The Role of Mutations on Gene KCNJ11 in Permanent Neonatal Diabetes Mellitus Syndrome
Shahin Asadi*, Elaheh Fayyazian and Sahel Kesrat
Medical Genetics-Harvard University. Director of the Division of Medical Genetics and Molecular Optogenetic Research.
Division of Medical Genetics and Molecular Pathology Research, Harvard University, Boston Children's Hospital
Received Date: 16/03/2022; Published Date: 30./03/2022
*Corresponding author: Shahin Asadi, Medical Genetics-Harvard University, Director of the Division of Medical Genetics and Molecular Optogenetic Research. Division of Medical Genetics and Molecular Pathology Research, Harvard University, Boston Children's Hospital, USA
Abstract
Diabetes mellitus is a type of diabetes that first appears in the first 6 months of life and lasts a lifetime. This form of diabetes is characterized by high blood sugar levels (hyperglycemia) caused by a lack of the hormone insulin. Few people with chronic neonatal diabetes syndrome have underdeveloped pancreas. Chronic neonatal diabetes syndrome may be caused by mutations in several genes. About 30% of people with chronic neonatal diabetes are caused by a mutation in the KCNJ11 gene, which is located in the short arm of chromosome 11 at 11p15.1.
Keywords: Permanent Neonatal Diabetes Mellitus Syndrome; KCNJ11 gene; Mutation; Hyperglycemia
Generalities of Permanent Neonatal Diabetes Mellitus Syndrome
Diabetes mellitus is a type of diabetes that first appears in the first 6 months of life and lasts a lifetime. This form of diabetes is characterized by high blood sugar levels (hyperglycemia) caused by a lack of the hormone insulin. Insulin controls the conversion of glucose (a type of sugar) from the blood to the cell for conversion into energy [1].
Clinical signs and symptoms of Permanent Neonatal Diabetes Mellitus Syndrome
People with chronic neonatal diabetes syndrome experience slow growth before birth (intrauterine growth retardation). Babies with hyperglycemia and dehydration (dehydration) are unable to gain weight and grow at the expected speed (lack of developmental progress) [1,2].

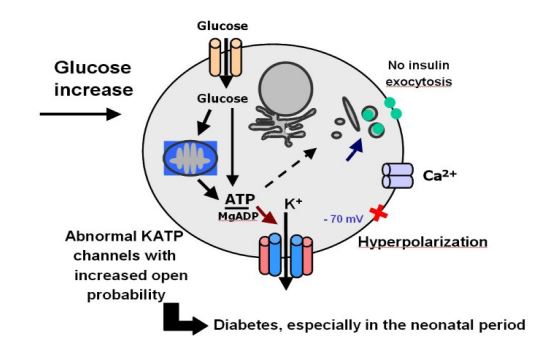
Figure 2: Schematic of the biochemical mechanism of beta pancreatic cells [1].
In some cases, people with chronic neonatal diabetes also have certain neurological problems, including stunted growth and recurrent seizures (epilepsy). This combination of growth retardation, epilepsy and neonatal diabetes is known as DEND syndrome. DEND syndrome mediates a similar combination but with mild growth retardation without epilepsy [1,2].
Few people with chronic neonatal diabetes syndrome have underdeveloped pancreas. Because the pancreas is responsible for digestive enzymes, as well as the secretion of insulin and the production of other hormones, people with this disease have digestive problems such as fatty stools and an inability to absorb fat-soluble vitamins [1,2].
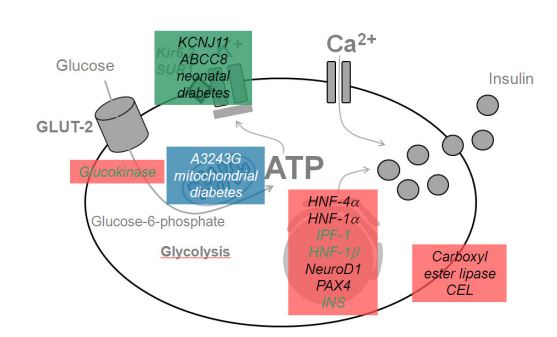
Figure 3: Schematic of the biochemical mechanism of KCNJ11 and ABCC8 genes in Permanent Neonatal Diabetes Mellitus Syndrome [1].
Etiology of Permanent Neonatal Diabetes Mellitus Syndrome
Permanent Neonatal Diabetes Mellitus Syndrome may be caused by mutations in several genes. About 30% of people with chronic neonatal diabetes are caused by a mutation in the KCNJ11 gene, which is located in the short arm of chromosome 11 at 11p15.1. Another 20 percent of people with chronic neonatal diabetes have a mutation in the ABCC8 gene. These genes provide the instructions for making potassium-sensitive ATP (K-ATP) channel subunits. Each K-ATP channel consists of eight subunits, four subunits produced by the KCNJ11 gene and four subunits of the ABCC8 gene [1,3].

Figure 4: Schematic view of chromosome 11 where the KCNJ11 and ABCC8 genes are located in the short arm of this chromosome as 11p15.1 [1].
K-ATP channels are found in cell membranes in pancreatic insulin-secreting beta cells. These channels open and close in response to the amount of glucose in the bloodstream. Closure of the ducts in response to an increase in glucose releases insulin from beta cells and enters the bloodstream, which helps control blood sugar [1,4].
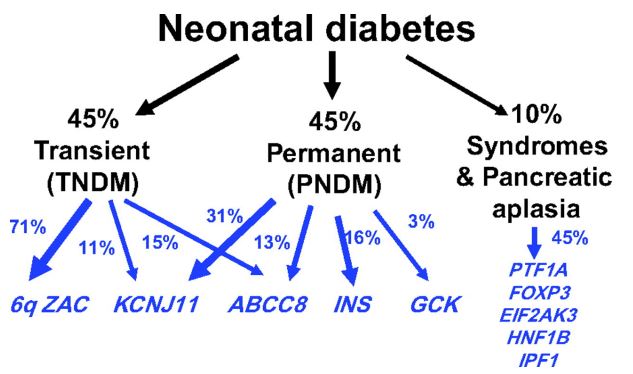
Figure 5: Schematic of the percentage of factors involved in neonatal diabetes [1].
Mutations in the KCNJ11 or ABCC8 gene, which causes neonatal chronic diabetes, lead to insulin secretion from beta cells into K-ATP channels that do not close and impair blood sugar control [1,5].
Mutations in the INS gene, located on the short arm of chromosome 11 at 11p15.5, which provides the instructions for making insulin, have been identified in about 20 percent of people with chronic neonatal diabetes. Insulin is produced as a precursor called proinsulin, which is made up of a single chain of protein building blocks (amino acids). The proinsulin chain is cut (split) to form separate pieces called chains A and B, which are joined together by connections called disulfide bonds to form insulin. Mutations in the INS gene are thought to disrupt the cleavage of the proinsulin chain or the binding of chains A and B to form insulin, leading to impaired glycemic control. It is worth noting that persistent neonatal diabetes can also be caused by mutations in other genes, some of which have not been identified [1,5].
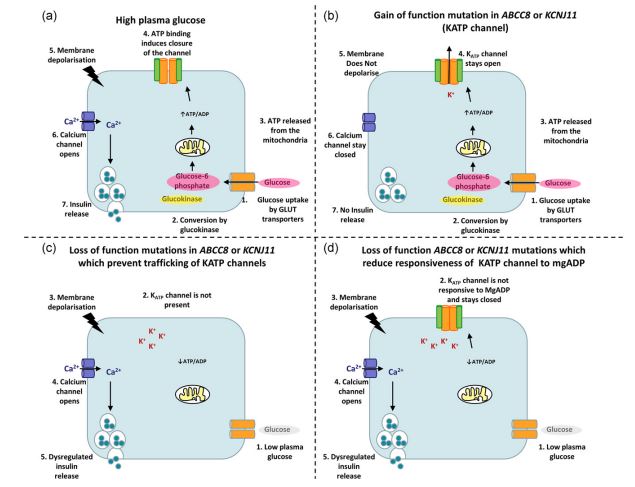
Figure 6: Schematic of healthy plasma glucose versus mutant function of KCNJ11 and ABCC8 genes [1].
Chronic Diabetes Syndrome can have different inherited patterns. When the disease is caused by a mutation in the KCNJ11 or INS gene, it follows an autosomal dominant inheritance pattern, meaning that one copy of the altered target gene in each cell (either parent) is sufficient to cause the disorder. And the chance of having a child with the disease in an autosomal dominant state is 50% for any possible pregnancy. In about 90% of these cases, the disease is caused by new mutations in the gene and occurs in people who do not have a family history of the disorder. In the remaining cases, the infected person inherits the mutation from the infected parents [1,6].
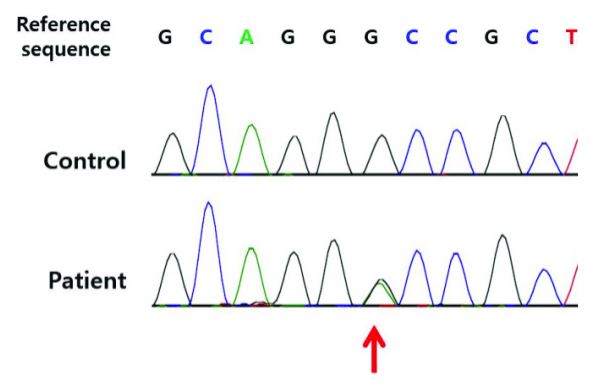

Figure 7: An example of the corresponding mutation in the KCNJ11 and ABCC8 genes [1].
When chronic neonatal diabetes syndrome is caused by a mutation in the ABCC8 gene, it may follow an autosomal dominant or autosomal recessive inheritance pattern. In an autosomal recessive pattern, rejection of two copies of the target mutant gene (one from the father and the other from the mother) is required to cause the disorder, and the chance of having a child with autosomal recessive disease is 25% for each possible pregnancy. The parents of a person with autosomal recessive disease each carry a copy of the mutated gene, but typically show no signs or symptoms of the disease [1,7]
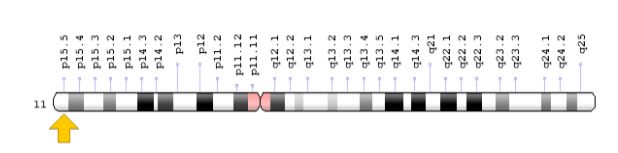
Figure 8: Schematic view of chromosome 11 where the INS gene is located in the short arm of this chromosome as 11p15.5 [1].
In less common cases, the disease is caused by mutations in other genes, and in these cases, it is inherited in an autosomal recessive pattern [1,7].
Prevalence of Permanent Neonatal Diabetes Mellitus Syndrome
About 1 in 400,000 babies develop diabetes in the first few months of life. However, about half of these babies have a transient state of diabetes, and their diabetes resolves on its own by 18 months. The rest of neonatal diabetes is thought to be permanent [1,8].
Diagnosis of Permanent Neonatal Diabetes Mellitus Syndrome
Chronic neonatal diabetes syndrome can be diagnosed based on the clinical findings of some patients and some pathological tests. The most accurate method of diagnosing this disease is molecular genetic testing for INS, ABCC8, KCNJ11 genes to check for possible mutations [1,9].
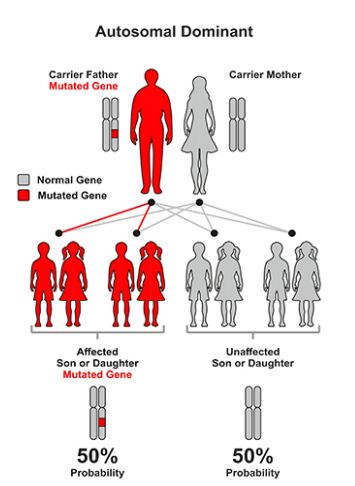
Figure 9: Schematic of the dominant autosomal inherited pattern followed by persistent neonatal diabetes syndrome [1].
Treatment routes for Permanent Neonatal Diabetes Mellitus Syndrome
The strategy for treating and managing chronic neonatal diabetes syndrome is symptomatic and supportive. Treatment should be regular and daily with special drugs to minimize the suffering of patients. Genetic counseling is also essential for all parents who want a healthy baby [1,10].

Figure 10: Schematic of the autosomal recessive inherited pattern that the neonatal chronic diabetes syndrome follows [1].
Discussion and Conclusion
People with chronic neonatal diabetes syndrome experience slow growth before birth (intrauterine growth retardation). In some cases, people with chronic neonatal diabetes also have certain neurological problems, including stunted growth and recurrent seizures (epilepsy). This combination of growth retardation, epilepsy and neonatal diabetes is known as DEND syndrome. The strategy for treating and managing chronic neonatal diabetes syndrome is symptomatic and supportive. Treatment should be regular and daily with special drugs to minimize the suffering of patients [1-11].
References
- Asadi S, Pathology in Medical Genetics Book, Amidi Publications Iran 2022; 20.
- Edghill EL, Flanagan SE, Ellard S. Permanent neonatal diabetes due to activating mutations in ABCC8 and KCNJ11. Rev Endocr Metab Disord, 2010; 11(3): 193-198. doi: 10.1007/s11154-010-9149-x.
- Edghill EL, Flanagan SE, Patch AM, Boustred C, Parrish A, Shields B, et al. Neonatal Diabetes International Collaborative Group, Hattersley AT, Ellard S. Insulin mutation screening in 1,044 patients with diabetes: mutations in the INS gene are a common cause of neonatal diabetes but a rare cause of diabetes diagnosed in childhood or adulthood. Diabetes. 2008; 57(4): 1034-1042.
- Ellard S, Flanagan SE, Girard CA, Patch AM, Harries LW, Parrish A, et al. Permanent neonatal diabetes caused by dominant, recessive, or compound heterozygous SUR1 mutations with opposite functional effects. Am J Hum Genet, 2007; 81(2): 375-382.
- Flanagan SE, Clauin S, Bellanné-Chantelot C, de Lonlay P, Harries LW, Gloyn AL, et al. Update of mutations in the genes encoding the pancreatic beta-cell K(ATP) channel subunits Kir6.2 (KCNJ11) and sulfonylurea receptor 1 (ABCC8) in diabetes mellitus and hyperinsulinism. Hum Mutat, 2009; 30(2): 170-180. doi: 10.1002/humu.20838.
- Flanagan SE, Edghill EL, Gloyn AL, Ellard S, Hattersley AT. Mutations in KCNJ11, which encodes Kir6.2, are a common cause of diabetes diagnosed in the first 6 months of life, with the phenotype determined by genotype. Diabetologia, 2006; 49(6): 1190-1197.
- Malecki MT, Mlynarski W. Monogenic diabetes: implications for therapy of rare types of disease. Diabetes Obes Metab, 2008; 10(8): 607-616.
- Osbak KK, Colclough K, Saint-Martin C, Beer NL, Bellanné-Chantelot C, Ellard S, et al. Update on mutations in glucokinase (GCK), which cause maturity-onset diabetes of the young, permanent neonatal diabetes, and hyperinsulinemic hypoglycemia. Hum Mutat, 2009; 30(11): 1512-1526. doi: 10.1002/humu.21110.
- Polak M, Cavé H. Neonatal diabetes mellitus: a disease linked to multiple mechanisms. Orphanet J Rare Dis, 2007; 2: 12.
- Rubio-Cabezas O, Klupa T, Malecki MT. CEED3 Consortium. Permanent neonatal diabetes mellitus--the importance of diabetes differential diagnosis in neonates and infants. Eur J Clin Invest, 2011; 41(3): 323-333. doi: 10.1111/j.1365-2362.2010.02409.x.
- Støy J, Steiner DF, Park SY, Ye H, Philipson LH, Bell GI. Clinical and molecular genetics of neonatal diabetes due to mutations in the insulin gene. Rev Endocr Metab Disord, 2010; 11(3): 205-215. doi: 10.1007/s11154-010-9151-3. Review. Erratum in: Rev Endocr Metab Disord, 2012; 13(1): 79-81.

