Pleiotropic Effect of Metformin in COVID-19
Adwiza Rai, Jaysheela J*, Somasundaram G*
Department of Pharmacology, Sri Lakshmi Narayana Institute of Medical Sciences, India
Received Date: 18/10/2021; Published Date: 16/11/2021
*Corresponding author: Dr. J Jayasheela, Assistant Professor, Department of Pharmacology, Sri Lakshmi Narayana Institute of Medical Sciences, Pondicherry, India
Dr. G Somsundram, HOD Pharmacology, Sri Lakshmi Narayana Institute of Medical Sciences, Pondicherry, India
Aditya Rai 4th Year MBBS, Sri Lakshmi Narayana Institute Of Medical Sciences
Abstract
The outbreak of the coronavirus disease COVID-19 is caused by Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) which is highly transmissable and has thus been an ongoing pandemic since 2019, with infected cases over 219 million and death count up to 4.9million leading to constant threat to human health and safety. Coronaviruses are enveloped positive sense RNA viruses, which replicate in the host cell cytoplasm and possess a 5’ capped RNA and also contain the longest RNA among all RNA viruses. The majority of COVID 19 cases are asymptomatic or show mild to moderate symptoms but a low percentage may experience severe respiratory failure. It is diagnosed by nasopharyngeal swab by real time reverse transcriptase polymerase chain reaction (RT PCR). COVID-19 produces symptoms like fever, dry cough, breathlessness, and loss of smell and taste. It can affect both upper and lower respiratory tract depending on viral load. There have been many clinical trials going on to test possible therapy in treatment, few older drugs have been approved by FDA that have shown positive response one of them is Metformin which is used in clinical practice as an anti-diabetic agent. Metformin has been suggested to have anti-inflammatory properties and exert antioxidant actions which could lend support to a potential benefit in the occasion of major inflammatory reactions occurring in COVID-19. Thereby serving a potential treatment for individual hospitalized of COVID-19 with obesity or type 2 diabetes.
Keywords: COVID-19; Diabetes; Drug repurposing; Metformin; SARS-CoV-2; T2DM; Cost effective
Introduction
Coronaviruses belong to the Coronaviridae family. Corona represents crown-like spikes on the outer surface of the virus; and thus, it was named coronavirus. Coronaviruses are 65-125 nm in diameter and have positive sense single-stranded RNA as nucleic material, size ranging from 26 to 32 kilo bases (kb) in length. The subgroups of the coronavirus family are alpha (α), beta (β), gamma (γ), and delta (δ)[1]. Being just like its homologous virus, SARS COV, which infected thousands of individuals in 2003, but COVID-19 has lower severity and mortality as compared to SARS COV but is more transmissive affecting all age groups. Counting SARS coronavirus 2 (SARS-Cov2), there are currently seven human coronaviruses strains known to infect humans which belong to the α and β coronavirus genera. Same as SARS and MERS, this new coronavirus, 2019-nCoV, is considered to be zoonotic in origin and could be transmitted through the respiratory tract, through direct contact, and possibly through patients’ excreta, which may contain the living virus [2]. Disease can be asymptomatic or symptomatic. Most common clinical symptoms are fever, dry cough, breathlessness in moderate to severe cases, and chills in other to non specific symptoms such as headache, fatigue, dyspnea, SARS-CoV-2 seems to have high pathogenicity and transmissibility [3]. It is a disease that causes severe lung injury and multiple organ damage in severe cases which necessitates the development of new drugs. Excessive inflammation is linked to the severity and fatality of COVID-19 cases. It has therefore become a global threat to human health and declared pandemic.
To deal with ongoing situation many potential treatments are imposed and undergoing clinical trials to test their efficiency. One of these is METFORMIN. Diabetes is a major comorbidity in COVID-19 patients [4] 20-50% of people had diabetes during COVID-19 pandemic depending on global region [5].
Metformin was initially discovered from plant Galega officinalis (also known as goat’s rue) and was a traditional herbal medicine in Europe. Found in 1918 as an anti-influenza medication and lowering glucose levels was one of its side effects [6]. This property was explored by French diabetologist Jean Sterne, who reported the use of metformin to treat diabetes for the very first time in 1957. Many of the inflammatory and coagulopathic molecules implicated in Covid-19 morbidity are secreted by adipocytes, specifically visceral adipocytes, including interleukin-6 (IL-6), tumour necrosis factor (TNFα),D-dimer, and others.TNFα has been particularly of Covid-19 patients lung tissue. TNFα contributes to insulin resistance, and levels are higher in people with type 2 diabetes (T2DM). T2DM and obesity both have lower levels of the anti-inflammatory cytokine IL-10. Metformin, being the first-line treatment for type 2 diabetes (T2DM), reduces TNFα and IL-6 while increasing IL-10 levels is currently approved by the Food and Drug Administration (FDA) as a first-line medication for type 2 diabetes [7-10].
It has an immunomodulatory activity that reduces the production of proinflammatory cytokines using macrophages and causes the formation of neutrophil extracellular traps (NETs). Different uses of metformin were identified over time, and the benefits of metformin for various diseases and even ageing were affirmed. Cancers (e.g., breast cancer, endometrial cancer, bone cancer, colorectal cancer, and melanoma), obesity, liver disease, cardiovascular disease, and renal disease are some of these diseases. Metformin exerts its effects via various signaling pathways.
Metformin was seen in animal studies to reduce inflammation and protect against acute lung injuries. First, metformin treatment significantly reduces lung destruction caused by lipopolysaccharide (LPS) and acute lung injury caused by paraquat [11]. Second, metformin protects the lungs from the high pressures of mechanical ventilation. Furthermore, in a bleomycin mouse model, metformin reverses established fibrosis of injured lungs. These findings, including the anti-inflammatory effects, suggest that metformin has the potential to reduce the inflammation and lung injuries associated with severe COVID-19 infection.
Chemical Structure
Metformin Hydrochloride is the hydrochloride salt of the biguanide metformin with antihyperglycemic and potential antineoplastic activities which is commonly used drug having a good efficacy and tolerability, few side effects, and also being reasonably priced. It is a white, hygroscopic crystalline compound. Chemically it is N, N-dimethylbiguanide hydrochloride. with a molecular formula of C4H11N5•HCl (Figure a and b) and a molecular weight of 165.63, freely soluble in water and is practically insoluble in acetone, ether, and chloroform.
Metformin is an unusually hydrophilic drug that mostly exists in a positively charged protonated form under physiological conditions with pKa (12.4) [12].
Wang and colleagues reported an endocytosis process, pH- and receptor-dependent. Endosomal pH is a crucial factor for virus survival within the host cell virus and the ACE2 receptor into the endosome and recycling of the ACE2.The vacuolar ATPase (V-ATPase) and endosomal Na+/H+ exchangers (eNHEs) as the primary regulators for endosomal pH are known targets for Metformin. Hence, metformin can increase the cellular and endosomal pH and suppresses the endocytic cycle and virion maturation receptors back to the surface of the cell surface membrane [13].

Figure a: Skeletal formula of Metformin [14]
Figure b: Ball-and-stick model of the metformin molecule, C4H11N5 [15].
Biochemistry
Metformin inhibits complex I (NADPH: ubiquinone oxidoreductase) of the mitochondrial respiratory chain, thereby increasing the cellular AMP to ATP ratio and leading to activation of AMP-activated protein kinase (AMPK) and regulating AMPK-mediated transcription of target genes.
Mechanism of Action
The AMPK-pathway plays an important role in metformin actions. Metformin improves glycemia by lowering hepatic gluconeogenesis, primarily through AMP-activated protein kinase (AMPK) Thr 172 [16]. AMPK activation helps improve hepatic insulin sensitivity and glucose usage in the gut, while also promoting glucagon-like peptide-1 (GLP-1) secretion and altering the gut microbiome. Metformin is shown to have cytokine-lowering effects in both diabetic and non-diabetic patients, that may be of prime significance in Covid-19. Treatment with metformin in covid-19 patients with diabetes increases protein level of phosphorylated AMPK in high-glucose-treated endothelial cells. The phosphorylated AMPK further phosphorylates multiple downstream effectors regulating cellular metabolism and energy homeostasis, thereby providing protective effect.
Figure 3: The primary action of metformin is to decrease gluconeogenesis in the liver, and this is believed to occur through inhibition of mitochondrial complex 1, resulting in changes in the ratio of AMP/ATP and ADP/ATP and the concomitant activation of AMPK. Activation of AMPK results in the activation or inhibition of numerous downstream pathways affecting liver metabolism, including gluconeogenesis. AMPK activity may also be modulated by metformin through kinases such as ATM or STK11. Metformin may also alter muscle glucose utilization through activation of muscle cell AMPK.

Administration [18]
Adult Dose: 500 mg orally twice a day or 850 mg orally once a day
Pediatric Dose (10years or older): 500 mg orally twice a day or 850 mg orally once a day
Pharmacokinetics
Metformin has an average half-life in plasma of about 6.2 hours and remains unmetabolized, hence excreted unchanged in urine by tubular secretion [19-20]. Metformin is distributed and appears to accumulate in red blood cells, with a significantly longer elimination half-life of 17.6 hours.
Contraindications & Side Effects
Drug is contraindicated in people with:
- Severe renal impairment [21]
- Those having known hypersensitivity to metformin [21]
- Acute or chronic metabolic acidosis [21]
It is also cautiously to be used in the people with less severe renal impairment, people with age 65years or [22], any hypoxic state, chronic alcohol consumption.
Metformin has some known side effects such as gastrointestinal irritation including diarrhea, nausea, vomiting, increased flatulence and potentially fatal lactic acidosis [23].
Lactic acidosis is characterized by an abnormal increase in lactic acid levels in the body, causing symptoms such as muscle pain, abdominal pain as well as respiratory distress. Lactic acidosis is also seen in COVID-19 infection; henceforth, metformin should be used with caution in this condition [24].
Clinical Evidence
According to recent reports both diabetes and obesity are associated to severe complications in SARS-COV-2 infection [25].
A retrospective observational study was conducted on diabetic patients with confirmed COVID-19 infection, discharged or died from January 27’2020 to March 24’2020 at Tongji hospital, Wuhan, China. All of the patients were kept anonymous. The study was approved by Tongji Hospital’s Ethical committee [26].
The inclusion criteria included any form of body contact with confirmed cases within 14 days were referred as an exposure history. Clinical features such as fever, computed tomography (CT) images with signs such as patch ground-glass opacities, and laboratory tests showing a decrease in both leukocytes and lymphocytes are included. A patient with an exposure history can be considered a suspect if any two of the clinical features appear but an exposure-free patient can only be suspected if all three clinical features are present.
According to the National Health Commission of China’s Diagnosis and Treatment of Pneumonia Infected by Novel Coronavirus patients were classified as
- Mildly ill (clinical symptoms mild, no signs of pneumonia on CT),
- Moderately ill (clinical symptoms like fever and respiratory distress, and CT images showing signs of pneumonia),
- Seriously ill (respiratory rate: >30 breaths/minutes; resting oxygen saturation: <93 percent; or PaO2/FiO2 ratio: <300 mmHg), and
- Critically ill (respiratory failure and mechanical ventilation, shock or intensive care required).
The exclusion criteria were a
- hospital stay or medication course of less than three days,
- an age of 85 years,
- and lack of information about laboratory parameters at admission.
Statistical Analysis [26]
Study included 283 diabetic patients who were infected with COVID-19.
For at least three days, 104 (metformin group) were given metformin alone or in combination with other medications. The remaining 179 patients (no-metformin group) were given one or multiple anti diabetic medication other than metformin.
53 (51.0%) of the metformin group and 103 (57.5%) of the non-metformin group participants were males (P = 0.28), and the mean age in the two groups were 63.0 (55.8–68.3) for metformin and 65.0 (57.5–71.0) years for non-metformin (P = 0.06).
At admission, there were no significant differences between the two groups in terms of gender, age, underlying diseases, clinical severity, or oxygen-support category.
Outcome- A total 104 out of 283 patients (36.74) in the metformin group compared to 179 of 283 (63.25) had COVID -19 related hospitalization or death from any cause (odd ratio; 4.36; confidence interval [CL] 1.22-15.59; P=0.02). There was no significant difference found in the hospital stay between the two groups (P=0.74) but a significant reduced in mortality of metformin group was observed 3/104 (2.9%) as compared to non-metformin group 22/179(12.3%) (P=0.01).
Cost Implications
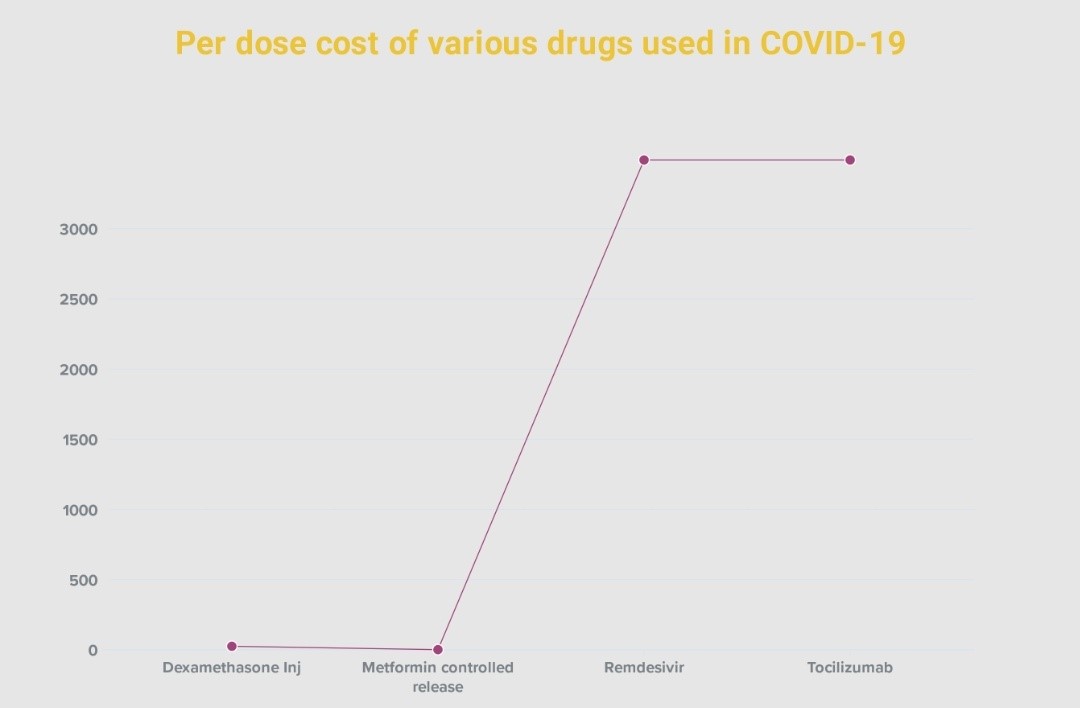
Along with therapeutic efficacy and safety, the third aspect of a successful medication regime is drug cost. The current medications used for COVID-19 treatment are not available in generic form, making them prohibitively expensive for patients in the middle/lower socioeconomic groups. Thus, in such emergency situations where recommended drugs are unaffordable or inaccessible due to a lack of supply, metformin, one of most affordable anti-diabetic drugs, can be reconsidered as an adjunctive therapy in treating COVID-19. Metformin, in its generic form, is widely prescribed. A 56-tablet pack of 500 mg dosage costs £1.67 in the United Kingdom; a 100-tablet pack of 500 mg costs around $8 in the United States; and a 10-tablet pack costs INR 22 in India [27].
Hill AM, Barber MJ, Gotham D estimated a cost of production of 148 medicines on essential medicine list of WHO that most of the essential drugs can be manufactured at low cost. They estimated the average of Average API price per kilogram was in the range of US$1–US$10/kg for 5 medicines which included metformin [28].
Chart 1: Described the cost comparison of various drugs like dexamethasone, metformin, remdesivir and tocilizumab which are prescribed in covid-19, remdesivir mrp was fixed by the major manufacturers after intervention of government on 17th april 2021 [30].
Conclusion
The world watches the safety and effectiveness of approved vaccines for protection against new SARS-CoV-2 infections, even though strict measures like worldwide lockdowns double masking, social distancing implemented to restrict the virus’s spread.
Despite the fact that key information about COVID-19 was collected in record time, much about how viral infection contributes to disease advancement and vastly differing severity in different individuals is still very much unknown.
However, it is evident that hyperglycemia and diabetes-related comorbidities accelerated severity of disease and mortality in COVID-19 patients.
Blood glucose levels must therefore be responsibly monitored and controlled in diabetic COVID-19 patients to improve prognosis and life expectancy. Metformin, an anti-diabetic drug, has potential antiviral, antioxidant, nephroprotective, cardioprotective, vasculoprotective, immunomodulatory, anti-inflammatory, antiproliferative effects and therefore could be re – purposed for the treatment of COVID-19. Besides this, metformin is considered to be safe, well-tolerated by patients, has very few side effects, it has been off-patent since 2002 29 (hence cost-effective), and can therefore be cost effective for the COVID-19 patients who may benefit from metformin intervention.
Thus, in detailed trials of metformin will help to understand its precise mechanism of action and efficacy enabling to gain approval for its use in the current pandemic.
Acknowledgement
References
- Shereen A, Khan S, Kazmi A, Bashir N, Siddique R. “COVID-19 infection: origin, transmission, and characteristics of human coronaviruses,” Journal of Advanced Research, 2020; 24: pp. 91–98.
- Zhang H-W, Yu J, Xu HJ, et al. “Corona virus international public health emergencies: implications for radiology management,” Academic Radiology, 2020; 27(4): pp. 463–467.
- Han Y, Yang H. “The transmission and diagnosis of 2019 novel coronavirus infection disease (COVID-19): a Chinese perspective,” Journal of Medical Virology, 2020; 92(6): pp. 639-644.
- Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020; 94: 91–95. Doi: 10.1016/j.ijid.2020.03.017
- Bornstein SR, Rubino F, Khunti K, Mingrone G, Hopkins D, Birkenfeld AL. Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol. 2020; 8(6): 546-550. doi: 10.1016/S2213-8587(20)30152-2
- Amin S, Lux A, O’Callaghan F. The journey of metformin from glycaemic control to mTOR inhibition and the suppression of tumour growth. Br J Clin Pharmacol. 2019; 85: 37-46. Doi: 10.1111/bcp.13780.
- Bray GA. Medical consequences of obesity. The Journal of clinical endocrinology and metabolism. 2004; 89(6): 2583–2589.
- Liu L, Feng J, Zhang G, et al. Visceral adipose tissue is more strongly associated with insulin resistance than subcutaneous adipose tissue in Chinese subjects with pre-diabetes. Current medical research and opinion. 2018; 34(1): 123-129.
- Hawley PC, Hawley MP. Difficulties in diagnosing pulmonary embolism in the obese patient: a literature review. Vasc Med, 2011; 16(6): 444-451.
- Ingraham NE, Lotfi-Emran S, Thielen BK, et al. Immunomodulation in COVID-19. Lancet Respir Med, 2020.
- Zhang, X, Shang, F, Hui, L, Zang, K, Sun, G. The alleviative effects of metformin for lipopolysaccharide-induced acute lung injury rat model and its underlying mechanism. Saudi Pharm J. 2017; 25: 666-670. Doi: 10.1016/j.jsps.2017.05.001
- Kim J, Lee H-Y, Ahn J, Hyun M, Lee I, Min K-J, et al. NHX-5, an Endosomal Na+/H+ Exchanger, Is Associated with Metformin Action. J Biol Chem. 2016; 291(35): 18591–18599. Doi: 10.1074/jbc.C116.744037. pmid:27435670o
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, et al. Role of AMP-activated Protein Kinase in Mechanism of Metformin Action. J Clin Invest, 2001; 108(8): 1167-1174. Doi: 10.1172/JCI13505.
- Fvasconcellos 2007; 21:15 (UTC).
- Hariharan M, Rajan SS, Srinivasan R. X-ray crystallographic data. “Structure of metformin hydrochloride”. Acta Cryst. 1989; C45 (6): 911-913. DOI:10.1107/S0108270188014246
- Rena G, Hardie DG, Pearson ER. The Mechanisms of Action of Metformin. Diabetologia, 2017; 60(9): 1577–1585. Doi: 10.1007/s00125-017-4342-z
- Gong Li, Goswami Srijib, Giacomini Kathleen M, Altman Russ B, and Klein Teri E. Metformin pathways: pharmacokinetics and pharmacodynamics. Pharmacogenetics and genomics. 2012.
- “Metformin: medicine to treat type 2 diabetes”. Nhs.uk. 2020.
- Bristol-Myers Squibb (27 August 2008). “Glucophage (metformin hydrochloride tablets) Label Information” (PDF). U.S. Food and Drug Administration (FDA). Archived (PDF) from the original on 22 September 2010. Retrieved 8 December 2009.
- Robert F, Fendri S, Hary L, Lacroix C, Andréjak M, Lalau JD. “Kinetics of plasma and erythrocyte metformin after acute administration in healthy subjects”. Diabetes & Metabolism. 2003; 29(3): 279-283. Doi:10.1016/s1262-3636(07)70037-x. PMID 12909816
- “Metformin: medicine to treat type 2 diabetes”. Nhs.uk. 25 February 2019. Retrieved 15 October 2020.
- Contraindications to the use of metformin BMJ 2003; 326: 4.
- Bolen S, Feldman L, Vassy J, Wilson L, Yeh HC, Marinopoulos S, et al. (September 2007). “Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus”. Annals of Internal Medicine. 2007; 147(6): 386-399. Doi:10.7326/0003-4819-147-6-200709180-00178. PMID 17638715.
- Kow CS, Hasan SS. Metformin use amid coronavirus disease 2019 pandemic [published online ahead of print, 2020 May 2019] J Med Virol 2020 doi:10.1002/jmv.26090
- Bornstein S, Rubino F, Khunti K, et al. Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol. 2020; 8(6): 546–550. Doi:10.1016/S2213-8587(20)30152-2
- Luo P, Qiu L, Liu Y, Liu XL, Zheng JL, Xue HY, et al. Metformin Treatment Was Associated with Decreased Mortality in COVID-19 Patients with Diabetes in a Retrospective Analysis. Am J Trop Med Hyg. 2020; 103: 69-72.
- Drug information on Glyciphage (500 mg) (Metformin) From Franco-Indian Pharmaceuticals Pvt. Ltd.
- Hill AM, Barber MJ, Gotham D. Estimated costs of Production and potential prices for the WHO Essential Medicines List. BMJ Global Health. 2018; 3: e000571.
- Samuel SM, Varghese E, Kubatka P, Triggle CR, Büsselberg D. Metformin: The Answer to Cancer in a Flower? Current Knowledge and Future Prospects of Metformin as an Anti-Cancer Agent in Breast Cancer. Biomolecules. 2019; 9(12): 846. Doi: 10.3390/biom9120846. PMID: 31835318; PMCID: PMC6995629.
- https://www.nppaindia.nic.in/wp-content/uploads/2021/04/Doc1128161044.pdf
- https://www.nppaindia.nic.in/en/utilities/list-of-notified-prices/dpco-2013/

