Infection Control Measures for Orthopaedic Operatories During Covid-19 Crisis: An Update
Sagar Chaudhari*, Sharayu Dhande, Ajit Govind Jangale and Sheetal Ajit Jangale
Department of Orthopaedics, Consulting Orthopaedic Surgeon, India
Department of periodontology and oral implantology, M A Rangoonwala College of Dental Sciences and Research Centre, India
Department of orthopaedics, Dr Vasanat Rao Pawar Medical College, India
Department of Pedodontist, Private practioner, India
Received Date: 21/03/2021; Published Date: 11/01/2022
*Corresponding author: Dr. Sagar Chaudhari, Chaudhari clinic, Department of Orthopaedics, Consulting Orthopaedic Surgeon, India.
Abstract
Surgical management of any trauma is a foundation of any healthcare system with both elective and emergency procedures contributing to the health of our patients. Healthcare professionals agree that stringent cleaning and disinfection of the orthopaedic operatories are essential elements of effective infection control programs. However, traditional manual cleaning and disinfection protocols carried out in operatories are often considered suboptimal at times. Factors influencing the risk to a healthcare professional include the number and types of blood contact experienced by the worker, risk of transmission of droplet infections, accidental blood splashes, sharp injuries, cutaneous scratches etc. However, operatories are high risk areas for transmission of respiratory infections given the urgency in management, the involvement of multiple staff, and the need for high transmission-risk activities such as airway management. The high prevalence of disease, limited resources, and staff under pressure, greatly increase the risk of transmission of coronavirus and also other healthcare associated pathogens, and this affects the efficacy of disinfection protocols. Advanced technologies in disinfection and newer disinfectants should be preferred in order to improve disinfection of surfaces in orthopaedic operatories.
Keywords: Infection Control; Orthopaedic Operatory; Occupational Exposure; Covid-19; Occupational Hazard
Introduction
Coronaviruses, a genus in the Coronaviridae family, are pleomorphic, enveloped viruses. Coronaviruses gained prominence during the Severe Acute Respiratory Syndrome (SARS) outbreaks of 2002–2003 [1,2]. On 11th February 2020, the World Health Organization (WHO) Director-General, Dr. Tedros Adhanom Ghebreyesus, named the disease caused by the SARS-CoV-2 as “COVID-19”, and by March 11th, 2020 when the total number of countries affected with it reached 114, with more than 118,000 cases rising daily along with 4000 deaths, the WHO declared it as a pandemic [3-9].Common symptoms at onset of illness were fever, cough, and myalgia; fewer common symptoms were sputum production, headache, haemoptysis, and diarrhoea, dyspnoea, lymphopenia, abnormal findings on chest CT [10-14].
The standard method of diagnosis is by real time reverse transcriptase polymerase chain reaction (rRT-PCR) from a nasopharyngeal swab. Virus‐specific nucleic acid sequences were detected in lung fluid, blood and throat swabs [15-18].The spread of covid is through air borne zoonotic droplets which spread through close contact with the infected individual [19].Close contact includes being within approximately six feet with an infected individual for a prolonged period of time or having direct contact with infectious secretions without wearing personal protective equipment [20].
Cleaning and disinfection are a critical component of any infection prevention program [21]. Newer Products and practices should be taken into consideration for surface disinfection in orthopaedic operatories like: Inactivation of emerging pathogens (e.g., CRE, C. auris), Technologies for terminal room decontamination (not including technologies with limited data), Ultraviolet light, Vaporized hydrogen peroxide, Continuous room decontamination technologies, Light disinfection, Low-concentration hydrogen peroxide, Self-disinfecting surfaces [22-29]. Touchless cleaning techniques provide an incremental benefit to manual practices by limiting cross-transmission of pathogens via environmental surfaces, though evidence of prevention of certain pathogens remains limited. These technologies include a variety of products including self-disinfecting surfaces along with few fumigation methods [30-35].
The main objective of this article is to summarize a variety of disinfectants and other factors that affect standard cleaning and disinfection practices, to put forth certain newer technologies that can supplement manual traditional cleaning and disinfection methods and thus support orthopaedic surgeons in their daily practice during this coronavirus pandemic [36-42].
Infection Control Measures: Personal Protective Equipment
Given the plentitude of challenges for achieving and maintaining adequate cleaning and disinfection in healthcare facilities, there is a need to consider the use of modern technologies designed to improve disinfection of surfaces in hospitals.
New technologies fall into several categories, including:
(A) Liquid surface disinfectants, (B) Improved methods for applying disinfectants, (C) Self-disinfecting technologies, (D) light-activated photosensitizers, (E) Automated or No-touch technologies.
These newer disinfectants have Environmental Protection Agency (EPA) safety rating of category IV (housekeepers do not need to wear any personal protective equipment while using these products) [43-46].
Chemical disinfection has always played a crucial role in eradication of micro-organisms. Newer hydrogen peroxide-based liquid surface disinfectants with a combination of peracetic acid and hydrogen peroxide have proved effective alternatives to disinfectants during the ongoing COVID-19 pandemic, also use of electrolyzed water (hypochlorous acid) and cold atmospheric pressure plasma have shown antibacterial effect.
Newer “no-touch” (automated) decontamination technologies include aerosol and vaporized hydrogen peroxide, mobile devices that emit continuous ultraviolet (UV-C) light, a pulsed-xenon UV light system, and use of high-intensity narrow-spectrum (405 nm) light. These “no-touch” technologies have been shown to reduce bacterial contamination of surfaces.
A micro-condensation hydrogen peroxide system has been associated in various studies with reductions in healthcare-associated colonization or infection, while there is more limited evidence of microbial decline by the pulsed-xenon system [47].
Touch (Wiping) Vs No-Touch (Mechanical) Disinfection
Reduction of microbial contamination is an important aspect of infection control program. The rate of hospital acquired infections is increasing dramatically in the past few years because multi-drug resistant strains of certain micro-organisms. Utilizing vapours for decontamination overcomes many limitations of traditional Touch (wiping) method of disinfection. Vapours have high potential to permeate or penetrate complex surfaces, albeit varying levels of uniformity.
No-Touch or Touchless technologies are recent innovation in disinfection technologies. They encompass a broad range of self-disinfecting surfaces and fumigation methods. They limit cross contamination of pathogens and hence an effective method of disinfection. Humphreys recently reviewed Self-disinfecting surfaces, but enough literature is yet to be published for the same.
Personal Protective Equipment
A comprehensive program for the use of PPE should be enforced. All the healthcare personnel should be trained in the use of PPE. They should be trained to clean, disinfect, store, and inspect their PPE. All staff should be strictly advised to use with the National Institute of Occupational Safety and Health (NIOSH)-certified N95 respirators. Personal goggles should be issued to every member of staff.
Hair covers or hoods should also be worn. Longer sleeved gloves are preferred to prevent exposure of the wrists with glove slippage. Alternately, vertical tape strips should be used to help keep gloves secured to the gown. Circumferential taping of gloves to the gown, such as used when wearing chemical PPE, is not necessary and makes gown and glove removal more challenging. Eye protection should include protection from side exposure with side shields or goggles. Full face shields advised since they help provide both eye protection and avoid facial and respirator contamination. Some disposable shoe covers may increase the risk of self-contamination during removal of protection clothing. Shoes worn should be impermeable to fluids and able to be decontaminated. Staff should wear operating room scrub suits or full coveralls under the PPE. Coveralls with an integrated hood may simplify the underlayer worn in conjunction with PPE, however the choice of product should be assessed for ease of removal to avoid contamination during removal. Hand hygiene must be performed after removing PPE, and in the event of inadvertent contamination of the hands by touching infected surfaces during PPE removal [48-50].
The Buddy System (two-person assistance system) with mutual supervision should be adopted unlike other countries. In addition, the Sky Eye monitoring system should be installed in nurse stations, physicians’ offices, PPE donning areas, and PPE-doffing areas to observe and monitor in real-time during arranging shifts of infection control teams on a 24hour basis. The staff should be reminded in a timely manner for the precautions to be taken during donning and doffing of the PPE to ensure their utmost safety. During doffing, the PPE should be gently rolled on the body and any vigorous movements should be avoided, also thus the soiled outside surface of PPE should be rolled inwards. A proper distance should be maintained when spraying the chlorine-based disinfectant to allow for full atomization and to achieve effective sterilization. Moreover, the spraying of disinfectant should avoid the head and face to prevent the disinfectant from irritating the respiratory tract and mucous membranes of the person. Healthcare professionals should avoid touching the side edges or front surfaces of face shield, eye-wear, headcaps, facemasks to prevent contamination. All healthcare professionals should strictly implement the seven-step handwashing technique for a minimum of 15 seconds [51].
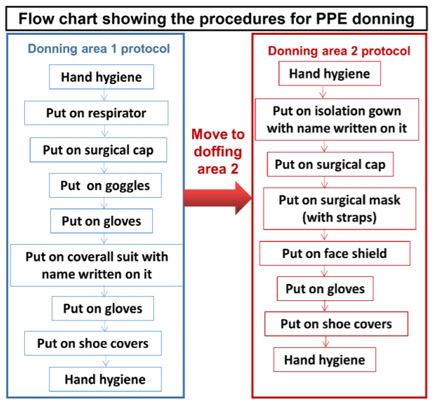
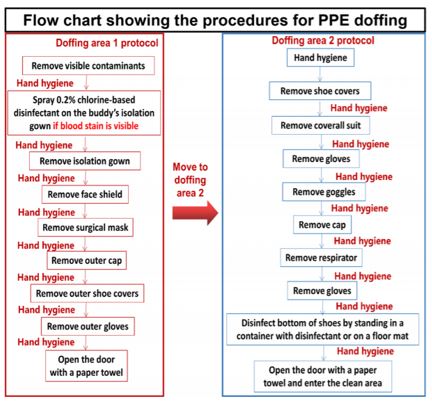
Flowchart enumerating procedures during donning and doffing of PPE kit [52].
The emergency response plan on exposure to contaminated PPE includes the following steps:
(1) Immediately suspend the doffing procedures once exposure occurs. The exposed area should be immediately disinfected by the buddy in the doffing area.
(2) If exposure occurs to the face or other skin surfaces, immediately apply 75% alcohol or ethanol-containing quick-drying hand sanitizer to wipe the exposed skin on the face or other area for 2 min.
(3) If exposure occurs to ocular mucosa, repeatedly rinse with normal saline and apply anti-infective eye drops.
(4) If exposure occurs to the oral mucosa, gargle with 75% alcohol once for 2 min, followed by gargling with normal saline three times.
(5) Continue doffing other PPE according to the procedures.
(6) Shower and change clothes.
(7) Finally, report the relevant information to the infection control team [52,53].
Recommended practices for extending the use and/or re-using an N95 respirator mask [54-57]:
- Avoid removing, adjusting, or touching the respirator (both outside and inside surfaces.
- Discard the respirator if it becomes grossly contaminated or damaged or if breathing through it becomes difficult.
Perform hand hygiene before and after handling/touching the respirator.
- Store the respirator in a clean, dry location to avoid contamination and maintain its integrity. It can be stored in a single-use breathable container, or hung in a designated area.
- Inspect the respirator and perform a seal check before each use.
There might be a risk of a shortage of N95 respirators during any pandemic, especially if it is extended for a prolonged period of time. In that case, alternatives such as Powered Air Purifying Respirator (PAPRs) may be used and practices may be introduced to extend the use of each N95 respirator [58].
Use of Powered air purifying respirators (PAPR) vs N95 mask as part of PPE during elective orthopaedic surgeries [59]:
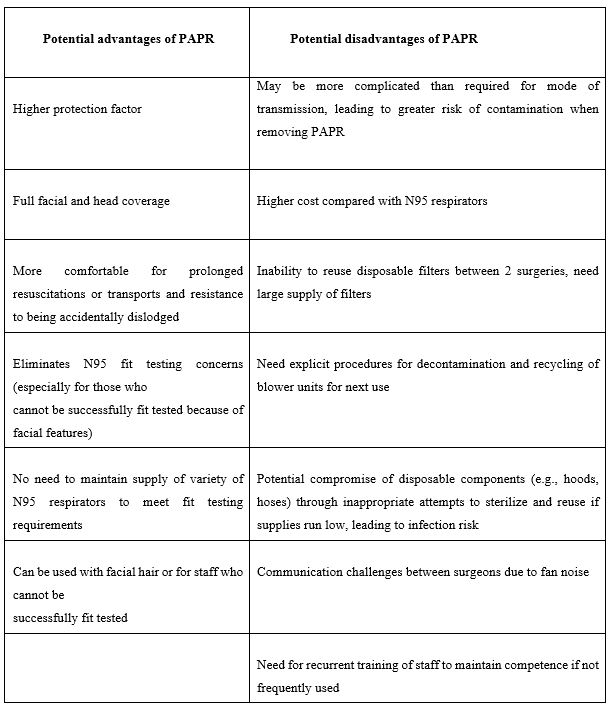
The Public Health Agency of Canada has Released Interim Infection Control Recommendations Related to COVID:
Localized aerosol may be generated during certain medical interventions like intubation, non-invasive ventilation, bag valve-mask ventilation that could result in airborne transmission of contagious diseases.
As earlier mentioned, bag mask ventilation could generate aerosols, prior to intubation due coughing of the patient during laryngoscopy. An exhalation filter can therefore, be attached between the mask or endotracheal tube and the bag to the resuscitation bag. Intubator could be placed at a higher risk in cases of inadequate sedation, which could result in patient agitation and also dislodgement of the PPE. Adequate pre-oxygenation refrains the risk of bag-mask ventilation. Video laryngoscopy should be used with a display for smooth intubation. A flexible bronchoscopic intubation with a help of video bronchoscope can be an effective alternative for complex cases. End-tidal carbondioxide should be detected to check the position of endotracheal tube placement [60,61].
Once intubated, lung protective mechanical ventilation strategies should be used (target tidal volume 6 mL_kg-1 predicted body weight, plateau pressure B30 cm H20, target SaO2 88–95% and pH C 7.25). All exhaled gas from the ventilator should be filtered. Pneumothorax was noted in some ventilated patients affected with SARS. Extrapolating to 2019-nCoV infected patients, clinicians should strongly consider pneumothorax in any ventilated patient with sudden respiratory deterioration. Given the potential delay in obtaining a chest x-ray for a patient in airborne isolation, portable ultrasound may be used to quickly assist in the diagnosis of a pneumothorax [62].
Conclusion
The Entire Orthopaedic Fraternity is continuing to develop strategies to deliver a safe musculoskeletal care during this ongoing COVID-19 crisis, while many surgeons of the orthopaedic workforce move to the front line. Orthopaedic surgeons and patients are having difficult choices about management options for a wide variety of orthopaedic injuries and urgent conditions due to the brisk nature of transmission of virus. In the current trend of evolving guidelines and infection control protocols about operative as well as non-operative management of COVID-19 patients, Orthopaedic surgeons are making a redoubled effort to get back to their interrupted practice amidst the pandemic. Utilization of newly developed disinfection practices will help deliver optimum treatment.
Conflict of interest: None
Author Contribution: All authors have equally contributed for completion of this manuscript.
References
- Peiris JS, Lai ST, Poon LL, Guan Y, Yam LY, Lim W, et al. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet, 2003; 361:1319-1325.
- Masters PS. The molecular biology of coronaviruses. Adv. Virus Res, 2006; 66: 193–292.
- World Health Organization Director-General’s Opening Remarks at the Media Briefing on COVID-19.
- Centres for Disease Control and Prevention (CDC). Update: Outbreak of severe acute respiratory syndrome--worldwide, 2003. MMWR Morb Mortal Wkly Rep, 2003; 52(12): 241-246.
- World Health Organization. Coronavirus never before seen in humans is the cause of SARS– update 31. Geneva: The Organization; 2003.
- World Health Organization. Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003.
- Drosten C, Günther S, Preiser W, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med, 2003; 348: 1967‐1976.
- Kumar D, Malviya R, Sharma PK. Corona virus: a review of COVID-19. EJMO, 2020; 4(1): 8-25.
- Cavanagh D. Coronaviridae: a review of coronaviruses and toroviruses. In: Schmidt A, Wolff MH, Weber O, eds. Coronaviruses with Special Emphasis on First Insights Concerning SARS. Switzerland: Birkhauser Verlog Basel; 2005.
- Hsu LY, Chia PY, Lim JF. The Novel Coronavirus (SARS-CoV-2) Pandemic. Annals Academy of Medicine Singapore, 2020; 49(105): 105-107.
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The lancet, 2020; 395(10223): 497-506.
- Li CK, et al. T cell responses to whole SARS coronavirus in humans. J. Immunol. 2008; 181: 5490–5500.
- Gu J, Korteweg C. Pathology and pathogenesis of severe acute respiratory syndrome. Am. J. Pathol. 2007; 170: 1136–1147.
- Chen J, Subbarao K. The immunobiology of SARS. Annu. Rev. Immunol. 25, 443–472 (2007).
- Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. Journal of medical virology, 2020; 92(4): 401-402.
- Perlman S, Netland J. Coronaviruses post-SARS: update on replication and pathogenesis. Nature reviews microbiology. 2009; 7(6): 439-450.
- Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. The lancet. 2020; 395(10224): 565-574.
- Yin Y, Wunderink RG. MERS, SARS and other coronaviruses as causes of pneumonia. Respirology. 2018; 23(2): 130-137.
- Kumar D, Malviya R, Sharma PK. Corona virus: a review of COVID-19. EJMO, 2020; 4(1): 8-25.
- Di Gennaro F, Pizzol D, Marotta C, Antunes M, Racalbuto V, Veronese N, Smith L. Coronavirus diseases (COVID-19) current status and future perspectives: a narrative review. International journal of environmental research and public health. 2020; 17(8): 2690.
- Practice Guidance for Healthcare Environmental Cleaning, 2nd Edition, AHA, 2012; Trillis F, Infect Control Hosp Epidemiol, 2008; Rutala WA, Am J Infect Control, 2014.
- Rutala WA, Weber DJ. Infect Control Hosp Epidemiol, 2014; 35: 855-865.
- Blazejewski C, Wallet F, Rouze A, et al. Efficiency of hydrogen peroxide in improving disinfection of ICU rooms. Crit Care, 2015; 19: 30.
- Falagas ME, Thomaidis PC, Kotsantis IK, Sgouros K, Samonis G, Karageorgopoulos DE. Airborne hydrogen peroxide for disinfection of the hospital environment and infection control: a systematic review. J Hosp Infect, 2011; 78: 171–177.
- Canadian Agency of Drugs and Technologies in Health. Nonmanual techniques for room disinfection in health care facilities: a review, 2014.
- Nerandzic MM, Cadnum JL, Pultz MJ, Donskey CJ. Evaluation of an automated ultraviolet radiation device for decontamination of clostridium difficile and other healthcare-associated pathogens in hospital rooms. BMC Infect Dis, 2010; 10: 197–2334.
- Nerandzic MM, Fisher CW, Donskey CJ. Sorting through the wealth of options: comparative evaluation of two ultraviolet disinfection systems. PLoS ONE, 2014; 9: e107444.
- Carling PC, Von Beheren S, Kim P, Woods C. Healthcare Environmental Hygiene Study Group. Intensive care unit environmental cleaning: an evaluation in sixteen hospitals using a novel assessment tool. J Hosp Infect, 2008; 68: 39–44.
- Boyce JM. Modern technologies for improving cleaning and disinfection of environmental surfaces in hospitals. Antimicrobial Resistance & Infection Control, 2016; 5(1): 1-10.
- Galvin S, Boyle M, Russell RJ, et al. Evaluation of vaporized hydrogen peroxide, citrox and pH neutral ecasol for decontamination of an enclosed area: a pilot study. J Hosp Infect, 2012; 80: 67–70.
- Havill NL, Moore BA, Boyce JM. Comparison of the microbiological efficacy of hydrogen peroxide vapor and ultraviolet light processes for room decontamination. Infect Control Hosp Epidemiol, 2012; 33: 507–512.
- Steindl G, Fiedler A, Huhulescu S, Wewalka G, Allerberger F. Effect of airborne hydrogen peroxide on spores of Clostridium difficile. Wien Klin Wochenschr, 2014.
- Rutala WA, Gergen MF, Tande BM, Weber DJ. Rapid hospital room decontamination using ultraviolet (UV) light with a nanostructured UV-reflective wall coating. Infect Control Hosp Epidemiol, 2013; 34: 527–529.
- Rutala WA, Gergen MF, Tande BM, Weber DJ. Room decontamination using an ultraviolet-C device with short ultraviolet exposure time. Infect Control Hosp Epidemiol, 2014; 35: 1070–1072.
- Rutala WA, Weber DJ, Gergen MF, Tande BM, Sickbert-Bennett EE. Does coating all room surfaces with an ultraviolet C lightnanoreflective coating improve decontamination compared with coating only the walls? Infect Control Hosp Epidemiol, 2014; 35: 323–325.
- Rutala WA, Weber DJ. Disinfectants used for environmental disinfection and new room decontamination technology. Am J Infect Control, 2013; 41: S36–41.
- Donskey CJ. Does improving surface cleaning and disinfection reduce health care-associated infections? Am J Infect Control, 2013; 41: S12–19.
- Dancer SJ. Controlling hospital-acquired infection: focus on the role of the environment and new technologies for decontamination. Clin Microbiol Rev, 2014; 27: 665–690.
- Han JH, Sullivan N, Leas BF, Pegues DA, Kaczmarek JL, Umscheid CA. Cleaning hospital room surfaces to prevent health care-associated infections. a technical brief. Ann Intern Med, 2015; 163: 598-607.
- Carling PC, Bartley JM. Evaluating hygienic cleaning in health care settings: what you do not know can harm your patients. Am J Infect Control, 2010; 38: S41–50.
- Boyce JM, Havill NL, Lipka A, Havill H, Rizvani R. Variations in hospital daily cleaning practices. Infect Control Hosp Epidemiol, 2010; 31: 99–101.
- Sitzlar B, Deshpande A, Fertelli D, Kundrapu S, Sethi AK, Donskey CJ. An Environmental Disinfection Odyssey: Evaluation of Sequential Interventions to Improve Disinfection of Clostridium difficile Isolation Rooms. Infect Control Hosp Epidemiol, 2013; 34: 459–465.
- Carling PC, Perkins J, Ferguson J, Thomasser A. Evaluating a new paradigm for comparing surface disinfection in clinical practice. Infect Control Hosp Epidemiol, 2014; 35: 1349–1355.
- Otter JA, Yezli S, Perl TM, Barbut F, French GL. The role of ‘no-touch’ automated room disinfection systems in infection prevention and control. J Hosp Infect, 2013; 83: 1–13.
- Ottawa (ON): Canadian Agency for Drugs and Technologies in Health. Non-manual techniques for room disinfection in healthcare facilities: a review of clinical effectiveness and guidelines, 2014.
- Andersen BM, Rasch M, Hochlin K, Jensen FH, Wismar P, Fredriksen JE. Decontamination of rooms, medical equipment and ambulances using an aerosol of hydrogen peroxide disinfectant. J Hosp Infect. 2006; 62:149–155.
- Otter JA, Yezli S, Perl TM, Barbut F, French GL. The role of ‘no-touch’ automated room disinfection systems in infection prevention and control. J Hosp Infect, 2013; 83: 1–13.
- Wax RS, Christian MD. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Canadian Journal of Anesthesia/Journal canadien d'anesthésie. 2020; 67(5): 568-576.
- Tang PF, Hou ZY, Wu XB, Zhang CQ, Wang JW, Xing X, et al. Expert consensus on management principles of orthopedic emergency in the epidemic of coronavirus disease 2019. Chinese medical journal. 2020; 133(9): 1096-1098.
- ACS. COVID 19: Consideration for Optimum Surgeon Protection. 2020.
- Ebola PPE Process “Buddy Checklist” per 10/21/14 CDC (10/27/14).
- Cheng L, Chen L, Xiao L, Zhang J, Cheng Y, Zhou L, et al. Problems and solutions of personal protective equipment doffing in COVID-19. Open Medicine. 2020; 15(1): 605-612.
- Fu Q, Zhang X, Li S. Risk management strategies for occupational exposure of medical staff with novel coronavirus infections. Chinese J Nosocomiol. 2020; 6: 1–5.
- Centers for Disease Control and Prevention. Recommended guidance for extended use and limited reuse of n95 filtering facepiece respirators in healthcare settings - NIOSH Workplace Safety and Health Topic, 2020.
- Radonovich LJ Jr, Cheng J, Shenal BV, Hodgson M, Bender BS. Respirator tolerance in health careworkers, JAMA 2009; 301: 36-38.
- Rebmann T, Carrico R, Wang J. Physiologic and other effects and compliance with long-term respirator use among medical intensive care unit nurses. Am J Infect Control, 2013; 41: 1218-1223.
- Fisher EM, Shaffer RE. Considerations for recommending extended use and limited reuse of filtering facepiece respirators in health care settings. J Occup Environ Hyg, 2014; 11: D115-28.
- Zamora JE, Murdoch J, Simchison B, Day AG. Contamination: a comparison of 2 personal protective systems. CMAJ, 2006; 175: 249-254.
- Government of Canada. Infection prevention and control for novel coronavirus (2019-nCoV): interim guidance for acute healthcare settings, 2020.
- Wax RS, Christian MD. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Canadian Journal of Anesthesia/Journal canadien d'anesthésie, 2020; 67(5): 568-576.
- Gottlieb M, Holladay D, Burns KM, Nakitende D, Bailitz J. Ultrasound for airway management: an evidence-based review for the emergency clinician. Am J Emerg Med, 2019.
- Fan E, Brodie D, Slutsky AS. Acute respiratory distress syndrome: advances in diagnosis and treatment. JAMA, 2018; 319: 698-710.

