients receiving placebo experience CDP for three months. Point-to-quarter estimates of time to CDP based on recurrence activity, disease progression and disease severity, exploratory analyses with recurrence or contrast enhancement up to 3mCDP, and post-hoc analyses up to 6-month CDP all demonstrate the superiority of Siponimod to placebo. ARR, increased Tz lesion and enhancing gadolinium lesions, and the rate of decrease in brain volume with Siponimod were lower than placebo. In contrast, the rate of serious adverse adverse event, seizures, hypertension and cardiovascular lesions are more reported in the use of Siponimod than placebo. [97,98] Adverse event in this drug includes headache, nasopharyngitis, urinary tract infection and fall and serious adverse event includes increased liver transaminases, basal multiple gait disturbance suicide attempts urinary tract infection depression concussion. Cell carcinoma sclerosis relapse and paraparesis [97,99]. Death from Siponimod due to metastatic gastro-intestinal melanoma septic shock in terminal colon cancer or suicide can occur infrequently. [97] The effect of Siponimod on preventing the development of disability is independent of its effect on disease recurrence. The spot effect of Siponimod shows a 14% to 20% reduction in quarterly CDP and a 29% to 33% reduction in 6-month CDP. While considering the recurrences during the study, patients fall into 3 categories: non-recurrent (83% 75%), definitely recurrent (11% -15%) and profitable (6% -6%) and reducing the risk of CDP in the 2017 quarter. ٪ And 6-month CDP is estimated at 29.320% compared to the placebo exposure period. The approximate correspondence of these statistics indicates the effect of Siponimod on disability independent of the effect on recurrence. [100,101] Siponimod can also be very cost-effective as an alternative to various treatments for RRMS and SPMS. Siponimod treatment strategy reduces overall treatment costs by decreasing the mean incremental costs of drug acquisition, the mean means incremental overall strategy costs, the mean incremental QALYS, and the incremental mean Lys [90].
Hematopoietic Stem Cells Transplantation
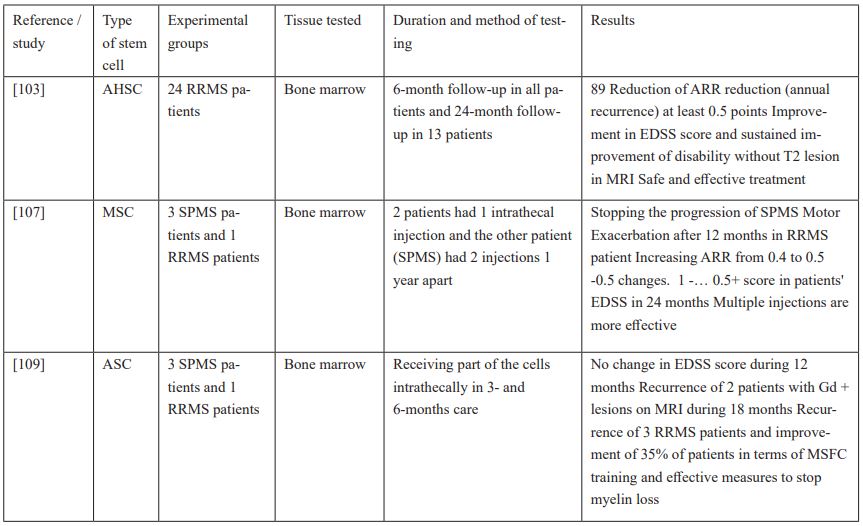
Mesenchymal and hematopoietic stem cell transplants were initially used to save patients from long-term bone marrow aplasia undergoing severe chemotherapy, but with advances in the method of this treatment, a relatively new approach to combat autoimmune disorders [102] MS, however, in Autologous Hematopoietic Stem Cell (AHSCT) transplantation in RRMS patients, a 89% reduction in disease recurrence and a 76.9% sustained improvement in disability were observed 24 months after transplantation. At 24 months after transplantation, there is little effect on information processing speed and visual memory and a significant effect on verbal learning. The most common side effect of this procedure is febrile neutropenia or FN (79). The second complication is reactivation of the nBarr Epstei virus (/ VA). Patients are positive for anti-EBV igG [105-103]. Acute respiratory distress syndrome (ARDS), idiopathic thrombocytopenic purpura (ITP), and haemorrhagic cystitis are rare. Given that most of these cases are treated with appropriate treatments, it can be said that this treatment is safe and effective; The effectiveness of this treatment method in the early stages of autoimmune disease has had better and more favourable results [103,106]. Intrathecal injection of mesenchymal stem cells (MSCs) in RRMS and SPMS patients in the first few hours shows mild fever and mild headache, but then stops the progression of the disease and improves recovery from disability, and as a conclusion on the topic Side effects of this method of treatment include fever, sepsis, and immunosuppression, which are also the most common [107,108]. This treatment provides a better response in SPMS patients and stops the progression of the disease in all of them. Be. In this link, the annual recurrence rate (ARR) increases from 0.4 to 0.5 and the EDSS changes. Multiple injections of MSC at one-year intervals are more effective than single injections and are a safe and uncomplicated treatment. Fat-derived stem cells (ASCs), which are a type of MSC, are isolated from adipose tissue by enzymatic digestion [107]. Intrathecal injection of ASC in patients with RRMS and SPMS who did not respond to first-, second-, and third-line therapies did not alter the level of disability (EDSS). This anti-inflammatory treatment is safe and slows the recurrence and progression of the disease. During the 18 months after treatment, recurrence was seen in only 15% of patients, and 35% of patients showed significant improvement in exploratory efficacy measures. No side effects are observed until 24 months after transplantation [109].
PGLA
In recent years, the use of PGLA nanoparticles has been proposed as a better carrier for the treatment of autoimmune diseases such as MS, although PGLA itself is FDA approved and also due to its biodegradability. Biocompatibility is an attractive carrier in this area, but nano-based therapies have not entered the clinical phase; On the other hand, the use of PGLA nanoparticles has a good effect on autoimmune encephalomyelitis in laboratory mice and the prevention and treatment of MS has also been successful [110-112].
References
- Gholamzad M, Ebtekar M, Ardestani MS, et al. “A comprehensive review on the treatment approaches of multiple sclerosis: currently and in the future.” Inflamm. Res. 2019; 68: 25–38.
- Javan M-R, Seyfizadeh N, Aslani S, Farhoodi M, Babaloo Z. “Molecular analysis of interleukin-25 exons 1 and 2 and its serum levels in Iranian patients with multiple sclerosis.” Am J Clin Exp Immunol, 2014; 3(2): 91.
- Javan MR, Shahraki S, Safa A, Zamani MR, Salmaninejad A, Aslani S. “An interleukin 12 B single nucleotide polymorphism increases IL-12p40 production and is associated with increased disease susceptibility in patients with relapsing-remitting multiple sclerosis.” Neurol Res, 2017; 39(5): 435–441.
- Javan MR, Aslani S, Zamani MR, Rostamnejad J, Asadi M, Farhoodi M, et al. “Downregulation of immunosuppressive molecules, PD-1 and PD-L1 but not PD-L2, in the patients with multiple sclerosis.” Iran J Allerg Asthma Immunol, 2016; 15(4): 296.
- Azimi M, Ghabaee M, Moghadasi AN, Noorbakhsh F, Izad M. “Immunomodulatory function of Treg-derived exosomes is impaired in patients with relapsing-remitting multiple sclerosis.” Immunol Res. 2018. 1–8.
- Faissner, Simon, et al. "Progressive multiple sclerosis: from pathophysiology to therapeutic strategies." Nature Reviews Drug Discovery, 2019; 18(12): 905-922.
- Dendrou CA, Fugger L, Friese MA. “Immunopathology of multiple sclerosis.” Nat Rev Immunol, 2015; 15(9): 545–558.
- Zéphir, Helene. "Progress in understanding the pathophysiology of multiple sclerosis." Revue neurologique, 2018: 174(6): 358-363.
- Bar-Or, Amit, et al. "Epstein–Barr virus in multiple sclerosis: theory and emerging immunotherapies." Trends in molecular medicine, 2020; 26(3): 296-310.
- Lublin FD, Reingold SC, Cohen JA, Cutter GR, Sørensen PS, Thompson AJ, et al. Defining the clinical course of multiple sclerosis the 2013 revisions. Neurology, 2014; 83(3): 278–286.
- Tanasescu R, Ionete C, Chou I-J, Constantinescu C. “Advances in the treatment of relapsing-remitting multiple sclerosis.” Biomed J, 2014; 37(2): 41.
- Preziosa, Paolo, et al. "Effects on cognition of DMTs in multiple sclerosis: moving beyond the prevention of inflammatory activity" Journal of neurology, 2021: 1-13.
- Thomas RH, Wakefield RA. “Oral disease-modifying therapies for relapsing-remitting multiple sclerosis.” Am J Health Syst Pharm, 2015; 72(1).
- Bascuñana, Pablo, et al. "Fingolimod as a treatment in neurologic disorders beyond multiple sclerosis." Drugs in R&D, 2020; 20(3): 197-207.
- Tintore Mar, Angela Vidal-Jordana, Jaume Sastre-Garriga. "Treatment of multiple sclerosis—success from bench to bedside." Nature Reviews Neurology, 2019; 15(1): 53-58.
- McGinley, Marisa P, Carolyn H. Goldschmidt, and Alexander D. Rae-Grant. "Diagnosis and treatment of multiple sclerosis: a review.", 2021; 325(8): 765-779.
- Ali R, Nicholas RSJ, Muraro PA. “Drugs in development for relapsing multiple sclerosis.” Drugs, 2013; 73(7): 625–650.
- Boster AL, Ford CC, Neudorfer O, Gilgun-Sherki Y. Glatiramer acetate: “long-term safety and efficacy in relapsing-remitting multiple sclerosis.” Expert Rev Neurother, 2015; 15(6): 575–586.
- Johnson KP, Brooks B, Cohen J, Ford C, Goldstein J, Lisak R, et al. “Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis results of a phase III multicenter, double-blind, placebo-controlled trial.” Neurology, 1995; 45(7): 1268–1276.
- Wynn, Daniel R. "Enduring clinical value of copaxone® (Glatiramer Acetate) in multiple sclerosis after 20 years of use." Multiple Sclerosis International 2019; 2019.
- Lugaresi A, Di Ioia M, Travaglini D, Pietrolongo E, Pucci E, Onofrj M.” Risk–benefit considerations in the treatment of relapsing-remitting multiple sclerosis.” Neuropsychiatr Dis Treat, 2013; 9: 893.
- Palace J, Duddy M, Bregenzer T, Lawton M, Zhu F, Boggild M, et al. “Effectiveness and cost-effectiveness of interferon beta and glatiramer acetate in the UK multiple sclerosis risk sharing scheme at 6 years: a clinical cohort study with natural history comparator.” Lancet Neurol, 2015; 14(5): 497–505.
- Dhib-Jalbut, Suhayl. "Mechanisms of action of interferons and glatiramer acetate in multiple sclerosis." Neurology, 2002; 58(8) suppl 4: S3-S9.
- Ford C, Goodman A, Johnson K, Kachuck N, Lindsey J, Lisak R, et al. “Continuous long-term immunomodulatory therapy in relapsing multiple sclerosis: results from the 15-year analysis of the US prospective open-label study of glatiramer acetate. “Mult Scler J, 2010; 16(3): 342–350.
- Sorensen, Per Soelberg. "New management algorithms in multiple sclerosis." Current opinion in neurology, 2014; 27(3): 246-259.
- Craddock J, Markovic-Plese S.” Immunomodulatory therapies for relapsing-remitting multiple sclerosis: monoclonal antibodies, currently approved and in testing.” Expert Rev Clin Pharmacol, 2015; 8(3): 283–296.
- Boneschi, Filippo Martinelli, et al. "Mitoxantrone for multiple sclerosis." Cochrane Database of Systematic Reviews, 2013; 5.
- Coles Alasdair J. "Alemtuzumab therapy for multiple sclerosis." Neurotherapeutics, 2013; 10(1): 29-33.
- Hoepner Robert, et al. "Efficacy and side effects of natalizumab therapy in patients with multiple sclerosis." Journal of central nervous system disease, 2014; 6: JCNSD-S14049.
- Baldassari Laura E, John W Rose. "Daclizumab: development, clinical trials, and practical aspects of use in multiple sclerosis." Neurotherapeutics, 2017; 14(4): 842-858.
- Steinman Lawrence. "Blocking adhesion molecules as therapy for multiple sclerosis: natalizumab." Nature reviews Drug discovery, 2005; 4(6): 510-518.
- Polman CH, O’connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, et al.” A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis.” N Engl J Med, 2006; 354(9): 899–910.
- Miller D, Soon D, Fernando K, MacManus D, Barker G, Yousry T, et al. “MRI outcomes in a placebo-controlled trial of natalizumab in relapsing MS.” Neurology, 2007; 68(17): 1390–1401.
- Rommer P, Zettl U, Kieseier B, Hartung HP, Menge T, Frohman E, et al. “Requirement for safety monitoring for approved multiple sclerosis therapies: an overview.” Clin Exp Immunol, 2014; 175(3): 397–407.
- McGuigan C, Craner M, Guadagno J, Kapoor R, Mazibrada G, Molyneux P, et al.” Stratification and monitoring of natalizumab associated progressive multifocal leukoencephalopathy risk: recommendations from an expert group.” J Neurol Neurosurg Psychiatry, 2015: jnnp-2015-311100.
- Bloomgren G, Richman S, Hotermans C, Subramanyam M, Goelz S, Natarajan A, et al. “Risk of natalizumab-associated progressive multifocal leukoencephalopathy.” N Engl J Med, 2012; 366(20): 1870–1880.
- Ho Pei-Ran, et al. "Risk of natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: a retrospective analysis of data from four clinical studies." The Lancet Neurology 2017; 16(11): 925-933.
- Plavina T, Subramanyam M, Bloomgren G, Richman S, Pace A, Lee S, et al.” Anti-JC virus antibody levels in serum or plasma further define risk of natalizumab-associated progressive multifocal leukoencephalopathy.” Ann Neurol, 2014; 76(6): 802–811.
- Yamout Bassem I, et al. "Efficacy and safety of natalizumab extended interval dosing." Multiple sclerosis and related disorders, 2018; 24: 113-116.
- Clerico, Marinella, et al. "Extending the interval of natalizumab dosing: is efficacy preserved?." Neurotherapeutics, 2020; 17(1): 200-207.
- Cohen Bruce A, et al. "The implications of immunogenicity for protein-based multiple sclerosis therapies." Journal of the neurological sciences, 2008; 275(1-2): 7-17.
- Torkildsen Ø, Myhr KM, Bø L. “Disease-modifying treatments for multiple sclerosis—a review of approved medications.” Eur J Neurol, 2016; 23(S1):18–27.
- Jones JL, Coles AJ. “Mode of action and clinical studies with alemtuzumab.” Exp Neurol, 2014; 262: 37–43.
- Singer BA, editor. Parenteral treatment of multiple sclerosis: “the advent of monoclonal antibodies.” Seminars in neurology; Thieme Medical Publishers, 2016.
- Coles Alasdair J. "Alemtuzumab treatment of multiple sclerosis." Seminars in neurology, Thieme Medical Publishers, 2013; 33(01).
- Hill-Cawthorne, Grant A, et al. "Long term lymphocyte reconstitution after alemtuzumab treatment of multiple sclerosis." Journal of Neurology, Neurosurgery & Psychiatry, 2012; 83(3): 298-304.
- Cohen JA, Arnold DL, Comi G, Bar-Or A, Gujrathi S, Hartung JP, et al. “Safety and efficacy of the selective sphingosine 1-phosphate receptor modulator ozanimod in relapsing multiple sclerosis (RADIANCE): a randomised, placebo-controlled, phase 2 trial.” Lancet Neurol, 2016; 15(4): 373–381.
- Coles AJ, Twyman CL, Arnold DL, Cohen JA, Confavreux C, Fox EJ, et al.” Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial.” Lancet, 2012; 380(9856): 1829–1839.
- Cohen JA, Coles AJ, Arnold DL, Confavreux C, Fox EJ, Hartung H-P, et al. “Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial.” Lancet, 2012; 380(9856): 1819–1828.
- Meltzer Ethan, et al. "Mitigating alemtuzumab-associated autoimmunity in MS: a “whack-a-mole” B-cell depletion strategy." Neurology-Neuroimmunology Neuroinflammation, 2020; 7(6).
- Bielekova B, Catalfamo M, Reichert-Scrivner S, Packer A, Cerna M, Waldmann TA, et al. “Regulatory CD56bright natural killer cells mediate immunomodulatory effects of IL-2Rα-targeted therapy (daclizumab) in multiple sclerosis.” Proc Natl Acad Sci, 2006; 103(15): 5941–5946.
- Wiendl Heinz, Catharina C. Gross. "Modulation of IL-2Rα with daclizumab for treatment of multiple sclerosis." Nature Reviews Neurology, 2013; 9(7): 394-404.
- Wynn D, Kaufman M, Montalban X, Vollmer T, Simon J, Elkins J, et al. Daclizumab in active relapsing multiple sclerosis (CHOICE study):” a phase 2, randomised, double-blind, placebo controlled, add-on trial with interferon beta.” Lancet Neurol, 2010; 9(4): 381–390.
- Gold R, Giovannoni G, Selmaj K, Havrdova E, Montalban X, Radue E-W, et al.” Daclizumab high-yield process in relapsing-remitting multiple sclerosis (SELECT): a randomised, double-blind, placebo-controlled trial.” The Lancet, 2013; 381(9884): 2167–2175.
- Giovannoni G, Gold R, Selmaj K, Havrdova E, Montalban X, Radue E-W, et al. Daclizumab high-yield process in relapsing-remitting multiple sclerosis (SELECTION): “a multicentre, randomised, double-blind extension trial.” Lancet Neurol, 2014; 13(5): 472–481
- Milo Ron. "The efficacy and safety of daclizumab and its potential role in the treatment of multiple sclerosis." Therapeutic advances in neurological disorders, 2014; 7(1): 7-21.
- Cortese I, Ohayon J, Fenton K, Lee C-C, Raffeld M, Cowen EW, et al. “Cutaneous adverse events in multiple sclerosis patients treated with daclizumab.” Neurology, 2016; 86(9): 847–855.
- Shirley Matt. "Daclizumab: a review in relapsing multiple sclerosis." Drugs, 2017; 77(4): 447-458.
- Baldassari LE, Rose JW. Daclizumab: “development, clinical trials, and practical aspects of use in multiple sclerosis.” Neurotherapeutics, 2017: 1-17.
- Li J-M, Yang Y, Zhu P, Zheng F, Gong F-L, Mei Y-W. “Mitoxantrone exerts both cytotoxic and immunoregulatory effects on activated microglial cells.” Immunopharmacol Immunotoxicol, 2012; 34(1): 36–41.
- Gonsette RE. "Mitoxantrone immunotherapy in multiple sclerosis." Multiple Sclerosis Journal, 1996: 1(6): 329-332.
- Millefiorini E, Gasperini C, Pozzilli C, D’andrea F, Bastianello S, Trojano M, et al.” Randomized placebo-controlled trial of mitoxantrone in relapsing-remitting multiple sclerosis: 24-month clinical and MRI outcome.” J Neurol, 1997; 244(3): 153–159.
- Hartung H-P, Gonsette R, Konig N, Kwiecinski H, Guseo A, Morrissey SP, et al.” Mitoxantrone in progressive multiple sclerosis: a placebo-controlled, double-blind, randomised, multicentre trial.” Lancet, 2002; 360(9350): 2018–2025.
- Jain Kewal K. "Evaluation of mitoxantrone for the treatment of multiple sclerosis." Expert Opinion on Investigational Drugs, 2000; 9(5): 1139-1149.
- Tanasescu R, Debouverie M, Pittion S, Anxionnat R, Vespignani H.” Acute myeloid leukaemia induced by mitoxantrone in a multiple sclerosis patient.” J Neurol, 2004; 251(6): 762–763.
- Bar-Or, Amit. "Teriflunomide (Aubagio®) for the treatment of multiple sclerosis." Experimental neurology, 2014; 262: 57-65.
- Tanasescu R, Evangelou N, Constantinescu CS. “Role of oral teriflunomide in the management of multiple sclerosis.” Neuropsychiatr Dis Treat, 2013; 9: 539
- Vermersch P, Czlonkowska A, Grimaldi LM, Confavreux C, Comi G, Kappos L, et al. “Teriflunomide versus subcutaneous interferon beta-1a in patients with relapsing multiple sclerosis: a randomised, controlled phase 3 trial.” Mult Scler J, 2014; 20(6): 705–716.
- Gold R, Wolinsky JS. "Pathophysiology of multiple sclerosis and the place of teriflunomide." Acta neurologica scandinavica, 2011; 124(2): 75-84.
- Miller AE, Wolinsky JS, Kappos L, Comi G, Freedman MS, Olsson TP, et al. “Oral teriflunomide for patients with a first clinical episode suggestive of multiple sclerosis (TOPIC): a randomised, double-blind, placebo-controlled, phase 3 trial.” Lancet Neurol, 2014; 13(10): 977–986.
- Comi G, Freedman MS, Kappos L, Olsson TP, Miller AE, Wolinsky JS, et al.” Pooled safety and tolerability data from four placebo-controlled teriflunomide studies and extensions.” Mult Scler Relat Disord, 2016; 5: 97–104.
- He Dian, et al. "Teriflunomide for multiple sclerosis." Cochrane database of systematic reviews, 2016; 3.
- Hauser Stephen L, et al. "Ofatumumab versus teriflunomide in multiple sclerosis." New England Journal of Medicine, 2020; 383(6): 546-557.
- Linker RA, Gold R.” Dimethyl fumarate for treatment of multiple sclerosis: mechanism of action, effectiveness, and side effects.” Curr Neurol Neurosci Rep, 2013; 13(11): 394.
- Prosperini, Luca, Simona Pontecorvo. "Dimethyl fumarate in the management of multiple sclerosis: appropriate patient selection and special considerations." Therapeutics and clinical risk management, 2016: 12: 339.
- Gold R, Kappos L, Arnold DL, Bar-Or A, Giovannoni G, Selmaj K, et al. “Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis.” N Engl J Med, 2012; 367(12): 1098–1107.
- Havrdova E, Hutchinson M, Kurukulasuriya NC, Raghupathi K, Sweetser MT, Dawson KT et al. “Oral BG-12 (dimethyl fumarate) for relapsing–remitting multiple sclerosis: a review of DEFINE and CONFIRM: Evaluation of: Gold R, Kappos L, Arnold D, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med, 2012; 367: 1098–1107; and Fox RJ, Miller DH, Phillips JT, et al. “Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis.” N Engl J Med, 2012; 367: 1087–1097. Expert Opin Pharmacother, 2013; 14(15): 2145–2156.
- Gold R, Arnold DL, Bar-Or A, Hutchinson M, Kappos L, Havrdova E, et al. “Long-term effects of delayed-release dimethyl fumarate in multiple sclerosis: Interim analysis of ENDORSE, a randomized extension study.” Mult Scler J, 2017; 23(2): 253–265.
- Sheremata, William, Andrew D. Brown, and Kottil W. Rammohan. "Dimethyl fumarate for treating relapsing multiple sclerosis." Expert opinion on drug safety, 2015; 14(1): 161-170.
- Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, et al. “The immune modulator FTY720 targets sphingosine 1-phosphate receptors.” J Biol Chem, 2002; 277(24): 21453–21457.
- Tanasescu R, Constantinescu CS. “Pharmacokinetic evaluation of fingolimod for the treatment of multiple sclerosis.” Expert Opin Drug Metab Toxicol, 2014; 10(4): 621–630
- Groves A, Kihara Y, Chun J. Fingolimod: “direct CNS effects of sphingosine 1-phosphate (S1P) receptor modulation and implications in multiple sclerosis therapy.” J Neurol Sci, 2013; 328(1): 9–18.
- Chun, Jerold, and Hans-Peter Hartung. "Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis." Clinical neuropharmacology, 2010; 33(2): 91.
- Kappos L, Radue E-W, O’connor P, Polman C, Hohlfeld R, Calabresi P, et al.” A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis.” N Engl J Med, 2010; 362(5): 387–401.
- Cohen JA, Barkhof F, Comi G, Hartung H-P, Khatri BO, Montalban X, et al. “Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis.” N Engl J Med, 2010; 362(5): 402–415.
- Lublin F, Miller DH, Freedman MS, Cree BA, Wolinsky JS, Weiner H, et al.” Oral fingolimod in primary progressive multiple sclerosis (INFORMS): a phase 3, randomised, double-blind, placebo-controlled trial.” Lancet, 2016; 387(10023): 1075–1084.
- yzenberg I, Hoepner R, Kleiter I. “Fingolimod for multiple sclerosis and emerging indications: appropriate patient selection, safety precautions, and special considerations.” Ther Clin Risk Manag, 2016; 12: 261.
- Pelletier, Daniel, and David A. Hafler. "Fingolimod for multiple sclerosis." New England Journal of Medicine, 2012; 366(4): 339-347.
- Meissner, Axel, Volker Limmroth. "Update on the cardiovascular profile of fingolimod in the therapy of relapsing-remitting multiple sclerosis (MS)." Multiple sclerosis and related disorders, 2016; 8: 19-26.
- Schur, Nadine, et al. "Cost Effectiveness and Budget Impact of Siponimod Compared to Interferon Beta-1a in the Treatment of Adult Patients with Secondary Progressive Multiple Sclerosis with Active Disease in Switzerland." PharmacoEconomics, 2021; 39(5): 563-577.
- Goodman, Andrew D., Nidhiben Anadani, and Lee Gerwitz. "Siponimod in the treatment of multiple sclerosis." Expert opinion on investigational drugs, 2019; 28(12): 1051-1057.
- Glaenzel U, Jin Y, Nufer R, Li W, Schroer K, Adam-Stitah S, et al.” Metabolism and disposition of siponimod, a novel selective S1P1/S1P5 agonist, in healthy volunteers and in vitro identification of human cytochrome P450 enzymes involved in its oxidative metabolism.” Drug metabolism and disposition. 2018; 46(7): 1001-1013.
- Kapoor, Raju, et al. "Serum neurofilament light as a biomarker in progressive multiple sclerosis." Neurology, 2020; 95(10): 436-444.
- Kappos L, Li DK, Stüve O, Hartung HP, Freedman MS, Hemmer B, et al. “Safety and efficacy of siponimod (BAF312) in patients with relapsing-remitting multiple sclerosis: dose-blinded, randomized extension of the phase 2 BOLD study.” JAMA neurology, 2016; 73(9): 1089-1098.
- Chaudhry, Burhan Z, Jeffrey A Cohen, Devon S. Conway. "Sphingosine 1-phosphate receptor modulators for the treatment of multiple sclerosis." Neurotherapeutics, 2017; 14(4): 859-873.
- Faissner, Simon, Ralf Gold. "Progressive multiple sclerosis: latest therapeutic developments and future directions." Therapeutic advances in neurological disorders, 2019; 12: 1756286419878323.
- Kappos L, Bar-Or A, Cree BA, Fox RJ, Giovannoni G, Gold R, et al.” Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomised, phase 3 study.” The Lancet, 2018; 391(10127): 1263-1273.
- Scott, Lesley J. "Siponimod: a review in secondary progressive multiple sclerosis." CNS drugs, 2020; 34(11): 1191-1200.
- Cao, Liujiao, et al. "Siponimod for multiple sclerosis." Cochrane Database of Systematic Reviews 11, 2021.
- Cree BA, Magnusson B, Rouyrre N, Fox RJ, Giovannoni G, Vermersch P, et al. “Siponimod: Disentangling disability and relapses in secondary progressive multiple sclerosis”. Multiple Sclerosis Journal, 2021; 27(10): 1564-1576.
- Schoedel, Kerri A, et al. "Abuse and dependence potential of sphingosine-1-phosphate (S1P) receptor modulators used in the treatment of multiple sclerosis: a review of literature and public data." Psychopharmacology, 2021: 1-13.
- Atkins Harold L, Mark S Freedman. "Hematopoietic stem cell therapy for multiple sclerosis: top 10 lessons learned." Neurotherapeutics, 2013; 10(1): 68-76.
- Giedraitiene N, Kizlaitiene R, Peceliunas V, Griskevicius L, Kaubrys G. “Selective cognitive dysfunction and physical disability improvement after autologous hematopoietic stem cell transplantation in highly active multiple sclerosis.” Scientific reports, 2020; 10(1): 1-9.
- Nourbakhsh B, Rutatangwa A, Waltz M, Rensel M, Moodley M, Graves J, et al. “Heterogeneity in association of remote herpesvirus infections and pediatric MS.” Annals of clinical and translational neurology, 2018; 5(10): 1222.
- Czarnowska A, Kapica-Topczewska K, Zajkowska O, Świerzbińska R, Chorąży M, Tarasiuk J, et al. “Herpesviridae seropositivity in patients with multiple sclerosis: first Polish study.” European neurology, 2018; 80(5-6): 229-235.
- Bakhuraysah, Maha M, Christopher Siatskas, Steven Petratos. "Hematopoietic stem cell transplantation for multiple sclerosis: is it a clinical reality?" Stem Cell Research & Therapy, 2016; 7(1): 1-12.
- Sahraian MA, Mohyeddin Bonab M, Baghbanian SM, Owji M, Naser Moghadasi A. “Therapeutic use of intrathecal mesenchymal stem cells in patients with multiple sclerosis: a pilot study with booster injection.” Immunological Investigations, 2019; 48(2):160-168.
- Kuan, Thomas Low Tat, Farahnaz Amini, Marjan Sadat Seghayat. "Feasibility and toxicity of hematopoietic stem cell transplant in multiple sclerosis." Iranian journal of basic medical sciences, 2017; 20(7): 729.
- Stepien A, Dabrowska NL, Maciagowska M, Macoch RP, Zolocinska A, Mazur S, et al. “Clinical application of autologous adipose stem cells in patients with multiple sclerosis: preliminary results.” Mediators of Inflammation, 2016; 2016.
- Gholamzad, Mehrdad, et al. “Prophylactic and Therapeutic Effects of MOG-Conjugated PLGA Nanoparticles in C57Bl/6 Mouse Model of Multiple Sclerosis.” Advanced pharmaceutical bulletin, 2021; 11(3): 505-513. doi:10.34172/apb.2021.058.
- Gholamzad, Mehrdad, et al. “Intravenous Injection of Myelin Oligodendrocyte Glycoprotein-coated PLGA Microparticles Have Tolerogenic Effects in Experimental Autoimmune Encephalomyelitis.” Iranian journal of allergy, asthma, and immunology, 2017; 16(3): 271-281.
- Chountoulesi, Maria, Costas Demetzos. "Promising nanotechnology approaches in treatment of autoimmune diseases of central nervous system." Brain Sciences, 2020; 10(6): 338.

