Application of Nanomedicine in Cancers
Alex avakian, Hadis Sadeghi, Ali Faraji, Romina Hamidi Rad, Seyyed Hamid reza Jalalian, Mohammad Hossein Ghanbari, Parmida Latifi, Marzieh Arab Loodaricheh, Armin Soleymani Fard, Ehsan Ababaf, Amir Gholamzad, Seyed Ali Hosseini Zavareh*
1Department of Medicine, Islamic Azad University Sari branch, Iran
2Department of Medicine, Tehran Medical Sciences Branch, Islamic Azad University, Iran
3Medical Student, Department of Medicine, Islamic Azad University of Medical Sciences, Iran
4Medical Student, Faculty of Medicine, Tehran Medical Sciences, Islamic Azad University, Iran
5Medical Student, Department of Medicine, Islamic Azad University Tehran Medical Sciences, Iran
6Medical Laboratory Science Student, Faculty of Allied Medicine, Tehran Medical Sciences, Islamic Azad University, Iran
7Medical Student, Department of Medicine, Islamic Azad University Tehran Medical Sciences, Iran
Received Date: 21/04/2022; Published Date: 05/05/2022
*Corresponding author: Sayed Ali Hosseini Zavareh, Department of Medicine, Islamic Azad University Tehran Medical Sciences, Iran
Abstract
Nano-drugs have always been used as a new treatment for various cancers. Recently, many advances have been made in the use of nano-drugs, which has led to the introduction of nano-drugs as an important factor in the front line. Has been treated. Also, some of the nanoparticles studied have a dual use in the treatment and diagnosis of cancer cells, most research. Nano-drugs have been developed for breast and stomach cancers that have gone through their clinical phases, but in other cancers, most drugs are in phases 2 and 3; Unfortunately, there is no definitive cure for cancer, but recent advances in nanotechnology and nanomedicine, which have led to the development of new drugs, are helping to provide a better and less complication-free treatment for cancer. In this article, we provide an overview of the use of nanodrugs, their reported results, and their function as the mainstay of cancer treatment.
Keywords: Nanomedicine; Cancer nanomedicine
Introduction
Cancer is not just a disease but a group of diseases. Cancer can originate in any tissue in the body; During this disease, a cell proliferates abnormally, eventually resulting in an irregular mass known as a tumor [1]. This finding has uncontrollable metabolic needs and also lacks the ability to differentiate; Therefore, tumor cells cannot function properly as adult cells [2]. During the process of changing a normal cell to a malignant one, the cell undergoes multiple mutations in different genes. Mutations can involve normal cell genes and eventually cause the conversion of proto-oncogene to oncogene; Also, mutations in tumor suppressor and anti-oncogen genes lead to loss of their regulatory function [2]. There are several treatments, including intravenous drug, inhalation, and oral. The treatment of cancer with nanoparticles is a new phenomenon in medicine that started 20 years ago and the most growth in the content about it in the last 5 years [3]. Nanodrugs provide a creative and effective therapeutic and diagnostic approach to cancers. [4] Have been studied on drug resistance [5]. The need to classify patients in oncology, to select the appropriate patient commercially and clinically, to integrate different treatment methods in cancers, as well as the ability to create nanoparticles in the immune system, makes NATO therapy significantly considered [6-9] Common cancers include lung, breast, and stomach cancers. Therefore, in this article, we have examined the effective and practical therapeutic approaches of nanodrugs for the treatment of these cancers.
Lung cancer
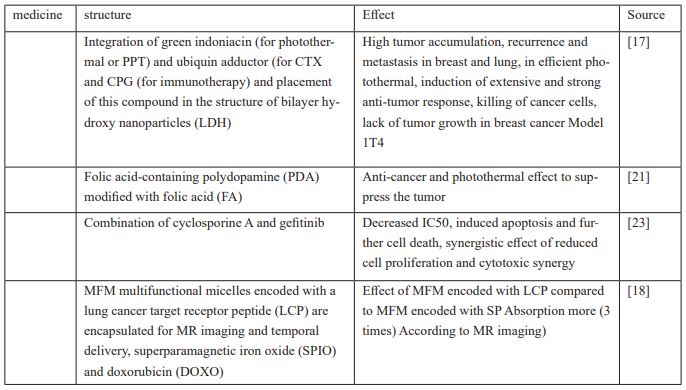
Lung cancer is a type of malignant tumor that has the highest incidence and mortality [10], which justifies the growing urgent need for new treatment strategies for lung cancer [11], targeted nanoparticles TNPs have the potential to overcome the limitations of efficacy and toxicity associated with traditional cytotoxic agents and also to inhibit the growth and proliferation of cancer cells directly by further transfection of the injected drug in a regulated and controllable manner [12,13] Gradual release enhances the stability of drug efficacy, especially in the treatment of solid tumors; NKO12 is a nanoparticle derived from the incorporation of SN-38 into micelles designed for prodrug delivery and gradual release. Releases the non-enzymatic form 38-SN in physiological [14] A study investigated PSMA target DPXL nanoparticles compared to non-target NPs, which have a longer blood circulation due to the gradual decrease in DTXL In the blood plasma, they have more antitumor activity, in general, this drug is used to treat patients with other solid tumors can be used. 1[5] HHTs reduce tumor growth, while the aptamer-modified HGT (Loaded PLGA-SS-PEG) nanopharmaceutical (EGFR) suppresses subcutaneous tumor growth and has a greater therapeutic effect; PLGA nanoparticles also have no effect on lung BEAS2 B cells and are biocompatible, and their use has fewer side effects and toxicity [16]. LDH was introduced. This drug stops lung cancer metastasis and prevents tumor growth in the 1T4 breast cancer model. It is a combination of green indoniacin for photothermal or PTT, doxorubicin, CTX and CPG for immunotherapy, which is composed of a bilayer of hydroxide nanoparticles (LDH).
It is effective for photothermal treatment due to high tumor accumulation. Releases DOX from DOX / DNA prodrug, which synergizes PTT / CTX to kill tumor cancer cells. On the other hand, CPG / LDH, by absorbing antigens produced by CTX and PTT, induces extensive and strong anti-tumor responses [17]. In another paper, pH was introduced as a multifunctional micelle (MFM) encoded with a lung cancer receptor-targeting peptide (LCP) enclosed for MR imaging and therapeutic delivery, magnetic iron superpara (SPIO) and doxorubicin (DOXO). LPC performance at the multifunctional micelle (MFM) surface triples MFM uptake compared to coded micelle or scrambled peptide (SP), resulting in more successful release of DOXO from the MFM and its accumulation in the nucleus. LCP-encoded MFMs also increase cytotoxicity compared to LPC-encoded MFMs. And has a significant role in accurate cell targeting [18] and in another study, a type of polypopamine nanoparticle containing cinobaphagin (PDA and modified with folic acid (FA)) along with photothermal therapy for the treatment of lung cancer was introduced [19,20] This drug has a multifaceted effect including anti-cancer and photothermal effects to suppress the proliferation of tumor cells and PDA due to its natural melanin compatible with nature and is biodegradable. Modification of PDA with FA improves therapeutic effects and reduces toxicity and side effects. If PDA is not corrected by FA, it prolongs blood circulation due to the effect of EPR. On the other hand, the fluorescence of ICG nanoparticles of PDA, when modified with FA, is much stronger at the tumor site than PDA without modification. PDA nanomedicine enters the cell through FA and FR and through cell endocytosis. Cancers become tumors. In 808 nm laser radiation, PDA has the potential to be used in photothermal therapy (PTT).
PDA suppression also increases in this laser radiation; This inhibitory effect of lung cancer cells is mainly due to thermal erosion. PDA nanoparticles produce higher selective toxicity for lung cancer cells with higher FR expression and lower pH than normal cells [21]. Expression of the TUSC2 gene induces apoptosis in cancer cells, but most The early stages of cancer spread are inactivated; DC lipid nanoparticles were designed to carry TUSC2 gene mRNA injected intravenously into tumor tissue that has low toxicity and can be administered repeatedly with an effective dose of other target molecules [22] HAN.W In its 2018 study, sought to investigate the synergistic effect of Cyclosporin A and Gefitinib against cancer cells, especially Gef-resistant NSCLC cells when nanoparticles (NPs) were co-delivered. In the laboratory, this study was performed on 3 nscle cells: EGFR - TKI - sensitive PC - 9 acquired - resistance PC - 9 primary - resistance H1975 cells.
Treatment with Gef-NPs induced apoptosis, although the addition of Csa to the np formulation showed higher rates of apoptosis in the same cells. Treatment with CsA / Gef - NPS resulted in 1.8 to 2.3 times more cell death compared to Gef - NPS in all nscle cells and showed a synergistic effect of reduced cell proliferation compared to Gef – NPS.
Table 1: Test results.

These 4 independent tests clearly confirm the cytotoxic synergy of a chemosensitizer and an MTA regardless of encapsulation in a ninovehicle [23]. In the process of cancer diagnosis and treatment, ZnO nanoparticles (ZnO) due to their unique properties such as improved properties Catalytic and better light absorption are used [24,25], so in a study, in order to synthesize zinc oxide nanoparticles, Mangifera indica leaf extract was used to increase the antioxidant activity of nanoparticles. This biosynthesis method reduces the toxicity of zinc oxide nanoparticles, fast and environmentally friendly. [26], gold nanoparticles are also used as a financial antimicrobial agent, making AUNPs by chemical reduction of HAUCIA by NABHA, which acts as both a reducing agent and a coating, increasing the anti-cancer properties against cells. Non-small cell lung cancer is A549, which stops the cell cycle in the G2 / M phase. Plasmid-enhanced green fluorescent protein (PEGFP) delivery (NCL) structure containing transferrin-containing ligands has been used that can bind to A549 cells through their TVRs and replicate genes involved in suppressing tumor growth. Easier to get to the cells [27,28].
Gastric cancer
Nanoparticles, which are referred to below, act in various ways such as increasing the absorption of radiation therapy, induction of apoptosis and disruption of the cell cycle of cells. Cell membrane proteins (CMs) are responsible for targeting tumors. [CM / SLN [ref is a type of CM that has more anti-toxicity and biocompatibility. CM / SLN / ICG is also a type of CM that increases cell death by increasing 3-caspase production. [29]. Caspase is a protein that increases the expression and production of anti-tumor proteins such as 2-bcl. [CM / SLN / ICG [ref] generally performs better than CM / SLN. According to experiments, CM / SLN focuses on liver and kidneys after injection, while DM / SLN / ICG focuses on cancerous tumors themselves. As a result, this nanoparticle will be used effectively in the treatment of gastric cancer [29] and in another study it was shown that miR - 200c - loaded gelatinase - stimuli PEG - Pep - PCLs NPs with accumulation in gastric cancer cells such as BGC823 and GES increases the sensitivity of these cells to radio waves. For example, experiments have shown that miR-200c nanoparticles have a significant effect on reducing the tumor mass of CD44 cells; These nanoparticles also cause the death of gastric cancer cells by increasing the expression of proteins involved in programmed death, such as caspase3,8,9. Other nanoparticles that induce apoptosis include DOC-PEG-PCL-mAb[30].
Showed that by increasing the expression of programmed death factors such as caspase3,8,9 as well as reducing the factors of planned death prevention and cell cycle arrest in the G2 stage of mitosis have a significant effect on death and cessation of cancer cell growth [31-34]. FesO-CMC-SFU is another type of nanoparticle that kills cancer cells through accumulation, programmed mitochondrial death, and DNA damage. The nanoparticles were injected into cancer cells by researchers in an experiment. In this experiment, Fero, CMC - SFU was injected at concentrations of 25.5, 50, 75% + and SC90 7901 cells for 72.48.24 hours. Fexo NPS was also injected into similar cells. After the experiment, it was found that Fesos-CMC-SFU has a greater effect than T. F. [35] PEG-amorphous TiO is a type of nanoparticle that is involved in the fight against cancer by reducing cell division. In these nanoparticles, the longer the placement time and concentration, the higher the lethality. The highest lethality of these nanoparticles is in MKN-45 cells at a concentration of 50 ng / ml for 72,48,24 hours. These values are 30 ng / ml for amorphous TiO2 for 72 hours. According to research at a concentration of 50 g / m for 72 hours, the lethality of PEG-amorphous TiO2 was 40% and 60%, and also this number was 25% and 75% in amorphous TiO2%. This statistic shows the effect of this nanoparticle and its relationship with concentration [36]. In another way, FesONPs use silver ion green synthesis to convert it to silver nanoparticles (SPR). By injecting these nanoparticles called T.Polium-AgNPs (130 Hg / ml) into 45-MNK cells, the viability of the cells reaches 26.1% and also the IC50 concentration reaches 68.2 g / ml within 48 hours. In addition, there is a direct relationship between dose (T.Polium - AgNPs (130 g / ml) and mortality rate - 45 - MKN; respectively, the higher the dose, 75, 25, 12.5, the lower the number of cancer cells, and this sign It gives a tremendous effect to this nanoparticle [37].
chitosan / heparin nanoparticle - encapsulated cytolethal distending toxin (CdtB - NPs) are nanoparticles or high biological activity that were tested in an experiment on their effect on cell cycle process. In this experiment, CdtB - NPS was injected into AGS cells, and within 24 hours, the researchers noticed an increase in cells stopped at the G2 / M stage [38]. 2 - Bcl in reducing cell death and Bak, Bax is involved in increasing cell death [ref] CdtB - NPS By increasing the expression of Bax and Bak and decreasing the expression of 2 - bel, increases the number of cells stopped in G1 and induces It becomes cell death. It can also inject -CdtB directly into the nucleus and initiate programmed death by phosphorylating CHK2, HAX, and Ps3 [38]۔ And c - Met expression in high gastric tumor cells [ref] Research has shown that si - c - Met dramatically reduces c - Met expression and increases sensitivity to chemotherapy. EXO si - c - Met significantly increases cisplatin inhibition rate, reduces cell viability and migration capacity, and also encourages cell death in SGC7901 cells, which in turn leads to a total cessation of tumor growth. [39] and nanoparticles alone could not block tumors, but better results were obtained when tumors were treated with TNPs and trastuzumab. Tumor growth was completely inhibited when the tumor was treated with nanoparticles -AMO-21/5-FU-TNPs and its efficacy was much higher than that of AMO-21/5-Fu-NPs + trastuzumab. Antibodies formed as fusion nanoparticles (such as trastuzumab combined with 21-AMO and FU-5 nanodarticles) were not only effective in treating gastric cell tumors but also sensitized cancer cells to trastuzumab and -5 FU. Increased anti-tumor properties. In a similar approach, a nanoparticle of PEG-PCL or trastuzumab polymer was combined, which if the ratio of the two is 50%, has the greatest effect on tumor binding. Therefore, PEG-PCLs combined with trastuzumab can be used to treat gastric cancers, which leads to increased anti-cancer properties in HER-2 positive cells and also by reducing the side effects of using trastuzumab against HER-2 positive cells. [40] In subsequent research, the anti-tumor properties of IRGD-TK-NPS against gastric cancer cells have been proven. Hypersensitivity of RGD - TK - NPS ROS can potentiate the rapid change of UA (Uronic Acid prodrugs to active drugs) and increase their blood retention time, which ultimately increases the antitumor activity of UAs. IRGD Combine with UA-based DPNS to increase tumor targeting; in fact, iRGD-optimized nanoparticles enter cancer cells more efficiently and selectively.
The value of iRGD-TK-NPS IC50 is 7.2 which is 1.4, 2.4, 2.4 and 15.7 less effective than UA alone, CC-NPS, TK-NPS and RGD-CC-NPS, respectively. UA alone had half the effect of stopping the tumor (TSR 45%) versus IRGD - TK - NPS had the best effect (TSR 86.5%) which is a confirmation of the rapid and complete release of the drug into the cancer cell [41], in a study Another, CD44 / CD133 - ATRA - PLPN has been used as a potent agent for targeting gastric cancer cells. It was observed that in MKN-45 CD133 cells, the toxicity of ATRA-PLPN was increased compared to ATRA, which proves the effectiveness of nanoparticles in delivering ATRA to gastric cancer cells [42]. In another study, researchers in a group of cancer patients compared treatment methods. The study found that people who received BCC1 capsules survived 112 days longer than those who received a placebo. Also, those with metastatic cancer lived 184 days longer than those who received a placebo. Also, people who took both BCC1 capsules and chemotherapy survived 195 days longer than the group. In another study, they found that people with metastatic cancer who took BCC1 at the same time had a 0.476-fold higher risk of death than the rest of the group. Also, in patients receiving chemotherapy, the death rate was 0.737 times higher than the experimental group. With the use of BCc1 capsules, the standard of living improved in both patients with metastatic cancer and non-metastatic patients. Also, half of those with cancer in the placebo group died within the first 30 days, but those who took the BCC1 capsule died within the first 50 days. This shows the tremendous effect of BCC1 capsules in the treatment of cancer, especially gastric cancer [43].
Breast Cancer
In a study to investigate the anti-metastatic effect of the lung on breast cancer and the eradication of primary cancer cells, CLM, which is drug-labeled micelles. CLM reduces the survival rate of tumor cells and can inhibit the migration and invasion of cancer cells [44], nanoscale and response to CLM stimuli facilitates on-site drug delivery and increases the antitumor effect of free drugs ; During intravenous injection, micelles containing the drug accumulate at the tumor site; Controlled release and release of their cargo is done by isolating hydrazion and GFLG BONDS bonds and by responding to stimuli in the tumor microenvironment [44]. It had a great anti-metastatic effect on orthotropic breast cancer and lung metastasis. A large number of apoptotic cells were induced and the pathway related to MMP metastasis and infiltration of MDSCs was inhibited. It also has good biocompatibility with major organs and reduces DOX cardiac toxicity [44]. Delivers tumors. The drug was formulated as tannomys using a polymer carrier that specifically targets tumors. [45] According to a 2019 study, the nanoparticle PEG-PAM-PAN @ DOX is not only effective against breast cancer, but also has an excellent sensitive effect on areas around the tumor for magnetic resonance imaging. This effect facilitates magnetic resonance imaging and collects more information about the area and function of tumors, which has a great impact on the effective treatment of cancers. This nanodrug is an amphiphilic copolymer containing aslamine (polyethylene glycol, polyacrylamide, polystyrene, [PEG-b-P [AM-co-AN) synthesized by polymerization of extra-fragmented chain transfer [46]. In terms of its anti-cancer effect, it has less cytotoxicity than free doxorubin, which allows it to enter the synoplasm and nucleus of cancer cells more effectively to kill it, and compared to traditional drugs, the time of exposure to chemotherapy drugs. It has a self-assembling nature that results in simple, effective and biocompatible synthase [46], [PEG - b - P [AM - co - AN has a chemical exchange saturation transfer (CEST) effect Which makes it possible to use CEST imaging to monitor the accumulation of nanocarriers and to collect molecular information from pathological tissues [46].
In another study on the effectiveness of anticancer drugs on drug-resistant breast cancer, ECG-ME microemulsion was used as an oral delivery system and the result showed that this drug as an oral formulation could be safe in the treatment of drug-resistant breast cancer. And be effective. The components of this drug are seed oil and ginsenoside rh2 loaded with autopsied, both of which have an anti-tumor synergistic effect with autopsied. The drug is absorbed in the intestine as nanoparticles, circulates in the bloodstream, and therefore has a longer shelf life in the blood and accumulates in the tumor area. ECG-ME treatment is almost safe and does not cause semen. ECG-ME has the property of controlled spatio-temporal release. This property inhibits P-BP by the initially released Barh2 and increases the intracellular accumulation of the released otoposide [47]. In the following, we will introduce a nano-drug platform from human serum albumin (HSA) that, along with two therapeutic drugs, growth factor antibodies and methotrexate (MTX) and cancer-targeted folic acid (FOL) targets breast cancer cells. Anti-growth factor antibody on hsa conjugate kills excess tgf-b produced by MDA-MB-231 cancer cells. The combination of FOL with hsa causes breast cancer cells to express more FOL receptors and absorb more FOL-HAS-MTX. The high affinity of folic acid for folate receptors in cancer cell membranes leads to the administration of anticancer drugs to cancer cells. FOL on the HSA conjugate by binding to folic acid receptors in cancer cells increases the uptake of the HAS conjugate through endocytosis by MDA-MB-231 cells. After endocytosis, MTX binds to dihydrofolate reductase on the HSA conjugate, causing cytotoxicity. This process disrupts cell growth and proliferation [48], recently in 2020 a nanomedicine called IPEDNPSCOM was studied for the multifaceted treatment of breast cancer. The structural basis of I - P @ NPSDM are nanoparticle particles PTX and IND, which are activated and released by tumor-activated oxygen (ROS) and glutathione (GSH) into tumor cells and directly combined with chemotherapy and photodynamic therapy. Destroy cancer cells. To increase intracellular maintenance and recovery, they produced ROS as nanofibrils by producing active oxygen by laser (IPCONPSM) and then covering the IPCDNPSOM with a macrophage membrane, which prolongs blood circulation, reduces toxicity of foreign and precise materials [49]. Finally, we use the high potential of hypoxic nanoparticles to accurately stimulate tumors and effectively suppress them.In this study, it was shown that drugs can be delivered effectively. Anticancer and optical sensors used hypoxic polymer rods into cancer cells, causing citric toxicity and cell death through chemotherapy and photodynamic therapy (PTD). Intracellular translocation increases cargo, which in combination with chemotherapy and PTD induces apoptosis. And 1Hypoxic AT to inhibit recurrence and eradication of breast cancer and metastasis in lung cancer, micelles combine with CPG and AaCTLA to increase the immune response [50].
Table 3: Drug information of the second part.

Cancer of the Liver
In this section, we reviewed the research conducted on the effect of nanoparticles combined and conjugated with chemotherapy drugs such as doxorubicin and mitoxantrone. DHAD - PBCA - NPs, mitoxantrone - loaded polybutylcyanacrylate nanoparticles were performed and the results show that the target response rate for the DHAD - PBCA - NPS group was 10.5%, 61.4% of patients with stable disease, and 28.1% of patients with progression. No objective response was found for the DHAD injection group (45% of patients had stable disease and 54.9% of patients had progression) [51]. There was a significant difference in stable and progressive disease between the two groups. (P <0.05) The mean survival period of DHAD - PBCA - NPS group and DHAD injection group was 5.46 months and 3.23 months, respectively. The use of this nanoparticle also reduced the side effects of chemotherapy. Leukopenia was observed in 47.4% and 74.5% of DHAD - PBCA - NPS group and DHAD injection group, respectively. Meanwhile, anemia was observed in 65% and 37.3% of the DHAD-PBCA-NPS group and the DHAD injection group, respectively [38]. In this study, the antitumor efficacy of doxycycline-loaded polyisohexyl cyanoacrylate nanoparticles (PIHCA-Dox) versus free doxorubicin (Dox) was investigated. In vitro and in vivo in vitro, PIHCA - Dox IC50 vs. Dox for Huh7 (1.7-fold decrease; P <0.001) HepaRG (4.5-fold decrease, HepG2> 0.01, (P (1.5-fold decrease, P And HepG2.2.15 (1.5-fold decrease: P <0.059) (Huh7, HepaRG, HepG2, and HepG2.2.15 - different HCC cell lines) [52] Application of transferrin-modified polymer nanoparticles (Tf) For doxorubicin (DOX) and then platinum (DDP), another method for achieving combined tumor therapy is the higher TP-DOX / DDP nanoparticles, which have higher cytotoxicity and increased antitumor activity, both in vitro and in vivo than their counterparts without [53] In another study, the use of doxorubicin (siRNA (DOX programmed death ligand 1 (NP DOX / siPD-L1)), (PD-L1) was shown to induce cell death. Immunogenic (ICD) and overexpression of PD - L1 in HCC.In vivo study showed that intravenous injection of NP DOX / siPD - L1 significantly found tumor volume and PD - L1 expression found in the tumor. Inhibited an animal model carrying an H22 tumor. In addition, NPDOX / SIPD-L1 treatment also regulated the population of adult dendritic cells and cytotoxic T cells and the production of cytokines in tumor tissues [54]. LDCPs act by inhibiting tumor permeability and cell activation by siRNA translocation of 1PD-L anti-ligands (ligands related to immunoassay) and immunotherapy of IL-2-encoded plasmid DNA for HCC (Hepatocellular carcinoma). They increase CD8 + T and complement the effectiveness of the cancer immunotherapy vaccine. This mechanism also stops the progression of HCC [55].
References
- Richardson P. What is cancer? Practice Nursing, 1997; 8(18): 27-29.
- Taylor M, Heller T, Bailey L, Pattison S. A Simplified Biology of Cancers, 1992.
- Shi J, Kantoff PW, Wooster R, Farokhzad OC. Cancer nanomedicine: progress, challenges and opportunities. Nature reviews cancer, 2017; 17(1): 20-37.
- Roma-Rodrigues C, Pombo I, Raposo L, Pedrosa P, Fernandes AR, Baptista PV. Nanotheranostics targeting the tumor microenvironment. Frontiers in Bioengineering and Biotechnology, 2019: 197.
- Markman JL, Rekechenetskiy A, Holler E, Ljubimova JY. Nanomedicine therapeutic approaches to overcome cancer drug resistance. Advanced drug delivery reviews, 2013; 65(13-14): 1866-1879.
- van der Meel R, Sulheim E, Shi Y, Kiessling F, Mulder WJ, Lammers T. Smart cancer nanomedicine. Nature nanotechnology. 2019; 14(11): 1007-1017.
- Dogra, Prashant, et al. "Mathematical modeling in cancer nanomedicine: a review." Biomedical Microdevices 2019; 21(2): 1-23.
- Beltrán-Gracia, Esteban, et al. "Nanomedicine review: Clinical developments in liposomal applications." Cancer Nanotechnology, 2019; 10(1): 1-40.
- Mohammadzadeh, Vahideh, et al. "Applications of plant-based nanoparticles in nanomedicine: A review." Sustainable Chemistry and Pharmacy, 2022; 25: 100606.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. CA Cancer J. Clin., 2018; 68: 394-424.
- Kawabata A, Baoum A, Ohta N, Jacquez S, Seo GM, Berkland C, et al. Intratracheal administration of a nanoparticle-based therapy with the angiotensin II type 2 receptor gene attenuates lung cancer growth. Cancer research, 2012; 72(8): 2057-2067.
- Farokhzad OC, Jon S, Khademhosseini A, Tran TN, LaVan DA, Langer R. Nanoparticle-aptamer bioconjugates: a new approach for targeting prostate cancer cells. Cancer research, 2004; 64(21): 7668-7672.
- Kolishetti N, Dhar S, Valencia PM, Lin LQ, Karnik R, Lippard SJ, et al. Engineering of self-assembled nanoparticle platform for precisely controlled combination drug therapy. Proceedings of the National Academy of Sciences, 2010; 107(42): 17939-17944.
- Hamaguchi T, Doi T, Eguchi-Nakajima T, Kato K, Yamada Y, Shimada Y, et al. Phase I study of NK012, a novel SN-38–incorporating micellar nanoparticle, in adult patients with solid tumors. Clinical Cancer Research, 2010; 16(20): 5058-5066.
- Hrkach J, Von Hoff D, Ali MM, Andrianova E, Auer J, Campbell T, et al. Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Science translational medicine, 2012; 4(128): 128ra39.
- Zhang Z, Cheng W, Pan Y, Jia L. An anticancer agent-loaded PLGA nanomedicine with glutathione-response and targeted delivery for the treatment of lung cancer. Journal of Materials Chemistry B, 2020; 8(4): 655-665.
- Zhang LX, Sun XM, Xu ZP, Liu RT. Development of multifunctional clay-based nanomedicine for elimination of primary invasive breast cancer and prevention of its lung metastasis and distant inoculation. ACS applied materials & interfaces, 2019; 11(39): 35566-35576.
- Guthi JS, Yang SG, Huang G, Li S, Khemtong C, Kessinger CW, et al. MRI-visible micellar nanomedicine for targeted drug delivery to lung cancer cells. Molecular pharmaceutics, 2010; 7(1): 32-40.
- Volk, Robert J, et al. "Effect of a patient decision aid on lung cancer screening decision-making by persons who smoke: a randomized clinical trial." JAMA network open, 2020; 3(1): e1920362-e1920362.
- Ashrafizadeh Milad, et al. "Versatile role of curcumin and its derivatives in lung cancer therapy." Journal of cellular physiology, 2020; 235(12): 9241-9268.
- Li J, Zhang Z, Deng H, Zheng Z. Cinobufagin-loaded and folic acid-modified polydopamine nanomedicine combined with photothermal therapy for the treatment of lung cancer. Frontiers in chemistry, 2021; 9: 117.
- Lu C, Stewart DJ, Lee JJ, Ji L, Ramesh R, Jayachandran G, et al. Phase I clinical trial of systemically administered TUSC2 (FUS1)-nanoparticles mediating functional gene transfer in humans. PloS one. 2012; 7(4): e34833.
- Han W, Shi L, Ren L, Zhou L, Li T, Qiao Y, et al. A nanomedicine approach enables co-delivery of cyclosporin A and gefitinib to potentiate the therapeutic efficacy in drug-resistant lung cancer. Signal transduction and targeted therapy, 2018; 3(1): 1-10.
- Anbuvannan M, Ramesh M, Viruthagiri G, Shanmugam N, Kannadasan N. Anisochilus carnosus leaf extract mediated synthesis of zinc oxide nanoparticles for antibacterial and photocatalytic activities. Materials Science in Semiconductor Processing, 2015; 39: 621-628.
- Nair, Shantikumar, et al. "Role of size scale of ZnO nanoparticles and microparticles on toxicity toward bacteria and osteoblast cancer cells." Journal of Materials Science: Materials in Medicine, 2009; 20(1): 235-241.
- Rajeshkumar S, Kumar SV, Ramaiah A, Agarwal H, Lakshmi T, Roopan SM. Biosynthesis of zinc oxide nanoparticles usingMangifera indica leaves and evaluation of their antioxidant and cytotoxic properties in lung cancer (A549) cells. Enzyme and microbial technology, 2018; 9117: 91-95.
- Ramalingam V, Revathidevi S, Shanmuganayagam TS, Muthulakshmi L, Rajaram R. Gold nanoparticle induces mitochondria-mediated apoptosis and cell cycle arrest in nonsmall cell lung cancer cells. Gold Bulletin, 2017; 50(2): 177-189.
- Han Y, Li Y, Zhang P, Sun J, Li X, Sun X, et al. Nanostructured lipid carriers as novel drug delivery system for lung cancer gene therapy. Pharmaceutical development and technology, 2016; 21(3): 277-281.
- Hu N, Yin JF, Ji Z, Hong Y, Wu P, Bian B, et al. Strengthening gastric cancer therapy by trastuzumab-conjugated nanoparticles with simultaneous encapsulation of anti-MiR-21 and 5-fluorouridine. Cellular Physiology and Biochemistry, 2017; 44(6): 2158-2173.
- Cui FB, Liu Q, Li RT, Shen J, Wu PY, Yu LX, et al. Enhancement of radiotherapy efficacy by miR-200c-loaded gelatinase-stimuli PEG-Pep-PCL nanoparticles in gastric cancer cells. International journal of nanomedicine, 2014; 9: 2345.
- Xu S, Cui F, Huang D, Zhang D, Zhu A, Sun X, et al. PD-L1 monoclonal antibody-conjugated nanoparticles enhance drug delivery level and chemotherapy efficacy in gastric cancer cells. International journal of nanomedicine, 2019; 14: 17.
- Wang, Zhi, et al. "The Mechanism of Growth-inhibitory Effect of DOC-2/DAB2 in Prostate Cancer: CHARACTERIZATION OF A NOVEL GTPase-ACTIVATING PROTEIN ASSOCIATED WITH N-TERMINAL DOMAIN OF DOC-2/DAB2∗." Journal of Biological Chemistry, 2002; 277(15): 12622-12631.
- Pasut, Gianfranco, Mauro Sergi, Francesco M Veronese. "Anti-cancer PEG-enzymes: 30 years old, but still a current approach." Advanced drug delivery reviews, 2008; 60(1): 69-78.
- Liu, Qin, et al. "Targeted delivery of miR-200c/DOC to inhibit cancer stem cells and cancer cells by the gelatinases-stimuli nanoparticles." Biomaterials, 2013; 34(29): 7191-7203.
- Liu X, Deng X, Li X, Xue D, Zhang H, Liu T, et al. A visualized investigation at the atomic scale of the antitumor effect of magnetic nanomedicine on gastric cancer cells. Nanomedicine, 2014; 9(9): 1389-1402.
- Nasr R, Hasanzadeh H, Khaleghian A, Moshtaghian A, Emadi A, Moshfegh S. Induction of apoptosis and inhibition of invasion in gastric cancer cells by titanium dioxide nanoparticles. Oman medical journal, 2018; 33(2): 111.
- Hashemi SF, Tasharrofi N, Saber MM. Green synthesis of silver nanoparticles using Teucrium polium leaf extract and assessment of their antitumor effects against MNK45 human gastric cancer cell line. Journal of Molecular structure, 2020; 1208: 127889.
- Lai CK, Lu YL, Hsieh JT, Tsai SC, Feng CL, Tsai YS, et al. Development of chitosan/heparin nanoparticle-encapsulated cytolethal distending toxin for gastric cancer therapy. Nanomedicine, 2014; 9(6): 803-817.
- Zhang Q, Zhang H, Ning T, Liu D, Deng T, Liu R, et al. Exosome-delivered c-Met siRNA could reverse chemoresistance to cisplatin in gastric cancer. International journal of nanomedicine, 2020; 15: 2323.
- Hu N, Yin JF, Ji Z, Hong Y, Wu P, Bian B, et al. Strengthening gastric cancer therapy by trastuzumab-conjugated nanoparticles with simultaneous encapsulation of anti-MiR-21 and 5-fluorouridine. Cellular Physiology and Biochemistry, 2017; 44(6): 2158-2173.
- Ma J, Chen Y, Liang W, Li L, Du J, Pan C, et al. ROS-responsive dimeric prodrug-based nanomedicine targeted therapy for gastric cancer. Drug Delivery, 2021; 28(1):1204-1213.
- Chen H, Lin J, Shan Y, Zhengmao L. The promotion of nanoparticle delivery to two populations of gastric cancer stem cells by CD133 and CD44 antibodies. Biomedicine & Pharmacotherapy, 2019; 115: 108857.
- Hafizi M, Kalanaky S, Khayamzadeh M, Noorian S, Kaveh V, Gharib B, et al. A randomized, double-blind, placebo-controlled investigation of BCc1 nanomedicine effect on survival and quality of life in metastatic and non-metastatic gastric cancer patients. Journal of nanobiotechnology, 2019; 17(1): 1-9.
- Luo L, Xu F, Peng H, Luo Y, Tian X, Battaglia G, et al. Stimuli-responsive polymeric prodrug-based nanomedicine delivering nifuroxazide and doxorubicin against primary breast cancer and pulmonary metastasis. Journal of Controlled Release. 2020; 318: 124-135.
- Madaan A, Singh P, Awasthi A, Verma R, Singh AT, Jaggi M, et al. Efficiency and mechanism of intracellular paclitaxel delivery by novel nanopolymer-based tumor-targeted delivery system, NanoxelTM. Clinical and Translational Oncology, 2013; 15(1): 26-32.
- Jia Y, Wang C, Zheng J, Lin G, Ni D, Shen Z, et al. Novel nanomedicine with a chemical-exchange saturation transfer effect for breast cancer treatment in vivo. Journal of nanobiotechnology, 2019; 17(1): 1-4.
- Qu D, Wang L, Liu M, Shen S, Li T, Liu Y, et al. Oral nanomedicine based on multicomponent microemulsions for drug-resistant breast cancer treatment. Biomacromolecules, 2017; 18(4): 1268-1280.
- Lee S. Human serum albumin: A nanomedicine platform targeting breast cancer cells. Journal of Drug Delivery Science and Technology, 2019; 52: 652-659.
- Liu R, An Y, Jia W, Wang Y, Wu Y, Zhen Y, et al. Macrophage-mimic shape changeable nanomedicine retained in tumor for multimodal therapy of breast cancer. Journal of Controlled Release, 2020; 321: 589-601.
- Liu J, Ai X, Cabral H, Liu J, Huang Y, Mi P. Tumor hypoxia-activated combinatorial nanomedicine triggers systemic antitumor immunity to effectively eradicate advanced breast cancer. Biomaterials, 2021; 273: 120847.
- Zhou Q, Sun X, Zeng L, Liu J, Zhang Z. A randomized multicenter phase II clinical trial of mitoxantrone-loaded nanoparticles in the treatment of 108 patients with unresected hepatocellular carcinoma. Nanomedicine: Nanotechnology, biology and medicine, 2009; 5(4): 419-423.
- Barraud L, Merle P, Soma E, Lefrançois L, Guerret S, Chevallier M, et al. Increase of doxorubicin sensitivity by doxorubicin-loading into nanoparticles for hepatocellular carcinoma cells in vitro and in vivo. Journal of hepatology, 2005; 42(5): 736-743.
- Zhang X, Li J, Yan M. Targeted hepatocellular carcinoma therapy: transferrin modified, self-assembled polymeric nanomedicine for co-delivery of cisplatin and doxorubicin. Drug Development and Industrial Pharmacy, 2016; 42(10): 1590-1599.
- Zhu H, Zhou W, Wan Y, Ge K, Lu J, Jia C. Nanomedicine-mediated induction of immunogenic cell death and prevention of PD-L1 overexpression for enhanced hepatocellular carcinoma therapy. Cancer Nanotechnology, 2020; 11(1): 1-4.
- Huang KW, Hsu FF, Qiu JT, Chern GJ, Lee YA, Chang CC, et al. Highly efficient and tumor-selective nanoparticles for dual-targeted immunogene therapy against cancer. Science advances. 2020; 6(3): eaax5032.

