From Extracellular Vesicles to Global Environment: A Cosmopolite Sars-Cov-2 Virus
Veronika Kralj Iglič1*#, Raja Dahmane1#, Tjaša Griessler Bulc1,2#, Polonca Trebše1#, Saba Battelino3,4, Mojca Bavcon Kralj1, Metka Benčina5, Klemen Bohinc1, Darja Božič1,6, Mojca Debeljak7, Drago Dolinar8,9, Aleš Iglič4,6, Darja Istenič1, Zala Jan1, Monika Jenko9, Marko Jeran1,6, Gregor Jereb1, Mojca Jevšnik1, Aleksandra Krivograd Klemenčič2, Tomaž Lampe1, Irina Milisav1,4, Andreea Oarga-Mulec10,11, Andrej Ovca1, Borut Poljšak1, Franja Prosenc1, Anna Romolo1, Nina Resnik12, Robert Sotler1, Anja Šoštarič1,8, Vid Šuštar13, Urška Šunta1, Urška Štibler6, Bojana Uršič1,4 and Domen Vozel3,4
1University of Ljubljana, Faculty of Health Sciences, Slovenia
2University of Ljubljana, Faculty of Civil and Geodetic Engineering, Slovenia
3University Medical Centre Ljubljana, Department of Otorhinolaryngology and Cervicofacial Surgery, Slovenia
4Faculty of Medicine, University of Ljubljana, Slovenia
5Jozef Stefan Institute, Jamova 39, SI-1000 Ljubljana, Slovenia
6University of Ljubljana, Faculty of Electrical Engineering, Slovenia
7University Rehabilitation Institute, Republic of Slovenia, Slovenia
8University Medical Centre Ljubljana, Department of Orthopaedic Surgery, Slovenia
9MD-RI Institute for Materials Research in Medicine, Bohoričeva 5, Slovenia
10Slovenian National Building and Civil Engineering Institute, Dimičeva ulica 12, SI-1000 Ljubljana, Slovenia
11University of Nova Gorica, Vipavska cesta 13, Slovenia
12Kostak Company, Utilities Management and Civil Engineering, Slovenia
13Lymphocyte Cytoskeleton Group, Medical University of Turku, Finland
14University Medical Centre Ljubljana, Clinical Department of Urology, Slovenia
# These authors have contributed equally to the work; other authors are given in alphabetical order.
Received Date: 13/08/2020; Published Date: 27/09/2020
*Corresponding author: Veronika Kralj-Iglič, University of Ljubljana, Faculty of Health Sciences, Zdravstvena pot 5, SI-1000 Ljubljana, Slovenia, veronika.kralj-iglic@fe.uni-lj.si
Abstract
Within the micro and nano world, tiny membrane-enclosed bits of material are more or less free to move and act as communication tools within cells, between cells, between different tissues and between organisms in global environment. Based on the mechanism of membrane budding and vesiculation that includes all types of cells, in this review, we attempted to present a review on SARS-CoV-2 virus actions in compartments of different scales (cells and their surroundings, tissues, organisms and society). Interactions of the virus with cells on a molecular level, with neural system, endothelium, hematopoietic system, gastrointestinal system and genitourinary system. Transmission route between organisms and between mother and fetus are considered. Also, transmission of virus through contact with materials and with environment, the suggested measures to prevent contamination with the virus and to support the organism against the disease are given.
Keywords: Virus, Extracellular vesicles; COVID-19; SARS-CoV-2; Membrane vesicluation
Lilliput Particles
Living cells are composed of constituents of immense diversity and the processes that take place in living organisms are amazingly complex. Yet, human have reached an understanding of some laws of nature in an intention to direct and change the time course of these processes, in particular, to cure the disease. The things are subject to laws of nature, therefore understanding of these laws is key in attempts to master the health and disease.
It is important to gather evidences on various features of the system of interest. However, stacking the facts is just a foundation that should be upgraded by synthesis to be useful for prediction. Specific and sophisticated descriptions can be of help to resolve well-targeted problems. However, despite a present emerging need to resolve the problem of COrona VIrus Disease 2019 (COVID-19), the narrow target has not yet been identified. One possibility is that with increasing effort and a large number of skilled scientists working on the problem, a specific target molecule and a specific mechanism will be identified and an effective cure made. It could be just a matter of time when the resolution would be first spotted by someone. The other possibility is that such a solution, in principle, does not exist within the considered specific substances and processes. Pieces of evidence are exposing this disease in several manifestations and involving different tissues (respiratory system, gastrointestinal system, urinary system, cardiovascular system, neural system, urinary system, visual system, bone marrow, and blood). It indicates that the roots of the disease may be a fundamental mechanism that underlies cells in general and is not specific for one particular molecule or some of them. However, it can be interpreted that the target may be sought by directing the attention also to the biophysical properties of the membranes.
Within the micro and nano world, tiny membrane-enclosed bits of material (let us name them lilliput particles) are more or less free to move. Based on the evidence on their morphology and composition, viruses can be described as lilliput particles of genetic material. Their size extends over more than an order of magnitude: from the smallest DNA containing Adeno-Associated Virus (AAV) with the average size of about 20 nm [1] to Mimivirus with the average diameter about 750 nm [2]. There is another family of kindred lilliput particles that are called Extracellular Vesicles (EVs), defined as »particles naturally released from the cell that is delimited by a lipid bilayer and cannot replicate [3]. However, the lilliput particles, including enveloped viruses, EVs, cells, and also artificial membrane vesicles, are all united in one essential feature. They are subjected to the laws that determine the morphology of their membranes. These membranes have in common the bilayered lipid backbone structure reflecting the hydrophobic effect that is imposed upon the constituents, and the crucial role of the curvature created by constituting molecules. Furthermore, as membrane constituents are more or less free to move laterally over the membrane, they sort into regions of favourable curvature. Lipids such as free fatty acids or lysophosphatidic acid (inverted cone lipids) are known to promote positive curvature by introducing inverted cone shape into the membrane, while cone-shaped lipids like phosphatidylethanolamine, lysolipids or phosphatidic acid tend to adopt negative curvature [4].
Curvature-driven redistribution of constituents, in turn, affects direct interactions between them and induces the formation of platforms that enable specific and non-specific functional roles. It was suggested that viruses bud from lipid rafts [5].
As the lilliput particles are smaller, the curvature effects become increasingly important as regards the sorting mechanism. Virions require a membrane configuration, favorable for fusion which is dependent on lipid composition [6]. The studies on EVs have shown that lipid components such as phosphatidylserine (PS) or cholesterol have an important role in membrane-particle uptake [7-9]. The adopted membrane composition therefore may contribute to the infectivity of the virus and is an important element in the virus pathogenicity; it was found that the composition of the isolated virion membrane differs from that of the host cells and differs also between different types of viruses whereas the shape of the lipid molecules could play an important role [4].
However, the shape itself is of crucial importance, and similar shapes can be attained by molecules with different primary structures. We can say that the effect is not specific as regards the chemical properties of the molecules, e.g., the chemical reactions that may take place, but is specific as regards the shape of membrane constituents that make up the membrane. Hitherto, this aspect seems to have been neglected comparing to the importance ascribed to genetic issues (classification of viruses according to the RNA/DNA content), membrane composition (chemical composition of the membranes) and chemical and biological manifestations connected with production of small particles (which is described as virion replication).
Virions cannot reproduce independently, so their life cycle depends on finding a suitable host, using its reproductive machinery for proliferation, and lysing the host to release its progeny into the environment. If the interaction of the virion with the cells targets specific cell types and the identity of the virion is continued with replication, a possibility of suppressing the effect by involving a particular immune response or a particular metabolic route would seem useful. Virion can contain a specific molecule or a group of molecules (receptors), which act as recognition structures. The adsorption of viruses is mediated by proteins, carbohydrates, or glycolipids on the host's membrane surface. After the virus attaches to the host's surface, its viral DNA or RNA may enter either by fusion with the surface or by endocytosis, and initiate a latent or lytic cycle. Latency is a dormant phase, during which the viral DNA is replicated alongside the host's DNA until a particular biochemical signal appears and induces the lytic phase.
The mechanisms of formation of lilliput particles are not fully understood. Furthermore, although it may help recognise generity, properties, and effects of different types of viruses, the classification does not necessarily pose sharp borders between particles found in reality. Therefore, for some diseases caused by viruses, the cure has not been found. For example, the Ebola virus was recognised as a highly pathogenic filovirus causing severe haemorrhagic fever with high mortality rates. The Ebola virus disease outbreak in Africa from 2013 recorded 28616 cases with a mortality rate of about 70% [10]. Marburg virus disease is a highly virulent disease in humans that causes haemorrhagic fever, with a fatality ratio of up to 88% in previous outbreaks. [11] The Marburg virus disease largest outbreak in 2005 in Angola recorded 274 cases with a mortality rate of 88%. [11] The most recent record of the Marbourg virus is from 2014 in Uganda, where there was one patient who died. [11] Both Ebola and Marburg viruses present with tubular particles of different lengths (Figure 1).
Clearly, the medical cause to better understand the mechanisms of the virus-induced diseases has long been present and considerable efforts have been exerted with that intention, yet to our best knowledge, an effective cure for some diseases based on a well-defined mechanism and targeted therapy does not exist. Facing a Severe Acute Respiratory Syndrome CoronaVirus 2 (SARS-CoV-2) and the COrona VIrus Disease19 (COVID-19) with a considerably large number of affected subjects worldwide (in May 18, 2020 the number of cases reported exceeds 4,800.000 with mortality rate about 7% [12]) and significant economic and social issues, this cause has become even more urgent. As the membrane plays an essential role in the formation of virions, their movement, and interaction with cells, attention should also be devoted to membrane properties that are specific concerning a very general and fundamental issue that shapes the world from strings to galaxies - the curvature.
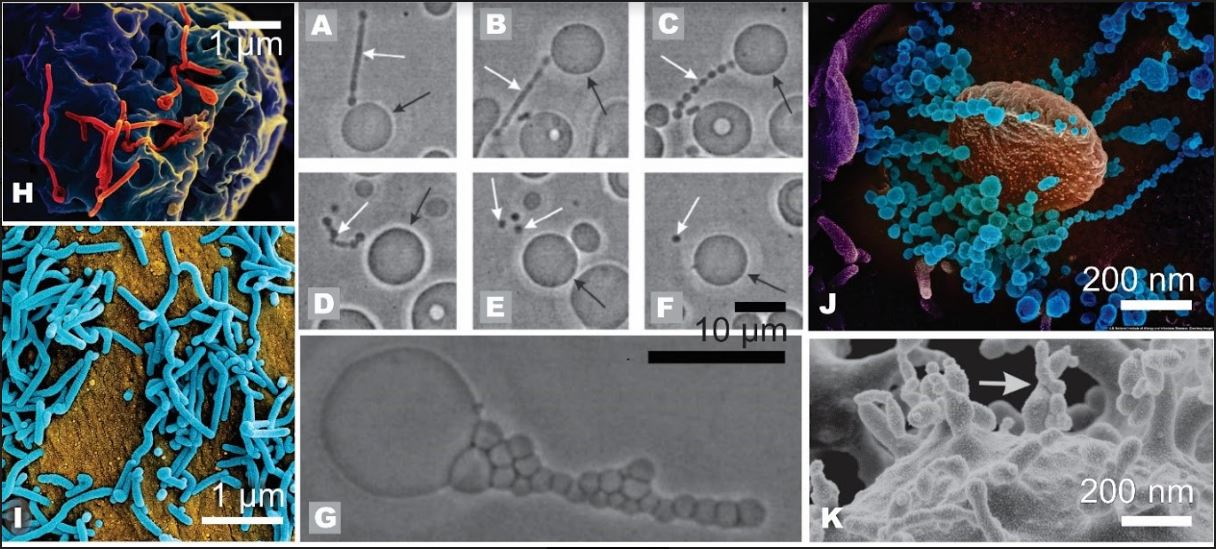
Figure 1. Lilliput particles in non-living and living systems: A-G: tubular budding of virions and spherical vesiculation in giant phospholipid vesicles (from ref.[13]); white arrows point to protrusions and daughter vesicles that are ultimately shed from the mother vesicle, black arrows point to the mother vesicle, H: tubular budding of Ebola virions (from ref.[14]), I: tubular budding of Marburg virions (from ref.[15]), J: SARS-CoV-2 virions (from ref.(16)), K: budding of the erythrocyte membrane induced by detergent (from ref.[17]). The scale measures in Panels H, I and J are tentative.
Leaving aside the characterisation and classification borders, the lilliput membrane-enclosed particles were found to act as communication tools within cells, between cells, between different tissues, and between organisms, taking with them also footprints of the environmental compartments. The SARS-CoV-2 virion has left evidences of its action in many systems. Based on the mechanism of membrane budding and vesiculation that includes all types of cells, in this review, we attempted to put together a mosaic of lilliput particles actions taking place in compartments of sizes ranging from nanometers to Earth environment, and the corresponding hypothetical mechanisms, with the emphasis on the SARS-CoV-2 which has exposed the life on Earth as one communicating compartment.
Mechanisms of Interaction of Virus with the Cell
SARS-CoV belongs to a family Coronaviridae causing respiratory infections in humans and animals [18]. Electron microscopic examination of infected cell samples showed lilliput particles within dilated cisternae of the rough endoplasmic reticulum of sizes 80~140 nm (Figure 1), with a halo indicating a protein corona [18]. Accumulating reports indicate that SARS-CoV-2 virions may adhere to the host cell by the interaction of its membrane Spike (S) protein with the host membrane protein angiotensin-converting enzyme 2 (ACE2), followed by the fusion of the viral and host membranes and inclusion of the virion contents into the cell. The translation of the replicase-transcriptase, replication of genome and transcription of mRNAs induces the assembly and budding of newly packaged virions [19].
Coronaviruses are enveloped RNA viruses; they are distributed and transmitted broadly among humans, other mammals, and birds. They cause respiratory, enteric, hepatic, and neurologic diseases [19] in human and animal [20]. Based on virus genome sequencing results and evolutionary analysis, the bat has been suspected as the natural host of virus origin, and it was found that the genome sequence of SARS-CoV-2 was 96.2% identical to a bat CoV RaTG13 [21]; SARS-CoV-2 could have been transmitted from bats to infect humans [22]. Alignment of receptor protein sequences implied high similarity between many species. This fact provides the possibility of alternative intermediate hosts, such as turtles, snakes, and pangolins [23]. SARS-CoV-2 S-protein efficiently interacts with the human ACE2 receptor [24,25], which is expressed in several organs, including the lung, heart, kidney, and intestine. However, the virus is expected to first get in contact with the epithelial barriers of the body – skin, respiratory and gastrointestinal tract or eye mucosa).
ACE2 expressing type II alveolar epithelial cells appear to be the prime target of viral invasion [26,27]. However, according to the reports on symptoms and receptor expression, the first line targets may also include a basal layer of non‐keratinizing squamous epithelium in the nasal and oral mucosa [26,28]. The symptoms include other organs expressing ACE2 (lung alveolar epithelial cells, endothelial cells, arterial smooth muscle cells, heart, kidneys, testes, and gastrointestinal system [26,29-34]. Previous studies on SARS-CoV, Middle East Respiratory Syndrome Corona Virus (MERS-CoV) and SARS-CoV-2 have shown that coronaviruses can infect even tissues in which the expected expression of the specific receptors is relatively low – namely liver and brain [35-38]. Lilliput particles were identified in multiple human organs of affected subjects. In lungs, SARS-CoV virions were located predominantly in the cytoplasm of bronchiolar and alveolar epithelial cells, but also in a part of macrophages and endothelial cells, in infiltrated lymphocytes, in renal tubular epithelial cells, in parenchymal cells, in sinusoid capillary endothelial cells of adrenal glands, in the cytoplasm of mucosal and crypt epithelial cells, in cardiomyocytes, in macrophages/histocytes, in sinusoid endothelial cells, in lymphocytes found in thoracic and celiac lymph nodes, in testicular epithelial cells and Leydig's cells [38]. SARS-CoV could attack multiple target cells, implicating that it might cause multi-organ damages, with lungs as the predominant organ of injury [38].
The S protein that enables SARS-CoV-2 entry into the cell is a homotrimer. Each subunit is functionally divided into two domains (S1 and S2). The S1 domain is enrolled in binding, while the S2 domain mediates membrane fusion [39]. According to the evidence, two proteolytic cleavages are needed for the activation of S proteins [40]. Availability of the S protein activating proteases is presumed to determine target tissues mainly and whether CoVs enter target cells through plasma membrane or endocytosis. It was observed that SARS-CoV-2 could be cleaved by serine proteases TMPRSS2 (Expressed in type II pneumocytes in the lung), and human airway trypsin-like protease [41], while it also can enter the cells through endocytosis and is cleaved by cathepsin L [24,25]. ACE2 and TMPRSS2 were found to be co-expressed by type II pneumocytes, facilitating infection by activating the SARS S protein for cell-cell and virus-cell fusion and diminishing viral recognition by neutralising antibodies [42]. However, it was found that due to the low thermostability of S-protein of SARS-CoV-2, the conformational changes needed for membrane fusion can occur directly upon the receptor binding, without exogenous protease priming or activation [25]. In this aspect, the fever generated in the infected body might promote viral infectivity through the activation of S-protein and also through the increase of membranes' fluidity (both viral and cell); further studies would be necessary to confirm this hypothesis. Besides ACE2, other receptors such as C-type lectin DC-SIGN (or CD209), human CD209L (or liver/lymph node-specific (L)-SIGN, and DC-SIGNR) and LSECtin were identified as interacting with S-protein of SARS-CoV [43].
Several innate immune signalling proteins are potentially targeted by SARS-CoV-2 virion proteins [44] supporting the hypothesis of inhibition of early immune reactions observed in previous coronavirus diseases [44,45]. The viral proteins are the key molecules in host immune modulation. The potential interactions of SARS-CoV-2 proteins with human proteins (in brackets) interfere with the IFN pathway by Nsp13 (TBK1 and TBKBP1), Nsp15 (RNF41 / Nrdp1) and Orf9b (TOMM70), and the NF-κB pathway by Nsp13 (TLE1, 3, and 5) and Orf9c (NLRX1, F2RL1, NDFIP2), two E3 ubiquitin ligases (TRIM59 and MIB1) regulating the antiviral immune signalling can be bound by Orf3a and Nsp9, respectively [44]. It was suggested that coronaviruses influence especially the Type I interferon recognition and signalling; Orf6 (binding to NUP98-RAE1, an interferon-inducible mRNA nuclear export complex) is expected to antagonize the host interferon signalling by perturbing the nuclear transport [44]. It was found that Type I interferon response in patients who died was lower than in patients that recovered from the SARS-CoV-2 infection. Antigen presentation via major histocompatibility complex classes I and II was downregulated when the macrophages or dendritic cells were infected [22, 46].
Likely, the extensive immune response is triggered mainly by viral-proliferation induced host-cell necrosis and vascular leakage. The macrophage mobilisation, virion-mediated downregulation of ACE2, and subsequent lymphocyte infiltration contribute to promoting the inflammation [47]. The resulting hyperinflammation burst importantly contributes to the manifestation of COVID-19; secondary inflammatory response with cytokine storm syndrome was observed in a subset of patients with severe COVID-19 that frequently lead to fatal outcome [48,49]. Haemophagocytic lymphohistiocytosis is a type of hyperinflammatory syndrome that is accompanied with unremitting fever, cytopenias, and hyperferritinaemia, with a fulminate hypercytokinaemia that may lead to fatal multiorgan failure [48].
Interactions of Virus with the Tissues
Effects of SARS-CoV-2 infection on nervous system
It was suggested that neural system impairment could be at least partially responsible for the respiratory failure in COVID-19 patients [49]. Several symptoms related to neural system impairment had been reported in patients with COVID-19, including headache, nausea, and vomiting, fatigue, olfactory and/or taste disorders, prominent agitation and confusion, corticospinal tract signs, and encephalopathy [46,50-53]. Hypoxic encephalopathy was observed in substantial number of deceased patients [54], and a case of COVID-19–associated acute haemorrhagic necrotizing encephalopathy was reported [55]. In the latter case the presence of virus in the brain was not confirmed, and such manifestations were presumed to be caused by the intracranial cytokine storms, resulting in blood-brain-barrier breakdown [55]. The reports on prevalence of neural system related symptoms are extremely variable (from few percent to over 90), with higher percentages observed in studies explicitly focused to the neural system involvement, indicating that results are strongly biased by devoted attention (and are therefore not re-presented in this review). Also, weather the observed symptoms are specific to SARS-CoV-2 infection, cytokines, or the effects or withdrawal of medication still needs to be clarified [53]. However, the foreseen impacts of SARS-CoV-2 virions on the central nervous system include (i) direct induction of neurological alterations (ii) worsening of the pre-existing neurological conditions (iii) increase of susceptibility to damage or aggravation of the damage caused by other insults [56]. Also, consecutive long-term neurological disorders could be expected [57].
The haematogenous or neural propagation were described in previously investigated coronaviruses and are predicted as possible pathways also in case of SARS-CoV-2 [58,59]. In viremia stage of infection, the virus spreads throughout the body. The susceptibility of endothelium for SARS-CoV-2 infection, can result in infection-induced brain capillary damage, enabling entering of the virus through the impaired barrier into the neuronal milieu [58]. On the other hand, it was observed that in mice, SARS-CoV virus entered the brain primarily via the olfactory bulb; direct transneuronal transmission of infection resulted in quick viral spread to connected areas of the brain, including cortex, basal ganglia, and midbrain, and neuronal death was observed even in absence of inflammation [60]. A study on plant-derived vesicles reported similar indications on transfer of intranasally administrated vesicles to the brain, bypassing the immune response [61] while Moriguchi et al. (2020) [62] reported on a case where SARS-CoV-2 RNA was not detected in the nasopharyngeal swab but was detected in the cerebrospinal fluid of a patient.
Effects of SARS-CoV-2 infection on endothelium
ACE2 receptors are expressed also by endothelial cells [63]. The endothelial cells make up the wall of the blood vessels by establishing a multifunctional, semi-permeable cellular barrier at blood-tissue interface. The large total surface of the endothelium is exposed to lilliput particles, their products as well as to agents of the activated host defence. Lilliput particles may interact with the endothelium in different ways such as by triggering chemical reactions that involve receptors, release of pore-forming toxins, inducing membrane budding and vesiculation and changing the cell function by using its resources for their replication. These pathophysiological effects determine the endothelial phenotype, resulting in endothelial barrier dysfunction which influences also its interaction with blood cells, in particular leukocytes and platelets. Moreover, endothelial responses retroact on the lilliput particles as well as on the blood cells in a complex intertwined way. Endothelial activation was found to contribute considerably to inflammation and coagulation in the vessels, and in resulting clinical manifestations [64].
When an organ is affected by a viral infection, the infected cells react by releasing proteins called inflammatory cytokines, including interleukin-6, into the surroundings and also in blood. When interacting with endothelial cells, cytokines create small gaps between the endothelial cells. Taking advantage of the gaps created, several cells of the immune system, including neutrophils, macrophages and lymphocytes that are activated by inflammatory cytokines pass from the blood to the infected organ and trigger an immune response against the virus. However, if the cytokine response is produced in exaggerated quantities, the gaps between the cells are too large and may lead to the accumulation of liquid.
Increased inflammatory response was observed in patients with COVID-19 [65]. The researchers from Wuhan, China reported in a study that included in the final analysis 191 patients (137 survivors and 54 non-survivors) that non survivors had at admission statistically significantly higher levels of an inflammation marker interleukin-6 protein in their blood than survivors (11.0 pg/mL vs. 6.3 pg/mL) [66]. As the disease progressed towards death, the levels of interleukin-6 have been further increasing compared to those of patients who healed, reaching the average value higher than 25 pg/mL 20 days from illness onset [66]. These data indicated the presence of an inflammatory storm.
When present in exaggerated quantities, inflammatory cytokines also damage the epithelial cells. Accumulation of fluid in the lungs and the destruction of the epithelial cells were found in patients who had respiratory distress [67]. Furthermore, inflammatory cytokines can be spread around the body through blood and cause a multi-organ failure [67].
Effects of SARS-CoV-2 Infection on hematopoietic System
Presence of virions in blood was detected in 1% of patients with COVID-19 [68]. Lymphopenia and thrombocytopenia in COVID-19 patients was on the average related to poorer outcome [69]. Some studies reported lymphocytopenia in about 40 % of patients with confirmed SARS-CoV-2 infection [66,70], while another study reported lymphocytopenia in over 80 % of 1099 hospitalized COVID-19 patients on admission [71]. In 35-40% patients of this cohort, mild thrombocytopenia was detected [71]. In the study of Zhou et al. (2020) [66] including 191 patients with laboratory confirmed SARS-CoV-2 infection, blood concentrations of interleukin-6 (IL-6) in patients were higher than 5 pg/L, and elevated d-dimer (marker of increased coagulation activity) over 1 µg/mL was found associated with fatal outcome of COVID-19; Besides Lymphocytopenia and high d-dimer concentrations, leucocytosis, elevated alanine transaminase, lactate dehydrogenase, high-sensitivity cardiac troponin I, creatine kinase, serum ferritin, IL-6, prothrombin time, creatinine, and procalcitonin were associated with death. Decreased platelet count and the d-dimer (biomarker of platelet activation) greater than 1 µg/mL were identified as risk factors related to poor prognosis of COVID-19 [66]. Three potential mechanisms for thrombocytopenia development were suggested by Xu et al. (2020) [72]: (i) reduction of platelet production: destroy of haematopoietic cells, due to the infection of bone marrow and following destroy of the infected cells in the cytokine storm- (ii) autoimmune destruction of platelets due to the mimicry and cross-reactivity, couplet by infection-related augmentation of autoantibodies and immune complexes, (iii) increased platelet consumption in infected organs: the damage of tissues (primary endothelium) activating aggregation of platelets and microthrombi formation. The elevation of d-dimer in patients supports this hypothesis of intravascular coagulation. The possible thromboembolism may contribute to the secondary tissue damages [72].
The morphological changes of blood cells in 40 patients with COVID-19 evaluated at admission, before antiviral and anti-inflammatory treatment, were described by Zini et al., (2020) [73]. Abnormalities of the neutrophil lineage (crowded, dark granulations in the cytoplasm with agranular peripheral regions, band forms of nuclei and dysmorphic cells with total absence of nuclear segmentation, consistent with pseudo‐Pelger morphology), and platelets (large, often hyperchromatic platelets, with protruding pseudopodia) were observed, along with frequent apoptotic cells (liquefied nuclear chromatin and granulated or basophilic cytoplasm, probably dependent on cell type) were found in blood smears. Immature cells were also frequently present. In 7 re-observed patients changes largely disappeared after 5-7 days of anti-viral and anti-inflammatory treatment [73].
Effects of SARS-CoV-2 Infection on Gastrointestinal and Genitourinary Tract
ACE2 that is essential for SARS-CoV-2 to enter into a host cell [74] was considered to play an important role in gastrointestinal (GI) tract infection with SARS-CoV-2 [75]. In case of SARS-CoV-2 virions, binding affinity to human ACE2 was found to be 10—20 times higher than in case of SARS-CoV [76]. Intestinal ACE2 has been proposed to interfere with uptake of dietary amino acids, regulating the antimicrobial peptides and therefore homeostasis of the gut microbiota is disturbed [77]. SARS-CoV virions were found in the liver of SARS patients [78] while not in the MERS-CoV patients [79]. ACE2 was reported to be expressed in liver cholangiocytes at levels comparable to the lung alveolar type 2 cell expression. The ACE2 expression was 20-times lower in the liver hepatocytes, which are the main drug metabolizers [80]. This finding also implies a possible route of infection and direct damage to the bile ducts by SARS-CoV and SARS-CoV-2 virions. The enzyme gamma-glutamyl transferase (GGT), a diagnostic biomarker for cholangiocyte injury, was elevated in 54% (30 out of 56) of patients with COVID-19 during hospitalization at The Fifth Medical Center of PLA General Hospital, Beijing [81]. SARS-CoV-2 may therefore target several tissues, possibly through the same entry route. Hofmann and co-workers recently reported the crucial roles of ACE2 in viral infection and of serine protease TMPRSS2 in protein priming; the authors also demonstrated that the inhibitor of TMPRSS2 (already approved for clinical use) blocked the viral entry thus paving a path for the development of possible treatment [24].
Some COVID-19 patients presented gastrointestinal (GI) symptoms such as nausea, vomiting, decreased appetite, diarrhoea, GI bleeding and abdominal pain [82]. It was suggested that initial digestive symptoms of COVID-19 could be used for early detection, early diagnosis, early isolation, and early intervention [83]. Specific mechanisms involved in diarrhoea pathogenesis are not yet known in full, however, it was speculated that viral infection may cause an alteration of intestine, which results in less efficient absorption by enterocytes [83]. Gao et al. (2020) [84] suggested that targeting gut microbiota could represent a new therapeutic option for COVID-19 treatment.
Due to the interaction of ACE2 on renal tubular cells SARS-CoV-2 is likely to invade the genitourinary system and cause acute kidney injury. A possible effect of COVID-19 on the genitourinary system is believed to be two-fold: COVID-19 can elicit a systemic inflammatory response syndrome (SIRS), sepsis and shock via systemic dissemination of the SARS-CoV-2 which damages distant organs. Nevertheless, some authors believe that virus could directly damage the kidney via the cytotoxic and immune-mediated mechanisms [85,86]. Moreover, histopathological studies of material obtained from deceased COVID-19 patients performed in Wuhan, China have shown that their kidney tissues revealed acute tubular necrosis, vacuole degeneration, tubulointerstitial lymphocyte infiltration and dilatated capillary vessels without a severe glomerular injury [86]. Immunohistochemistry studies detected the SARS-CoV-2 nucleocapsid protein antigen accumulation in renal tubules, but not in the glomeruli [86]. These findings provide the evidence of the SARS-CoV-2 virion invasion into the kidney, especially into renal tubules. Furthermore, the presence of nucleocapsid protein antigen in urine could have potential diagnostic value.
Transmission of Virus
Transmission of Sars-Cov-2 Infection from Mother to Child
During pregnancy, physiological changes in women’s immune and cardiopulmonary system occur, therefore there is greater risk for women to develop severe illness after being infected with respiratory virus [87]. In 1918 influenza pandemic, mortality rate for women aged between 15 and 49 was 4.9 per thousand, however that of pregnant women was between 5.3 and 5.7 per thousand [88]. In 2009, pregnant women accounted for 1 % of patients infected with influence A subtype H1N1 virus and represented 5 % of all H1N1 related deaths [89]. Before SARS-CoV-2, SARS-CoV and the MERS-CoV were responsible for death and life-threatening complications during pregnancy, including renal failure and difficulty in breathing, which resulted in the need for endotracheal intubation and admission to an intensive care unit [90,91]. found no data on preterm birth or perinatal complications in cases where infection was acquired during the first or early second trimester of pregnancy, but data exist for women in late second or third trimester of pregnancy: higher risk for more severe respiratory complications [92] and risk for health of fetus or some complications in newborn [93] was reported when pregnant women were infected with SARS-CoV-2. A study of 41 pregnant women showed that they had an increased risk of miscarriage, preterm birth, preeclampsia and Caesarian delivery; the risk was especially high in pregnant women with pneumonia [93]. Mullins et al. (2020) [94] reported that 47% women affected by COVID-19 delivered preterm while asymptomatic and those with mild conditions were reported to have fewer complications [93]. Pregnant patients have been treated with ribavirin, corticosteroids and antibiotics; however, optimal treatment has not yet been established [95]. Chen et al. (2020) [96] reported on nine women in third semester of pregnancy that mainly had two symptoms – fever and cough, some of them also complained about muscle pain, malaise, sore throat, shortness of breath and diarrhea. Possible symptoms of pregnant patients are also fatigue, headache and reduced fetal movement [97]. However, there were no cases of fetal death. Similar clinical manifestations were reported by Yu et al. (2020) [95] who monitored seven patients in third trimester of pregnancy. Chen et al. (2020) [96] also reported pregnancy complications in two of nine patients. Laboratory tests data confirmed lymphocytopenia and increase in C-reactive protein level and multiple patchy ground-glass shadows in lungs were detected by CT scans as reported by Chen et al. (2020) [96]. These authors also reported that the infection was not fatal for any of patients, also, none of them developed severe COVID-19 pneumonia [96].
Severe birth defects and pregnancy loss [98] or stillbirth [93] was found in SARS-CoV-2 infected mothers. It was suggested that the virions crossing the placental barrier is less likely and that the virions accessed decidua and placenta by passing from the lower reproductive tract or by blood, depending on gestational age, viral entry receptor expression, inutero environment and maternal immune response to the virus [98]. There has been no direct evidence of intrauterine transmission of SARS-CoV-2 in pregnant women who developed COVID-19 pneumonia in third trimester of pregnancy, but there are still reasonable concerns that COVID-19 could be contracted in the womb [96]. It was suggested that Immunoglobulin G (IgG) antibodies can be passively transferred across the placenta from mother to fetus from the end of second trimester until birth, when high levels are reached [99]. However, with it’s molecular weight of 900 kDa (app. six-times greater than IgG antibodies) [100], immunoglobulin M (IgM) antibodies were considered too large to be transmitted from mother to fetus [101]. Zeng et al. (2020) [101] speculated that there is a possibility for virus to cross the placental barrier, as they detected IgM antibodies in serum of two infants, few hours after being born to an infected mother. They suggested that IgM could have been produced by the fetus after the virus crossed the placenta. Similar conclusions were reported also by Dong et al. (2020) [102] who monitored a neonate with abnormal cytokine test results and elevated antibody levels two hours after birth. The laboratory results displaying inflammation and liver injury, beside the detection of IgM antibodies in neonates, indirectly supported the possibility of a transmission from the mother, since IgM antibodies usually do not appear until three to seven days after infection [89]. After the neonate tested positive for SARS-CoV-2, Wang et al. (2020) [103] performed nucleic acid tests for SARS-CoV-2 on cord blood and placental specimens that were retained during the Cesarean delivery, and the results were negative.
Chen et al. (2020) [96] reported Caesarean delivery in all nine of monitored. Four of them had preterm birth, none of which they found directly connected to SARS-CoV-2 infection. They did not collect the samples of vaginal mucosa and therefore could not assess whether transmission of SARS-CoV-2 was likely to take place during vaginal delivery. As indicated by previous studies, there are no reports of congenital infection with SARS-CoV-2 due to passage of fetus through the birth canal [104]. Dong et al. [102] suspected that the infant can get infected at delivery, but then tested mother’s vaginal secretions which were negative for SARS-CoV-2. Also, elevated IgM antibodies two hours after birth indicates possibility that infants contracted SARS-CoV-2 infection prior to the delivery [102]. It was also reported that the presence of virus was not detected in colostrum of infected pregnant women [95,103]. Fan et al. (2020) [105] suggested that breastfeeding could even have a protective effect. Due to immune response to SARS-CoV-2 infection, it would be possible that the mother produced sufficient neutralizing antibodies without developing serious conditions and pass them to the infant via breastfeeding.
Transmission of SARS-CoV-2 Infection via Genitourinary Tract
A suspicion of transmission of virus to other organisms via urine has been raised [106]. Paoli et al. (2020) [107] found that urine samples of a volunteer with confirmed SARS-CoV-2 infection was SARS-CoV-2 RNA negative. The COVID-19 investigation team in the USA performed a study which included the first 12 positive patients in their country; they have used real-time reverse-transcription polymerase chain reaction (rRT-PCR) to detect SARS-CoV-2 in urine specimens; in all hospitalized patients the rRT-PCR results were negative [108]. On the other hand, SARS-CoV-2 has been detected in the restrooms of COVID-19 positive patients [109]. The presence of SARS-CoV-2 RNA was found in urine of a COVID-19 patient without symptoms of urinary tract impairment [110]. Furthermore, SARS-CoV-2 RNA with low but detectable viral load was found in the urine of a patient with symptoms for COVID-19, positive SARS-CoV-2 RNA oropharyngeal swabs and opacities in the CT images of the lungs, on day 12 post infection and then periodically for one month[111]. Vero E6 cells infected with the urine sample exhibited cytopathic effects after 3 days [111]. Cell culture supernatant was negatively stained and visualized by transmission electron microscopy (TEM) showing presence of particles with characteristic shapes of coronavirus [111]. It is important to be aware that the virus could be transmitted via urine. As the route of transmission of SARS-CoV-2 is not yet fully understood, further research is required.
COVID-19 Co-Infections
With the weakened immunity of human body after viral infection, there is a greater risk for secondary bacterial infections. The trans-species interactions of viruses with bacteria are associated with various zoonotic viral diseases and disease progression [112]. Viral respiratory infections increase the incidence and severity of secondary bacterial complications, such as pneumonia and sepsis [113-115]. Although the pathogenesis of these complications is not completely understood, viral infection of respiratory tract promotes bacterial adhesion and colonization [113].
In case of SARS-CoV-2 infection, severely ill patients suffered a higher rate of co-infections with bacteria or fungi and they were more likely to develop complications [116]. Secondary bacterial infection increases risk for fatal outcome of the disease. Zhao et al. (2020) [66] reported that secondary bacterial infection occurred in 28 of 191 (15 %) COVID-19 patients admitted to hospitals in China and 27 of those 28 patients died. Different microorganisms that cause bacterial or fungal respiratory co-infections have been observed: Candida albicans [117], Enterobacter cloacae [117] and Acinetobacter baumannii [117,118], Klebsiella pneumonia [118], Aspergillus fumigatus [118] and Legionella pneumophila [95]. Patients may also be suffering from urinary tract or blood stream secondary co-infections [119]. Bacterial or fungal co-infection in COVID-19 patients appeared to be low (8 %); broad-spectrum antimicrobial therapy was applied in 72 % of these cases [119]. To avoid microbial resistance, antimicrobial therapy must be prescribed and used cautiously. For optimization of antimicrobial drug prescription in COVID-19, procalcitonin – specific bacterial biomarker could be of help [120]. Procalcitonin supports differentiation between bacterial and viral infection and helps to set early cessation of antibiotic without negative effects on patient’s health [119,121].
Interaction of Virus with Environment
SARS-CoV-2 Presence in Wastewaters
Both viable SARS-CoV-2 and viral RNA may enter waste waters (WW) as body excretions (saliva, sputum, urine and faeces). There are some reports on the detection of SARS-CoV-2 in WW in the Netherlands, USA, France, and Australia [122-126]. Ahmed et al. (2020) [126] claimed that the information on the presence of CoVs in WW is largely limited likely due to the lack of previous environmental investigations focusing on CoVs (spread via person-to-person contact rather than the faecal-oral route). During the SARS outbreak in 2004 in China, SARS-CoV RNA was detected in 100% (10/10) of untreated and 30% (3/10) of disinfected WW samples collected from a hospital in Beijing, China receiving SARS patients [127]. WW was also believed to be at least partly responsible for an exemplary SARS outbreak due to a faulty ventilation and plumbing system [128]. Several publications demonstrate the applicability of WW-based epidemiology (WBE) for COVID-19 surveillance as a potential tool for public health monitoring at the community level in order to understand the prevalence of viruses in each WW treatment plant (WWTP) catchment population [129,130]. WBE is especially useful for early warning of disease outbreaks and informing the efficacy of public health interventions, as previously demonstrated for enteric viruses, such as norovirus, hepatitis A virus, and poliovirus [126,131,132].
Understanding the inactivation of SARS-CoV-2 and its RNA would improve control measures and WW treatment requirements, but just a few documents on the persistence of CoVs in water and WW matrices are present [133-137]. Results of Wang et al. (2005) [133] on persistence of SARS-CoV in different samples (hospital WW, domestic sewage, tap water, phosphate buffered saline, faeces, urine, water, and WW at high various concentrations of 5, 10, 20 and 40 mg/L of chlorine and chlorine dioxide) and the effect of contact time on its inactivation in WW with different chlorine dioxide concentrations have shown that coronavirus persisted longer at 4°C (14 days in wastewater and at least 17 days in feces or urine) compared to 20°C (2 days) in hospital WW, domestic sewage, and dechlorinated tap water. At 20°C, SARS-CoV persisted in three fecal samples for 3 days and two urine samples for 17 days (inoculated titer of 105 TCID50). Free chlorine was more effective in inactivating SARS-CoV than chlorine dioxide [133].
Gundy et al. (2008) [134] determined the survival of human CoV 229E and enteric feline CoV (ATCC-990) in water and WW using plaque assay or median culture infectious dose (TCID50) technique; the survival of both human and feline CoVs showed similar patterns and was highly dependent on water temperature, level of organic matter, and biological activity; CoVs were inactivated rapidly in WW, with T99 values of <3 days.
A technical brief from WHO provides no evidence about the survival of SARSCoV-2 in WW or drinking water. [135] It is likely believed CoVs are less stable in the environment and are more susceptible to chlorine, pH, and temperature than most of non-enveloped enteric viruses [136]. Another publication suggests that an animal CoV remain infectious in water environments for days to weeks, depending on temperature and other physicochemical factors and their persistence could still be of concern for WW treatment facilities, storm water overflows, and WW intrusion in drinking water [137].
At present, significant knowledge gaps exist on the potential role of WW in the transmission of SARS-CoV-2. Survival of SARS-CoV-2 in environmental media, including WW and water, remains mostly unknown [138]. The persistence of SARS-CoV-2 needs to be determined in WW and environmental water for tropical, sub-tropical and temperate climatic zones as the persistence may be highly variable in different temperatures, as demonstrated in a recent study [138]. Moreover, the persistence of SARS-CoV-2 in WW and receiving waters and inactivation mechanisms, such as predation, UV, sunlight, and disinfection should be investigated [139].
COVID-19 Risks
COVID-19 Outbreak in 2019
A cluster of respiratory infection cases caused by a newly identified β-coronavirus occurred in Wuhan, China, at the end of 2019. World Health Organization (WHO) initially named this coronavirus as the 2019-novel coronavirus (2019-nCoV) on Jan 12, 2020. WHO officially named the disease as COVID-19, and Coronavirus Study Group (CSG) of the International Committee proposed to name the new coronavirus as SARS-CoV-2, both issued on Feb 11, 2020. SARS-CoV-2 is the seventh member of the group of coronaviruses that infect humans, and it is different from both MERS-CoV and SARS-CoV.
Risk of Sars-Cov-2 Infection through Faecal-Oral Transmitting Route
Since January 2020 when faeces samples tested positive for SARS-CoV-2, faecal-oral transmitting route of the virus gained serious attention [124].
Although there is no evidence of SARS-CoV-2 transmission by food so far [140] the virus is present in stool samples and can thus potentially spread via faeces [141-143]. Transmission from infecting intestines by faecal-oral-route was reported also for other coronavirus family members [83,144]. At a later stage of infection, there were even more faecal samples positive than the oral ones [145]. Several other studies confirmed these findings [144,146,147]. About a quarter of 73 hospitalized patients remained positive in stool samples even after being negative in the respiratory ones [148,149]. The nucleocapsid protein of SARS-CoV-2 was detected in glandular cells of gastric, duodenal and rectal epithelia [148] which correlated with the distribution of ACE2 in glandular cells previously implicated as a receptor for SARS-CoV-2 cell entry [21]. Therefore, SARS-CoV-2 can infect and replicate in the gastrointestinal tract, and thus this region is important for infection control. Anal swabs should complement detection of SARS-CoV-2 RNA from oral swabs used for infection diagnosis in the case of negative oral samples [145]. According to Zhang et al. (2020) [145], infected children discharged from hospital with negative SARS‐CoV‐2 results within 10 days tested positive in stool samples in spite of them remaining negative for nucleic acid presence in throat swab specimens. In adults, similar results were found by Chen et al. (2020) [150] when 64% of the tested patients were positive for virus in stool samples and negative in pharyngeal swabs, with duration of 7 days of viral shedding from faeces after conversion in pharyngeal swabs to negative. The fact that the stool specimens of children remain positive for a longer time than those of adults was discussed by Ma et al. (2020) [151]. The reasons found were that the children maintain a poorer hygiene than the adults, that the expression of ACE2 in the intestine of children may differ from that of adults and that children are more prone to silent aspiration favouring the entry of virus from saliva to the gastrointestinal tract through swallowing. Despite this, the detection of viral genetic material in faecal matter does not automatically indicate viable infectious virions. However, the virus may spread through faecal transmission and there are already studies that confirmed live viruses in faeces [141].
Worldwide untreated human excreta are and it will continue to be a major problem since 2.5 billion people still do not have basic sanitation facilities such as toilets or latrines and of these, 673 million are without access to appropriate sanitation among which one billion people still defecate in the open (e.g. in the street gutters, behind the bushes, into open water bodies) (WHO, 2019). When current situation revealed millions of people affected by SARS-CoV-2 all over the world, in their own countries or displaced (e.g. immigrants), and with the consideration of the possibility of faecal-oral transmitting route of the virus, areas with poor sanitation might become hazardous hot spots for spreading of SARS-CoV-2.
Faecal-oral transmitting route of SARS-CoV-2 may be a potential problem in developed countries in the case of poorly treated or non-treated domestic WW released into the environment, but also in the case of appropriate sewage system application. Excreta of the affected people eventually end up in municipal WW, sewage sludge and consequently in the environment [152] and the presence of SARS-CoV-2 in sewage was already reported [122,123,126]. However, the authors concluded, based on the lack of epidemiological evidence or case reports that sewage does not seem to be a transmission pathway of significance for SARS-CoV-2 [122,123]. Studies on novel coronaviruses including severe acute respiratory syndrome CoV (SARS-CoV) highlighted the persistence of these viruses in aquatic environments and WW treatment plants fed by the faecal discharge of infected people. It was proven that SARS-CoV in faeces samples can survive for 3 days [133] to 4 days [153] and that it can be infectious in water and sewage for days to weeks [154], or that several days are needed to lose 99% of the infectivity at room temperature, in pure water, or pasteurized settled sewage [155]. Especially hospitals with high concentration of infected people could pose higher risk of spreading the disease through sewage.
Even if the data on the viability of SARS-CoV-2 are for now poor, there is a crucial necessity for upgrading and improving current technologies and management of municipal WW collection and treatment, which should take in consideration possible virus contamination to ensure the correct treatment and disposal, even if the viruses for instance can survive in the environment for a few days only. Few days are enough for the viruses to reach other hosts, to proliferate and even mutate. There are numerous studies reporting on removal of enteric viruses at conventional activated sludge wastewater treatment plants (WWTP) reaching 30-90% efficiency. The removal of SARS-CoV-19 is expected to be in the same range if treated conventionally. However, there are several treatment options to inactivate the novel virus, such as the employment of disinfection units in drinking and conventional WWTPs and membrane bioreactors (MBR) where membrane pore sizes determine efficiency level of virus removal or UV-based advanced oxidation processes [156]. Decentralized separation WW treatment systems [157] can also be a viable option to be taken in consideration. Separation of WW fractions means a better control and it can result in decentralized virus inactivation and prevention of further transmission into the environment.
Green treatment technologies like extensive waste stabilization ponds, algae ponds and constructed wetlands also enable removal of viruses via natural processes and can be applied as decentralized solutions; however hydraulic retention time, system design and operation have significant influence on pathogen removal. To our best knowledge there are so far no data on removal of coronaviruses with green technologies. In open water treatment systems viruses can be trapped within bio-flocs or adsorbed to suspended particles such as organic matter, algae, bacteria and colloidal materials [158]; however according to Verbyla and Mihelcic [159] sedimentation of flocs with trapped viruses may not be a significant virus removal mechanism. Other mechanisms include direct and indirect sunlight-mediated mechanisms, temperature and interactions with other microorganisms and macroinvertebrates. Recent research has also revealed that direct and indirect sunlight-mediated mechanisms are not only dependent on pond water chemistry and optics, but also on the characteristics of the virus and its genome [159].
The removal efficiency of viruses in constructed wetlands can be highly uncertain and depends on environmental and operational factors including types of macrophytes and sunlight exposure. In subsurface flow wetland, macrophytes enhance the removal efficiency while in free water surface flow system, their contribution is passive and sun exposure has a bigger role; therefore, plant selection is an essential consideration in wetland design [160].
Sources and Transmission Routines of SARS-CoV-2 in Society
The source(s) and transmission routine(s) of SARS-CoV-2 remain elusive. In the beginning, an animal originated outbreak has been suspected, while most infected patients had visited a common seafood market within a limited period indicating highly viremic animal or contaminated food [161]. Human-to-human transmission occurred mainly between family members, including relatives and friends, with intimate contacts with patients or incubation carriers. Transmission between healthcare workers amounted to 3.8% of COVID-19 patients, as reported by the National Health Commission of China on Feb 14, 2020. Direct contact with intermediate host animals or consumption of wild animals was suspected to be the main route of SARS-CoV-2 transmission [22]. Considering the main symptoms in patients, the mode of transmission was found unlikely to be oral via food. However, a related virus SARS-CoV is listed by WHO as a potential foodborne transmitted because of its long survival [162]. According to the WHO report [163], the nose and mucosal membranes in the eyes are the primary sources for viruses' transmission. Fewer than 1% of infected individuals report conjunctivitis, according to the WHO report [163]. The mouth is another potential route for infection, especially when food handlers are hyper salivating, chewing, or swallowing (i.e., by sucking a finger, biting nails, or any other activity that increases exposure time also increases risk). Therefore, frequent and proper hand washing is the primary preventive measure that can help prevent the spread of pathogens aside from coronavirus.
Transmission of SARS-CoV occurs mainly after days of illness. It is associated with modest viral loads in the respiratory tract early in the illness, with viral loads peaking approximately ten days after symptom onset [164]. Higher viral loads of SARS-CoV-2 were detected soon after symptom onset, with higher viral loads detected in the nose than in the throat. These findings suggest that transmission may occur early in the course of infection [165]. As an emerging acute respiratory infectious disease, COVID-19 primarily spreads through the respiratory tract by droplets, respiratory secretions, and direct contact [165]. Mullis et al. (2012) [166] have shown that coronavirus can be stable on the surface of lettuce in laboratory conditions with infectivity retained for at least 14 days. The Survival rates of pathogenic microbes, including viruses on fresh produce, such as raw fruits and vegetables, are usually high; therefore, adequate minimal food processing steps, including washing, should be undertaken [167]. It should be practised, especially because, as demonstrated by Mullis et al. (2012) [166], the laboratory procedure does not entirely remove residual viruses. Epidemiologic significance of remaining infectious particles cannot be evaluated, as the coronavirus infectious dose is not known. Temperatures for coronavirus inactivation are not yet fully determined; current scientific guidance is that a temperature of 65°C for at least 3 minutes suffices. Researchers assume that the SARS-CoV-2 will respond as microorganisms do and that higher temperatures will require less time, but there are no reports on the experimental results to prove this premise [168].
SARS-CoV-2 can also reach the liver, which is the premier site for drug metabolism. Currently, there is no definite cure for this virus, and treatments previously effective against related coronaviruses, like SARS-CoV and MERS-CoV, are being used [169]. These medicines, e.g., Oseltamivir, Lopinavir/Ritonavir, Ribavirin, as well as chloroquine phosphate and hydroxychloroquine sulphate that are prescribed to COVID-19 patients, are metabolised in the liver. Unfortunately, 60% of patients who suffered from SARS had a liver impairment, and some liver impairment was also present in MERS-CoV patients [81]. Liver injuries were also reported in patients with COVID-19 patients; 2-11% had liver comorbidities, while 14-53% of the cases had abnormal blood levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) indicating a liver injury according to the data from The Fifth Medical Center of PLS General Hospital, Beijing [81] and the rate of liver dysfunction seemed to increase with the severity of the COVID-19. Underlying causes were suggested to be either a viral infection or medication side-effects.
Interaction of Sars-Cov Virions with Material
Decontamination of Surfaces Exposed to SARS-CoV-2 Infection
The suitability of individual decontamination method is implied by several factors: the targeted pathogen, in our case a completely new virus, the ease of installation and use, the rate of sterilization, the material to be sterilized (surface, polymeric content), the qualities related to the decontamination technique itself, and the cost [171-178]. Many control techniques could reduce risk from common viral infection on surfaces. Heating sterilization of objects is one of the oldest [170] followed by ultraviolet germicidal irradiation used for removal of viruses [171-177]. The main principle is the destruction of the viral DNA upon the absorption of the UV irradiation. The third way of virus decontamination is the use of chemical disinfectants, which were found to inactivate also SARS-CoV, regardless of type (45% isopropanol, 30% n-propanol, 80% -95% ethanol) [178].
Another issue during global emergencies coming from the virus SARS-CoV-2 is the over-stressed health care systems, which consequently enters the crisis mode. It requires the decontamination of single-use Personal Protective Equipment (PPE) to keep up with the growing demand from health care workers and patients. Unfortunately, in some cases also unverified methods are being applied, resulting in an incomplete decontamination with catastrophic results [179]. Several proposals suggested sterilization of used PPE and also N95 respirators with agents ranging from ethylene oxide, UV or gamma irradiation, ozone, hydrogen peroxide vaporization, microwave generated steaming, dry heating and alcohol, but all of them can degrade or/and decrease the efficacy of reprocessed PPE [179,180].
Heating
SARS-CoV-2 is, as reported by Chin et al. (2020) [181], highly stable at 4°C but sensitive to heat, where the virus inactivation was achieved at 70 °C in 5 minutes. Heating is one of commonly used, easily deployable, scalable disinfection technique, but should be applied with care not to damage the PPE. Heating (≤85 °C) under various humidities (≤100% relative humidity) was the most promising (up to 50 cycles), non-destructive method for the preservation of filtration properties in melt-blown fabrics as well as N95-grade respirators. Compared to UV radiation (degradation noticed after 20 cycles), according to Liao et al. (2020) [182], heating was twice more efficient.
UV-C radiation
Some hospitals have already begun using UV-C radiation in biosafety cabinets (BSCs), which showed to be efficient to decontaminate N95 respirator or surgical masks and provide a wide margin of safety exposure against SARS-CoV-2 [183]. Depending on the dimensions of BSCs and lamp intensities, authors report doses from 1 Jcm−2 (from 20 minutes to 4) [183], to more than 10-times lower dose (60 mJcm−2 12 mJ/min) [184]. However, it should be taken into account, that polymers are susceptible to UV-C radiation. With higher doses of UV-C radiation (120-950 Jcm−2) they may change also structural integrity and performance of respirators [177] but with lower doses, it seems to be one of the best practice available now [185].
Hydrogen Peroxide (H2O2)
Another well-known technology adapted for new uses is the application of H2O2 vapour, plasma generation and ionization, which was proven to be an efficient method of decontamination [186]. In the shortage of medical supplies, over 100 respirators were decontaminated and reused employing H2O2 [187,188]. The effective reuse of respirators was tested on the fit, the filter efficiency and the decontamination/disinfection level [189]. A more than 6 log reduction in bacterial spores and more than 3.8 log reduction in the infectious SARS-CoV2 load on N95 respirators were noticed in at least one cycle [189]. Hydrogen peroxide vapour decontamination technique, which is normally used to fumigate hospital rooms can be scaled to permit simultaneous sterilization. After 5 cycles applying Clarus C system® (Bioquell, Horsham, PA), the respirators appeared similar to new with no deformity [190,191]. After 10 cycles applying 7% H2O2 solution via the Pathogo Curis® (Curis) a complete decontamination of multiple virus species was achieved [192]. The disinfection process is faster using ionized hydrogen Peroxide (iHP) SteraMist®, which uses a higher concentration of H2O2 vapour (30% - 35%). The drawback is the concentration of remaining H2O2 immediately after use (0.6 ppm) [193].
Cleaning Agents
Previous studies on SARS-CoV-1 found surface disinfectants with 62–71% ethanol, 0.5% hydrogen peroxide or 0.1% sodium hypochlorite effective and expect a similar effect against the SARS-CoV-2 [194]. The virus is susceptible to many other active ingredients, such as povidone-iodine (1% iodine), chloroxylenol (0.24%) and benzalkonium chloride (0.05%). In radiology the equipment was effectively disinfected with isopropyl alcohol (70%), followed by a terminal cleaning using diluted bleach solution (6 mg chlorine releasing disinfectant tablet to 1,000 mL water) [109,195].
Disinfection of Wastewater from Hospital
The discharge of hospital WW, especially those without appropriate treatment is the most important route of the public exposure to SARS-CoV-2 [196]. In order to reduce the health risks to the public and environment, the appropriate disinfection WW should be performed before discharge. There are several technologies, commonly used for hospital WW disinfection, such as ozone, ultraviolet irradiation, liquid chlorine, chlorine dioxide, and sodium hypochlorite [196,197]. The general WW disinfection system in the hospital, described by Wang et al. (2020) [198] includes the following steps: primary disinfection, sedimentation, dechlorination, moving bed biofilm reactor, and re-disinfection. Every disinfection technology has unique advantages and disadvantages. The utilization of a certain type of disinfection technology should be determined by the comprehensive consideration of both economic and feasible factors risks, such as the amount of WW, safety conditions, the supply of disinfectants, etc. [198].
Bleaching powder is commonly used for the chlorination pre-treatment [199]. For general hospital with infectious disease area, 1 kg of bleaching powder containing 25% of available chlorine per 10 beds should be added 3 to 4 times before further disinfection. The optimal addition time is at the end of the peak period of the restroom use. Chlorine is a strong oxidizer, which is one of the most early used disinfection methods in disinfecting hospital WW [195]. Usually, 30 mg/L-50 mg/L and 15 mg/L-25 mg/L chlorine is added to WW after primary treatment and secondary treatment, respectively [199]. When using liquid chlorine for WW disinfection, a special vacuum chlorinator must be used, and the outlet of the chlorine injection pipe should be submerged in the WW [200]. The pipe materials may be affected by chlorine gas corrosion and from that reason they must stay resistant. Chlorine dioxide is known as one of the efficient disinfectants with high oxidization capability even under acidic conditions [201]. Its solubility is five times that of chlorine and its oxidization capacity is 2.63 times that of chlorine gas. It is generally recommended that the amount of chlorine dioxide used to treat hospital WW is 1/2.5 that of the available chlorine [199]. Chlorine dioxide destroys the anabolic pathways of protein and thus kills the microorganism, including bacteria, viruses, fungi, spores, and clostridium botulinum [202]. Chlorine dioxide advantage is in its high efficiency and low operation costs, but on the other hand its disadvantage is inconvenient storage and transport. Sodium hypochlorite disinfectant could be prepared using standard NaClO generator, which could significantly reduce the costs [203]. Ozone is a disinfectant with high bactericidal effect and is widely used in water supply WW treatment [204,205]. Application of UV irradiation with the wavelength between 200 nm and 300 nm could damage the structure of both DNA and RNA of the bacteria, viruses, and single-celled microorganisms and thus inhibit the protein synthesis. UV-B and UV-C have the best bactericidal effect [206]. For application of ultraviolet light there are low investment and operation costs, but disadvantage is in inadequate depth of penetration and occupational health [206].
Prevention and Treatment of Sars-CoV-2 Infection
Hygienic and Nutritional Interventions
Protective measures against the SARS-CoV-2 include necessary hygienic measures coupled with physical interventions (introduction of barriers) to prevent or reduce the contact between people [207]. In addition to hand washing and hand disinfection, other measures, such as respiratory hygiene, social distancing, isolation, Quarantine, and usage of personal protection, are necessary.
Hands are known vector for the transmission of different infections [208,209]. Carabin et al. (1999) [210] and Ladegaard and Stage (1999) [211] have shown that handwashing can significantly reduce the spread of different respiratory viruses by as much as 20%. During the current pandemic, WHO [207] and other international and national health bodies (CDC, ECDC, Slovenian National Institute of Public Health) recommended a frequent hand washing with soap and water for at least 20 seconds as an effective preventive measure. Soaping (mechanical friction and dispersion of soap across hand surfaces) should last for at least 20 to 30 seconds [212-215]. When soap and water are not available, an alcohol-based hand sanitizer that contains at least 65% of alcohol should be used [207]. Since alcohol-based disinfectant destroys viruses by contact, the sufficient amount of alcohol hand rub (3-5 mL) [215-217] and adequate spreading through the entire hand is more critical than mechanical [216]. Ma et al. (2020) [218] investigated efficiency of hand wiping by a wet towel soaked in water containing 1.00% soap powder, 0.05% active chlorine, or 0.25% active chlorine from sodium hypochlorite and reported removal of 98.36%, 96.62%, and 99.98% of the avian influenza virus from hands by the above agents, respectively. The authors proposed a similar efficiency also for the SARS-CoV-2.
Mouth and/or nose should be covered with the upper bend of the elbow while coughing or sneezing [207,219]. Also, tissue for single-use should be used. Hand washing or disinfection should be implemented after handling with the tissue.
The basic idea behind "social distancing" is to reduce interactions between people in the observed (or controlled) community to prevent the person-to-person spread of disease, especially in the case of droplet transmission. Social distancing means mainly closure of places (or activities) where people are gathering in close contacts (such as schools, kindergartens or office buildings, suspension of public markets, public transport, and cancellation of different gatherings) [220,221]. Social distancing also means the physical distance between people in public places. For the COVID-19 epidemic, a social distance of at least 1 m [207,219] or 2 m [222] is recommended. A distance between individuals should be maintained both indoor as well as outdoor. To prevent social interactions and spreading COVID-19 also crowd gathering in most places is prohibited or postponed [223].
Rather well-known techniques of physical interventions are isolation and quarantine. Isolation means the physical separation of the ill person from healthy ones to prevent transmission of disease [224]. In the case of SARS-CoV-2, there is no need for a negative pressure room in hospitals [220], however since SARS-CoV-2 is highly contagious, early detection and strict isolation is crucial to prevent the uncontrolled spread of the virus. The incubation period is reported to be from 2 to 14 days with a mean of around five days [225]. In the case of SARS-CoV-2 due to different incubation time and mainly due to presymptomatic or asymptomatic carriers [226] monitoring and movement restriction of healthy individuals with possible virus contact (i.e., the quarantine period) is of great importance. Quarantine means segregation and movement restriction of healthy individuals with potential connection with infectious disease [224]. In the case of the SARS epidemic in Singapore in 2003, quarantine had positive results [227].
Contaminated surfaces are important transmission root of the virus through hand contact by initiate self-inoculation of mucous membranes of the nose, eyes, or mouth. Several researchers investigated the persistence of coronaviruses on different surfaces. According to Kampf et al. (2020), [94] coronaviruses can remain infectious on different types of materials from 2 hours up to 9 days. According to van Doremalen et al. (2020) [228], under the experimental conditions, the SARS-CoV-2 virus had similar stability in aerosol as well as on different materials (plastic, stainless steel, copper, and cardboard) as SARS-CoV-1. Both viruses were more stable on smooth surfaces (plastic and stainless steel) than on copper or cardboard. In both cases, researchers observed an exponential decay across time, and however, the virus was still detected up to 72 hours after application in all observed materials. Therefore hand hygiene (including handwashing as well as avoidance of unnecessary touching of mouth, eyes, and nose) is of great importance. However, also disinfection of frequently touched objects and surfaces in the immediate patient surrounding can reduce the viral load on surfaces where the highest viral concentrations can be expected. The WHO [229,230] recommends consistent and thorough cleaning and disinfection procedures, especially in hospitals and other health care facilities using commonly used hospital-level disinfectants. Kampf et al. (2020), [194] in their review, summarized necessary conclusions regarding the efficiency of different disinfectants. According to Sattar et al. (1989), [231] the concentration of 0.1% of sodium hypochlorite is effective for coronavirus in 1 min; similarly, the effect could be achieved using 70% ethanol for disinfecting small surfaces (WHO, 2014) [232] or other commonly used disinfectants [194]. According to EPA [233] the products that could be used for surface disinfection for COVID-19 prevention include chlorine dioxide, quaternary ammonium, citric acid, ethanol, ethyl alcohol, glycolic acid, hydrochloric acid, hydrogen peroxide, peroxyacetic acid, triethylene glycol, thymol, sodium hypochlorite, sodium carbonate, sodium dischloroisocyanurate dehydrate, iodine; proper concentrations, as well as contact time, must be followed. However, EPA does not recommend the use of fumigation or wide-area disinfection of the outdoor environment to control COVID-19.
Although some governments declared obligatory usage of facemasks and gloves in indoor public places, WHO still does not recommend the use of facemask by healthy people [207,219]. Offeddu et al. (2017) [231,234] in their systematic review, recommended the use of respiratory protection for health care workers in the case of SARS. However, their report is concluded by the remark of inconclusive and inconsistent evidence within and across different studies. Also, other authors report insufficient protection by a medical mask compared to N95 respirator [235] or even homemade mask [234] in the case of COVID-19 pandemic strongly recommended face mask for all public places, since mask-wearing can rise individual vigilance and prevent direct hand to face contact. More importantly, a face mask can reduce air contamination of pathogens from infected people to the environment [218,236].
Medical Procedures
The transmission route of a virus is through the direct person-to-person interaction. The virions are transmitted by various bodily fluids (cough, sneeze, vomit, saliva and blood) [68]. The SARS-CoV2 transmission route depends on the size of the lilliput particles in the fluids which can be small and intermediate particles and large droplets. Small particles follow airflow streamlines and are capable of short and long-range transmission. Particles smaller than 5 μm are capable to reach alveoli and smaller than 10 μm can pass through the glottis. Therefore, viruses included in small particles are the most contagious. Large (> 20 μm) sized droplets follow a more ballistic trajectory which is dependent upon the gravitational force. Therefore, they are not capable to penetrate as deep as small particles, i. e. they do not pass through the glottis. The intermediate, 10-20 μm sized particles share some properties of small particles and large droplets. Additionally, a person can get infected via particles (i.e. via fomites), deposited on other surfaces which resembles the faecal-oral transmission [237].
According to the most recent findings, the main transmission route of SARS-CoV-2 is via droplets [238], however, all secretions (except sweat) should be considered as potentially infectious [239]. It is of utmost importance to be aware of the presence of the virus in small particles (i.e. aerosols), that are generated also during some medical procedures [228].
Since the transmission route of virions can be via the upper aerodigestive tract, the management of the diseases involving head and neck by a physician presents a significant risk to develop the COVID-19. Namely, an adequate otorhinolaryngological examination involves a close contact of the physician with the upper aerodigestive tract of the patient. Although the viral load is highest in the secretions collected directly in the flow from the lungs (i.e. bronchoalveolar lavage fluid samples) [68] the SARS-CoV-2 virions have been isolated from fibrobronchoscope brush biopsy samples and oropharyngeal and nasopharyngeal swabs [68,240]. Therefore, every otorhinolaryngological examination or procedure in the region of the head and neck presents a high risk of COVID-19 transmission [240].
The risk of transmission in otorhinolaryngological practice is especially high when managing the patients presenting at the check-up with the cough, nasal discharge, sore throat, anosmia, bleeding or vomiting. Cough can be a presenting symptom of different upper aerodigestive tract disorders, however, it is also the main symptom of COVID-19 [240]. Also nasal discharge or nasal congestion and sore throat have been identified as a presenting symptom in COVID-19 [241]. Vomiting can be the initial symptom of the COVID-19 [242], which could mislead to the management of the acute vestibular disorder. To our best knowledge, nosebleed has not been reported as the presenting symptom of COVID-19, so far. However, SARS-CoV-2 causes thrombocytopenia [72] and predisposes patients to the nosebleed. Otorhinolaryngological procedures present high risk for COVID-19 infection also due to aerosol-generating procedures (mastoidectomy, anterior skull-base surgery, nasal endoscopy, laryngoscopy, tracheostomy, tracheal tube replacement, tracheostomy care and other procedures, potentially provoking coughing, sneezing, vomiting or bleeding) and it considered that these procedures should be applied only if urgently required, as the SARS-CoV-2 virions transmit via aerosols [240]. The virus can be present in the blood and mucosa of head and neck, therefore aerosol-generating procedures (e.g. electrocautery, suction with the fenestrated instruments and drilling) should be avoided or performed with extreme caution by using an appropriate protective personal equipment. Since the COVID-19 patients can be asymptomatic, the care about possible transmission of SARS-CoV-2 virions is even more important.
Food Safety and Good Hygiene Practices in the Food Supply Chain
In the period of self-isolation and Quarantine, food supply chains have to ensure that safe food is available in every household. Therefore, everything in the food supply chain, from the food itself to the people working with food has to meet food safety requirements. Although it seems that the primary transmission of SARS-CoV-2 is not by food or food packaging, functional food hygiene is essential to protect the public from other foodborne pathogens, like norovirus, hepatitis A, harmful bacteria, and others. However, the results from a recent study by van Doremalen et al. (2020) [228] about SARS-CoV-2 stability on surfaces made of various materials raised concerns that food packaging could contribute to the spread of the virus. The results by van Doremalen et al. (2020) [228] were obtained under controlled laboratory conditions. No research study investigating the virus stability on food packaging surfaces in real-world conditions has been published so far to model a transmission across the food supply chain. The use of reusable packaging, where consumers bring their container for shopping as part of their zero-waste lifestyle, may decrease the consumer's contact with infected packaging.
Raspor (2008) [243] emphasized that food safety in the food supply chain is a result of several elements. Legislation should provide minimum hygiene requirements; official controls should be in place to check food business operators’ compliance, and food business operators should establish and maintain HACCP (Hazard Analysis and Critical Control Points) system. In the field of food safety, Codex Alimentarius provides guidelines for managing various risks along food supply chain [244]. Besides other food safety hazards, the human factor is the least controlled in the food supply chain [245]. Undetected factors often cause foodborne diseases; for example, food handlers often spread microorganisms by cross-contamination. The risk of cross-contamination depends on multiple factors, such as the time from contamination, the cleaning frequency, and the surface properties of the contaminated object (e.g., porousness and moisture). Because of the many critical situations in the food safety area, food business operators must align existing programs and HACCP procedures to meet the SARS-CoV-2 challenge. Therefore, food business operators should:
- Review the requirements and procedures for personal hygiene and employee's behaviour;
- Provide additional training for all employees regarding the personal hygiene procedures and proper conduct rules (with the emphasis on hand washing and health status of employees);
- Check up on employees health at all stages of the food supply chain (e.g., health questionnaires, including the question on possible contacts with potentially infected persons);
- Enable frequent hands disinfection (at building entrances, offices, dressing rooms, in production, etc.);
- Ensure proper use of working clothes, masks, gloves by all employees;
- Provide regular ventilation of the food establishments;
- Review existing cleaning and disinfection procedures;
- Review training programs for cleaning and disinfecting personnel;
- Update processes for the prevention of cross-contamination;
- Evaluate the working process and review the procedures;
- Review the requirements and procedures for foodstuffs' ingredients, packaging materials, and waste;
- Review the conditions and procedures for cleaning of transport vehicles;
- Review the requirements and procedures for returnable packaging;
- Review the crisis management and establish a preventive action plan regarding SARS-CoV-2;
- Review and update the risk analysis, critical control points, and all HACCP documents.
Food handlers along the food supply chain must perform their work strictly according to reviewed food safety requirements and hygiene procedures and take care to avoid direct person to person contacts. The threat of the SARS-CoV-2 transmission requires the introduction of additional hygiene and sanitation measures to ensure the protection of food and food handlers in the food supply chain [246].
During the food preparation, standard preventive measures target (among others) the prevention of faecal-oral transmission of pathogenic microorganisms. So far, no cases of SARS-CoV-2 transmission via this route has been reported [247]. However, this route of contamination is in line
with previous studies on SARS-CoV and MERS-CoV [75] as well as with recent findings by Wu et al. (2020) [247] who observed that faecal samples over half of the patients remained positive for SARS-CoV-2 RNA for a mean of 11.2 days after the respiratory tract samples became negative. These findings are implying that the virus is replicating in the gastrointestinal tract even after the viral clearance in the respiratory tract. Faecal-oral transmission may occur in contained living premises, such as hostels, dormitories, trains, buses, and cruise ships. The transmission potential is even higher in the areas with poor sanitation.
Nutritional Interventions
Many nutritional interventions, like Vitamin A, C, D, E, B, Omega‐3 polyunsaturated fatty acids, Selenium, Zinc, and Iron, have been reported to be successful in combating Influenza virus, MERS‐CoV, Measles virus, human immunodeficiency virus, Avian coronavirus, Bovine coronavirus and others (reviewed in Zhang and Liu (2020) [248]).
Many studies revealed that supplementation with the nutrients mentioned above reduced morbidity and mortality in different infectious diseases, such as lung diseases, measles, severe diarrhoea, measles‐related pneumonia, human immunodeficiency virus (HIV) infection, malaria [249,250]. There are no completed human interventional trials that would assess particular nutritional intervention during a SARS-CoV-2 infection. Participants are currently recruited for interventional studies of Vitamin D, Vitamin C, and Zinc as dietary supplements on patients infected with SARS-CoV-2 [251].
Vitamin D binds to the ACE2 receptor [252,253] that is used by SARS-CoV virions to enter the by binding via the S protein [254,255]. Calcitriol (25-OH vitamin D3), the active form of vitamin D that is usually made in the kidney, exerts protective effects on lung injury through modulating components of the renin-angiotensin system (RAS) such as ACE1 and ACE2, renin and angiotensin II [252]. 1, 25-dihydroxy vitamin D suppresses renin gene expression, thereby inhibiting the renin-angiotensin system and reducing the activity of the angiotensin-converting enzyme [256,257]. Vitamin-D deficiency may also pose an increased susceptibility to enveloped virus infections such as HIV, Hepatitis, Dengue, and Respiratory Syncytial virus infection [258].
The following studies report an indirect role of vitamin D on coronaviral infections. A meta-analysis from 2018 implied that the risk of infection with enveloped viruses is increased by 50% in people with low vitamin D levels [258]. Additionally, Vitamin D acts also as an immunomodulator through suppressing pro-inflammatory cytokines. According to the World Health Organisation (WHO) report [259]. Vitamin D deficiency can affect the immune system as vitamin D has an immunomodulation role [260-262]. Namely, studies have shown that innate immunity (monocytes and macrophages) successfully removes germs (bacteria, viruses) at their entry point (in mucous membranes of the respiratory tract) at optimal levels of Vitamin D 25 (OH) in the serum. Activation of 25 (OH) Vit D and 1, 25 (OH) Vit D by 1-alpha hydroxylase in macrophages induces the formation of endogenous antimicrobials and enable the macrophages to continue to work successfully against the invaders. Furthermore, the protective effects of Vitamin D in preventing respiratory tract infections was observed by many meta-analyses [263-266]. It can be concluded that although there is no reliable evidence that vitamin D can be used to prevent the SARS-CoV-2 infection, it is better to be optimally supplied with vitamin D. If the serum range of 25 (OH) Vit D is above 75 nmol / L or 30 ng/ml, the respiratory viral infections, in general, are better fought.
Vitamin C and Zinc both cooperate with the cellular antioxidative defence scavenge damaging reactive oxygen species, thus protecting the body's cells and tissues from oxidative damage. Vitamin C and Zinc also reinforce the immune system and ameliorate the symptoms caused by COVID-19 and might thus provide some protection against the infection caused by coronaviruses [267-270]. A recent clinical trial (Identifier: NCT04264533) will investigate the effects of vitamin C in patients infected by SARS-CoV-2 in Wuhan, China.
Future Prospects
Interaction of Virus with Extracellular Vesicles
The production and release of membrane-bound vesicles is an evolutionary conserved process that takes place in cells from all domains of life. In aquatic environments, lilliput particles are highly abundant and carry out several important functions. Firstly, they allow for efficient and highly concentrated transport of biological molecules to the target cells. Secondly, the cargo packaged inside the lilliput particles is protected from degradation due to external factors in the environment. Thirdly, the differences in protein and lipid composition in lilliput particles enable targeted transport of molecules to a specific subset of cells in the environment, thereby increasing the chances of their cargo reaching the recipient cells [271]. Through viral infections, viruses manipulate the composition of the communities, influence the microbial population and their genetic evolution, and also affect the biogeochemical cycles. Hence, the underlying mechanisms of host-virus interactions and environmental factors are two emerging areas of research [272].
Intriguingly, in the recent years, the researchers began to notice and draw a parallel between virions and extracellular vesicles (EVs). EVs are of equal size as virions, under the electron microscope, they closely resemble the virions of enveloped viruses. The biogenesis of EVs occurs either through the release from multivesicular bodies or by budding from the surface of plasma membrane [273]. Both, virions and EVs carry molecular cargoes (e.g. nucleic acids, proteins, sugars and lipids) which provides them with an important role in intercellular communication. Once released by the cells, they either become degraded or transport their cargo to recipient cells by either fusing with their cytoplasmic membrane and/or by the process of endocytosis [273,274].
In comparison to viruses, EV’s are generally considered to have a wider range of different cell types that they can enter. However, in some cases their uptake can also be partially dependent on specific ligand-receptor recognition [275,276]. Akin to the entry of viral genetic material into the cell, the cargo carried by EVs that enters the recipient cells similarly has a modifying effect on cells’ physiological functions [274]. Despite the close resemblance of viruses and EVs, there is one fundamental difference that sets them apart – EVs do not replicate [277].
Not only are there similarities between viruses and extracellular vesicles, but there is also evidence that they interact with each other. Besides being involved in cell-cell communication, EVs can also act as vectors for gene transfer and pathogenicity [271]. It was found that in eukaryotes, some cells that are infected with viruses produce EVs that can contain parts of viral genetic material as well as viral proteins [276-278]. Hence, the exploitation of EVs by viruses as a mode of enhancing viral infections is becoming increasingly acknowledged. An example of this phenomenon was described in marine microalgae Emiliania huxleyi [271,279]. The virus EhV from the family Phycodnaviridae is highly specific and only infects Emiliania huxleyi [280]. A study by Schatz et al. (2017) [280] has reported that the production of algal EVs is elevated during viral infections or exposure of bystander cells to infochemicals originating from infected cells. They found that, in comparison to viruses and infected cells, the lipid composition of these vesicles was different and their cargo contained specific small RNAs, which were proposed to alter the lipid metabolism and cell-cycle pathways in recipient host cells. This allows for faster lytic rate and increased viral production upon subsequent viral infection. In addition, EVs were shown to prolong the EhV half-life in the extracellular environment. Altogether, in this way, the EVs can accelerate the dynamics of viral infection, allowing more efficient viral propagating across Emiliania huxleyi blooms [280].
On the other hand, EVs were also found to suppress viral infections [281]. For instance, in marine cyanobacteria Prochlorococcus, EVs were suggested to act as baits for the binding of phages [281]. Such mechanism could have a protective role against phage infection. Bacterial EVs contain outer membrane material that matches the one on bacteria, meaning that there are protein receptors present, which the phages use for host identification [281]. Binding of phages to vesicles is thus favorable to bacteria as it reduces the chances of them becoming infected and destroyed [281]. A similar effect was also achieved in in vitro experiment with CD4+ T cells and EVs containing the HIV receptor CD4. These EVs were found to successfully bind viral particles, reducing the quantity of virions, which would otherwise infect the CD4+ T [277].
The accumulated evidences indicate that the viruses have developed an intricate relationship with microorganisms and EVs. Deepening our understanding into EV functions could potentially lead towards the development of therapeutic approaches for treatment of viral infections [277,282].
Interactions of Lilliput Particles with New Materials
In the future it will be important to develop novel diagnostic methods based on nanotechnology to quantitatively determine the amount of lilliput particles in the human body. Candidates for reliable and simple sensors of lilliput particles in biofluids are Titanium and Titanium Dioxide (TiO2) nanostructured surfaces which bind lilliput particles [283-287]. Figure 2 shows EV isolate from blood on TiO2 nanotubular surface. Globular structures heterogeneous in size were identified as EVs (white arrow) while material deposited also on the tips of the tubes in a toroid-like structures (black arrow).
Figure 2: Scanning electron microscope images of TiO2 nanotubular surface (A) and the TiO2 nanotubular surface that was exposed to the isolate of EVs from blood (B). A: adapted from Kulkarni et al., (2020)288, B: adapted from Lampe et al. (in preparation) [289].
Conclusions
Interplay between lilliput particles in a cell, organism and society derives from the same basic mechanisms consistent with laws of nature that govern organic and inorganic matter. These mechanisms involve biological membranes that are subject to physical laws, e.g. the first and second law of thermodynamics, given in terms of statistical physics. In order to master the manipulation of lilliput systems, the membranous nanostructure mechanisms should be better understood and novel technologically advanced methods should be developed. EVs could be used as platforms for virus detection and transformation. Devotion of attention and time to better understanding of basic mechanisms seems prerequisite to overcome confusion and loss of health and life for common debilitating diseases as well as at outbreaks such as the COVID-19.
Acknowledgements
Authors acknowledge EU Commision H2020 grant Ves4usP3-0388, ARRS grants P2-0232, P2-0273, P3-0388, J1-9162, J2-8162, Slovenia-Russia bilateral project and project No. C3330-17-529033 "Raziskovalci-2.0-ZAG-529033", co-financed by Ministry of Education, Science and Sport of Republic of Slovenia and the European Regional Development Fund.
References:
- Atchison, BC Casto, WM Hammon, Adenovirus-associated defective virus particles, Science, 149 (1965) 754–756, doi: https://science.sciencemag.org/content/149/3685/754.
- D Arslan, M Legendre, V Seltzer, et al., Distant Mimivirus relative with a larger genome highlights the fundamental features of Megaviridae, Proc. Natl. Acad. Sci. USA, 108 (2011) 17486–17491, doi: https://www.pnas.org/content/108/42/17486
- C Théry, KW Witwer, E Aikawa, et al., Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines, J. Extracell. Vesicles., 7 (2018) 1535750, doi: https://www.tandfonline.com/doi/full/10.1080/20013078.2018.1535750
- P. T. Ivanova, D. S. Myers, S. B. Milne et al., Lipid composition of viral envelope of three strains of influenza virus – not all viruses are created equal, ACS Infect. Dis., 1 (2015) 399–452, doi: https://pubs.acs.org/doi/10.1021/acsinfecdis.5b00040
- K. Simons, R. Ehehalt, Cholesterol, lipid rafts, and disease, J. Clin. Invest., 110 (2002) 597–603, doi: https://www.jci.org/articles/view/16390
- M. M. Lorizate, H.-G. Kräusslich, Role of lipids in virus replication, Cold. Spring. Harb. Perspect. Biol, 3 (2011) a004820, doi: https://cshperspectives.cshlp.org/content/3/10/a004820
- J. C. Voyta, D. P. Via, C. E. Butterfield, B. R. Zetter, Identification and isolation of endothelial cells based on their increased uptake of acetylated-low density lipoprotein, J. Cell Biol., 99 (1984) 2034–2040, doi: https://doi.org/10.1083/jcb.99.6.2034
- H. Costa Verdera, J. J. Gitz-Francois, R. M. Schiffelers, P. Vader, Cellular uptake of extracellular vesicles is mediated by clathrin-independent endocytosis and macropinocytosis, J. Controll. Rel, 266 (2017) 100–108, doi: https://doi.org/10.1016/j.jconrel.2017.09.019
- M. Durak-Kozica, Z. Baster, K. Kubat, E. Stępień, 3D visualization of extracellular vesicle uptake by endothelial cells, Cell. Mol. Biol. Lett., 23 (2018) 57, doi: https://doi.org/10.1186/s11658-018-0123-z
- T. A. M. Bharat, T. Noda, J. D. Riches et al., Structural dissection of Ebola virus and its assembly determinants using cryo-electron tomography, Proc. Natl. Acad. Sci. USA, 109 (2012) 4275–4280, doi: https://doi.org/10.1073/pnas.1120453109
- World Health Organization: Marburg virus disease. https://www.who.int/health-topics/marburg-virus-disease/#tab=tab_1, 04.05.2020
- World Health Organization: Coronavirus disease (COVID-19) pandemic. https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- J. Urbanija, N. Tomsic, M. Lokar et al., Coalescence of phospholipid membranes as a possible origin of anticoagulant effect of serum proteins, Chem. Phys. Lipids, 150 (2007) 49–57, doi: https://doi.org/10.1016/j.chemphyslip.2007.06.216
- ScienceSourceImages: Ebola Virus, SEM. https://www.sciencesource.com/archive/Ebola-Virus--SEM-SS2656904.html, 08.05.2020
- National Institute of Allergy and Infectious Diseases (NIAID): Marburg Virus Particles. https://www.flickr.com/photos/niaid/30971360137, 05.05.2020
- National Institute of Allergy and Infectious Diseases (NIAID), Novel Coronavirus SARS-CoV-2. https://www.flickr.com/photos/nihgov/49565158953, 05.04.2020
- R. Stukelj, V. Sustar, A. Mrvar-Brečko et al., Suppression of membrane vesiculation as anticoagulant and anti-metastatic mechanism. Role of stability of narrow necks, Gen. Phys. Biophys., 32 (2013) 33–45, doi: https://doi.org/10.4149/gpb_2013007
- V. C. C. Cheng, S. K. P. Lau, P. C. Y. Woo, K. Y. Y Cheng, Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection, Clin. Microbiol. Rev., 20 (2007) 660–694, doi: https://doi.org/10.1128/CMR.00023-07
- E. Prompetchara, C. Ketloy, T. Palaga, Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic, Asian Pac. J. Allergy Immunol., 38 (2020) 1–9, doi: https://doi.org/10.12932/AP-200220-0772
- S.R. Weiss, J. L. Leibowitz, Coronavirus pathogenesis, Adv. Virus Res., 81 (2011) 85–164, doi: https://doi.org/10.1016/B978-0-12-385885-6.00009-2
- P. Zhou, X.L. Yang, X.G. Wang et al., A pneumonia outbreak associated with a new Coronavirus of probable bat origin, Nature, 579 (2020) 270–273, doi: https://doi.org/10.1038/s41586-020-2012-7
- Y. R. Guo, Q. D. Cao, Z. S. Hong et al., The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil. Med. Res., 7 (2020) 11, doi: https://doi.org/10.1186/s40779-020-00240-0
- Z. Liu, X. Xiao, X. Wei et al., Composition and divergence of coronavirus spike proteins and host ACE2 receptors predict potential intermediate hosts of SARS-CoV-2, J. Med. Virol., (2020), doi: https://doi.org/10.1002/jmv.25726
- M. Hoffmann, H. Kleine-Weber, S. Schroeder et al., SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor, Cell, 181 (2020) 271–280.e8, doi: https://doi.org/10.1016/j.cell.2020.02.052
- X. Ou, Y. Liu, X. Lei et al., Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV, Nat. Commun., 11 (2020) 1620, doi: https://doi.org/10.1038/s41467-020-15562-9
- I. Hamming, W. Timens, M. L. Bulthuis et al., Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis, J. Pathol., 203 (2004) 631–637, doi: https://doi.org/10.1002/path.1570
- X.H. Yao, Z.C. He, T.Y. Li, et al., Pathological evidence for residual SARS-CoV-2 in pulmonary tissues of a ready-for-discharge patient, Cell Res., (2020) doi: https://doi.org/10.1038/s41422-020-0318-5
- N. Sungnak, C. Huang, C. Bécavin et al., SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes, Nat. Med., 26 (2020) 681–687, doi: https://doi.org/10.1038/s41591-020-0868-6
- S. Kowalczuk, A. Bröer, N. Tietze, et al., A protein complex in the brush‐border membrane explains a Hartnup disorder allele, FASEB J., 22 (2008) 2880–2887, doi: https://doi.org/10.1096/fj.08-107300
- M. Donoghue, F. Hsieh, E. Baronas et al., A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9, Circ. Res., 87 (2000) E1–9, doi: https://doi.org/10.1161/01.RES.87.5.e1
- S.R. Tipnis, N.M. Hooper, R. Hyde et al., A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase, J. Biol. Chem., 275 (2000) 33238–33243, doi: https://www.jbc.org/content/275/43/33238
- G.C. Douglas, M.K. O'Bryan, M.P. Hedger,et. al., The novel angiotensin-converting enzyme (ACE) homolog, ACE2, is selectively expressed by adult Leydig cells of the testis, Endocrinology, 145 (2004) 4703–4711, doi: https://doi.org/10.1210/en.2004-0443
- D. Harmer, M. Gilbert, R. Borman, K.L. Clark, Quantitative mRNA expression profiling of ACE2, a novel homologue of angiotensin converting enzyme, FEBS Lett., 532 (2002) 107–110, doi: https://doi.org/10.1016/S0014-5793(02)03640-2
- L.M. Burrell, J. Risvanis, E. Kubota et al., Myocardial infarction increases ACE2 expression in rat and humans, European Heart Journal, Eur. Heart J., 26 (2005) 369–375, doi: https://doi.org/10.1093/eurheartj/ehi114
- Y. Ding, L. He, Q. Zhang et al., Organ distribution of Severe Acute Respiratory Syndrome (SARS) associated Coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways, J. Pathol., 203 (2004) 622–630, doi: https://doi.org/10.1002/path.1560
- G.A. Farcas, S.M. Poutanen, T. Mazzulli et al., Fatal severe acute respiratory syndrome is associated with multiorgan involvement by coronavirus, J. Infect. Dis., 191 (2004) 193–197, doi: https://doi.org/10.1086/426870
- H. Chu, J.F.-W. Chan, T.T.-T. Yuen et al., Comparative tropism, replication kinetics, and cell damage profiling of SARS-CoV-2 and SARS-CoV with implications for clinical manifestations, transmissibility, and laboratory studies of COVID-19: an observational study, The Lancet Microbe, http://www.sciencedirect.com/science/article/pii/S2666524720300045, 05.05.2020
- J. Gu, E. Gong, B. Zhang et al., Multiple organ infection and the pathogenesis of SARS, J. Exp. Med., 202 (2005) 415–424, doi: https://doi.org/10.1084/jem.20050828
- P.S. Masters, S. Perlman, Coronaviridae, In D.M. Knipe, P. Halowley (ed.), Fields virology 6th ed., Wolters Kluwer Health/Lippincott Williams & Wilkins, Philadelphia, 2013, 825–858
- S. Belouzard, V.C. Chu, G.R. Whittaker, Activation of the SARS coronavirus spike protein via sequential proteolytic cleavage at two distinct sites, Proc. Natl. Acad. Sci. USA, 106 (2009) 5871–5876, doi: https://www.pnas.org/content/106/14/5871
- S. Bertram, I. Glowacka, M.A. Müller et. al., Cleavage and activation of the Severe Acute Respiratory Syndrome Coronavirus Spike protein by human airway trypsin-like protease, J. Virol., 85 (2011) 13363–13372, doi: https://jvi.asm.org/content/85/24/13363
- I. Glowacka, S. Bertram, M.A. Müller et al., Evidence that TMPRSS2 activates the Severe Acute Respiratory Syndrome Coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response, J. Virol., 85 (2011) 4122–4134, doi: https://jvi.asm.org/content/85/9/4122
- J.M. van den Brand, B.L. Haagmans, D. van Riel et al., The pathology and pathogenesis of experimental Severe Acute Respiratory Syndrome and influenza in animal models, J. Comp. Pathol., 151 (2014) 83–112, doi: https://doi.org/10.1016/j.jcpa.2014.01.004
- D.E. Gordon, G.M. Jang, M. Bouhaddou et al., A SARS-CoV-2-human protein-protein interaction map reveals drug targets and potential drug-repurposing, bioRxiv, (2020), doi: https://doi.org/10.1101/2020.03.22.002386
- M. Frieman, R. Baric, Mechanisms of Severe Acute Respiratory Syndrome pathogenesis and innate immunomodulation, Microbiol. Mol. Biol. Rev., 72 (2008) 672–685, doi: https://mmbr.asm.org/content/72/4/672
- Y.C. Li, W.Z. Bai, T. Hashikawa, The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients, J. Med. Virol., 92 (2020) 552–555, doi: https://doi.org/10.1002/jmv.25728
- Y. Fu, Y. Cheng, Y. Wu, Understanding SARS-CoV-2-mediated inflammatory responses: from mechanisms to potential therapeutic tools, Virol. Sin., (2020) 1-6, doi: https://doi.org/10.1007/s12250-020-00207-4
- P. Mehta, D.F. McAuley, M. Brown et al., COVID-19: consider cytokine storm syndromes and immunosuppression, Lancet., 395 (2020) 1033–1034, doi: https://doi.org/10.1016/S0140-6736(20)30628-0
- Q. Ruan, K. Yang, W. Wang et al., Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China, Intensive Care Med., (2020), doi: https://doi.org/10.1007/s00134-020-05991-x
- Giacomelli, L. Pezzati, F. Conti et. al., Self-reported olfactory and taste disorders in SARS-CoV-2 patients: a cross-sectional study, Clin. Infect. Dis., (2020) ciaa330, doi: https://doi.org/10.1093/cid/ciaa330
- J.R. Lechien, C.M. Chiesa-Estomba, D.R. De Siati et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study, Eur. Arch. Otorhinolaryngol., (2020), doi: https://doi.org/10.1007/s00405-020-05965-1
- G. Spinato, C. Fabbris, J. Polesel et al., Alterations in smell or taste in mildly symptomatic outpatients with SARS-CoV-2 infection, JAMA, (2020) e206771, doi: https://jamanetwork.com/journals/jama/fullarticle/276518 3
- J. Helms, S. Kremer, H. Merdiji et al., Neurologic features in severe SARS-CoV-2 Infection, New Engl. J. Med., (2020) NEJMc2008597, doi: https://www.nejm.org/doi/full/10.1056/NEJMc2008597
- T. Chen, D. Wu, H. Chen et. al., Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study, The BMJ, 368 (2020) m1091, doi: https://www.bmj.com/content/368/bmj.m1091
- N. Poyiadji, G. Shahin, D. Noujaim et al., COVID-19–associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features, Radiology., (2020) 201187, doi: https://doi.org/10.1148/radiol.2020201187
- F.G. De Felice, F. Tovar-Moll, J. Moll et. al., Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and the central nervous system, Trends Neurosci., (2020), doi: https://doi.org/10.1016/j.tins.2020.04.004
- M. Desforges, A. Le Coupanec, J.K. Stodola et al., Human coronaviruses: viral and cellular factors involved in neuroinvasiveness and neuropathogenesis, Virus Res., 194 (2014) 145–158, doi: https://doi.org/10.1016/j.virusres.2014.09.011
- A.M. Baig, Neurological manifestations in COVID-19 caused by SARS-CoV-2, CNS Neurosci. Ther., 26 (2020) 499–501, doi: https://onlinelibrary.wiley.com/doi/full/10.1111/cns.13372
- G. Conde Cardona, L.D. Quintana Pajaro, I.D. Quintero Marzola et al., Neurotropism of SARS-CoV 2: Mechanisms and manifestations, J. Neurol. Sci., 412 (2020) 116824, https://doi.org/10.1016/j.jns.2020.116824
- J. Netland, D.K. Meyerholz, S. Moore, Severe Acute Respiratory Syndrome Coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2, J. Virol., 82 (2008) 7264–7275, doi: https://jvi.asm.org/content/82/15/7264
- X. Zhuang, Y. Teng, A. Samykutty et al., Grapefruit-derived nanovectors delivering therapeutic miR17 through an intranasal route inhibit brain tumor progression, Mol. Ther., 24 (2016) 96–105, doi: https://doi.org/10.1038/mt.2015.188
- T. Moriguchi, N. Harii, J. Goto et al., A first case of meningitis/encephalitis associated with SARS-Coronavirus-2, Int. J. Infect. Dis., 94 (2020) 55–58, doi: https://doi.org/10.1016/j.ijid.2020.03.040
- C.M. Ferrario, J. Jessup, M.C. Chappell et al., Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2, Circulation, 111 (2005) 2605–2610, doi: https://doi.org/10.1161/CIRCULATIONAHA.104.510461
- S. Hippenstiel, N. Suttoport, Interaction of pathogens with the endothelium, Thromb. Haemost., 89 (2003) 18–24. doi: https://doi.org/10.1055/s-0037-1613538
- J. R. Tisoncik, M. J. Korth, C. P. Simmons et al., Into the Eye of the Cytokine Storm, Microbiol. Mol. Biol. Rev., 76 (2012) 16–32, doi: https://doi.org/10.1128/MMBR.05015-11
- F. Zhou, T. Yu, R. Du et al., Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study, Lancet, 395 (2020) 10229, 1054–1062, doi: https://doi.org/10.1016/S0140-6736(20)30566-3
- M.A. Matthay, R.L. Zemans, The acute respiratory distress syndrome: pathogenesis and treatment, Annu. Rev. Pathol., 6 (2011) 147–1163, doi: https://doi.org/10.1146/annurev-pathol-011110-130158
- W. Wang, Y. Xu, R. Gao et. al., Detection of SARS-CoV-2 in different types of clinical specimens, JAMA, (2020), doi: https://doi.org/10.1001/jama.2020.3786
- E. Terpos, I. Ntanasis-Stathopoulos, I. Elalamy et al., Hematological findings and complications of COVID-1, Am. J. Hematol., (2020), doi: https://doi.org/10.1002/ajh.25829
- B.E. Fan, V.C.L. Chong, S.S.W. Chan et al., Hematologic parameters in patients with COVID-19 infection, Am. J. Hematol., (2020), doi: https://doi.org/10.1002/ajh.25774
- W.J. Guan, Z.Y. Ni, Y. Hu et al., China Medical Treatment Expert Group for Covid-19, Clinical Characteristics of Coronavirus Disease 2019 in China, N. Engl. J. Med., 382 (2020) 1708–1720, doi: https://doi.org/10.1056/NEJMoa2002032
- P. Xu, Q. Zhou, J. Xu, Mechanism of thrombocytopenia in COVID-19 patients, Ann. Hematol., (2020) 1–4, doi: https://doi.org/10.1007/s00277-020-04019-0
- G. Zini, S. Bellesi, F. Ramundo, G. d’Onofrio, Morphological anomalies of circulating blood cells in COVID‐19, Am. J. Hematol., (2020) 1–3, doi: https://doi.org/10.1002/ajh.25824
- J. Cui, F. Li, Z.L. Shi, Origin and evolution of pathogenic coronaviruses, Nat. Rev. Microbiol., 17 (2020) 181–192, doi: https://doi.org/10.1038/s41579-018-0118-9
- C. Yeo, S. Kaushal, D. Yeo, Enteric involvement of coronaviruses: is faecal-oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol Hepatol, 5 (2020) 335–337, doi: https://doi.org/10.1016/S2468-1253(20)30048-0
- D. Wrapp, N. Wang, K.S. Corbett et al., Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation, Science, 367 (2020) 1260–1263, doi: https://doi.org/10.1126/science.abb2507
- T. Hashimoto, T. Perlot, A. Rehman, et al., ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation, Nature, 487 (2012) 477–481, doi: https://doi.org/10.1038/nature11228
- T.N. Chau, K.C. Lee, H. Yao et al., SARS-associated viral hepatitis caused by a novel coronavirus: Report of three cases, Hepatology, 39 (2004) 302–310, doi: https://doi.org/10.1002/hep.20111
- K.O. Alsaad, A. H. Hajeer, M. Al Balwi et al., Histopathology of Middle East respiratory syndrome coronovirus (MERS-CoV) infection - clinicopathological and ultrastructural study, Histopathology, 72 (2018) 516–524, doi: https://doi.org/10.1111/his.13379
- X. Chai, L. Hu, Y. Zhan et al., Specific ACE2 Expression in Cholangiocytes May Cause Liver Damage After 2019-nCoV Infection, Genomics, bioRxiv, (2020), doi: https://doi.org/10.1101/2020.02.03.931766
- C. Zhang, L. Shi, F.-S. Wang, Liver injury in COVID-19: management and challenges, Lancet Gastroenterol. Hepatol., 5 (2020) 428–430, doi: https://doi.org/10.1016/S2468-1253(20)30057-1
- Y. Tian, L. Rong, W. Nian, Y. He, Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission, Aliment Pharmacol. Ther., 51 (2020) 843–851, doi: https://doi.org/10.1111/apt.15731
- J. Gu, B. Han, J. Wang, COVID-19: Gastrointestinal manifestations and potential fecal-oral Transmission, Gastroenterology, 158 (2020) 1518–1519, doi: https://doi.org/10.1053/j.gastro.2020.02.054
- Q.Y. Gao, Y.X. Chen, J.Y. Fang, Novel coronavirus infection and gastrointestinal tract, J. Dig. Dis., 21 (2020) 125–126, doi: https://doi.org/10.1111/1751-2980.12851
- H. Li, D. Zhang, J. Xu et al., SARS-CoV-2 and viral sepsis: observations and hypotheses, The Lancet, 395 (2020) 1517–1520, maj 2020, doi: https://doi.org/10.1016/S0140-6736(20)30920-X
- B. Diao, C. Wang, R. Wang, et al., Human Kidney is a Target for Novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection, medRxiv (2020), doi: https://doi.org/10.1101/2020.03.04.20031120
- H. Yang, C. Wang, L. C. Poon, Novel coronavirus infection and pregnancy, Ultrasound Obstet. Gynecol., 55 (2020) 435–437, doi: https://doi.org/10.1002/uog.22006
- A. Reid, The effects of the 1918-1919 influenza pandemic on infant and child health in Derbyshire, Medical history, 49 (2005) 29-54. doi: https://doi.org/10.1017/s0025727300008279
- A.M. Siston, S.A. Rasmussen, M.A. Honein et al., Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United States, JAMA, 303 (2010) 1517–1525, doi: https://doi.org/10.1001/jama.2010.479
- S.F. Wong, K.M. Chow, T.N. Leung, et al., Pregnancy and perinatal outcomes of women with Severe Acute Respiratory Syndrome, Am. J. Obstet. Gynecol., 191 (2004) 292–297, doi: https://doi.org/10.1016/j.ajog.2003.11.019
- S.H. Alfaraj, J.A. Al-Tawfiq, Z.A. Memish, Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infection during pregnancy: report of two cases & review of the literature, J. Microbiol. Immunol. Infect., 52 (2019) 501–503, doi: https://doi.org/10.1016/j.jmii.2018.04.005
- M. Karimi-Zarchi, H. Neamatzadeh, S.A. Dastheib et al., Vertical Transmission of Coronavirus Disease 19 (COVID-19) from Infected Pregnant Mothers to Neonates: A Review, Fetal. Pediatr. Pathol., (2020), 1–5, doi: https://doi.org/10.1080/15513815.2020.1747120
- D. Di Mascio, A. Khalil, G. Saccone et al., Outcome of Coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis, Am. J. Obstet. Gynecol. MFM, (2020) 100107, doi: https://doi.org/10.1016/j.ajogmf.2020.100107
- E. Mullins, D. Evans, R. M. Viner, et al., Coronavirus in pregnancy and delivery: rapid review, Ultrasound Obstet. Gynecol., 55 (2020) 586–592, doi: https://doi.org/10.1002/uog.22014
- N. Yu, W. Li, Q. Kang, et al., Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: a retrospective, single-centre, descriptive study, Lancet Infect. Dis., 20 (2020) 559–564, doi: https://doi.org/10.1016/S1473-3099(20)30176-6
- H. Chen, J. Guo, C. Wang, et al., Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records, Lancet, 395 (2020) 809–815, doi: https://doi.org/10.1016/S0140-6736(20)30360-3
- Y. Chen, H. Peng, L. Wang, et al., Infants born to mothers with a new Coronavirus (COVID-19), Front. Pediatr., 8 (2020) 104, doi: https://doi.org/10.3389/fped.2020.00104
- K. Racicot, G. Mor, Risks associated with viral infections during pregnancy, J. Clin. Invest., 127 (2017) 1591–1599, doi: https://doi.org/10.1172/JCI87490
- P.F. Kohler, R.S. Farr, Elevation of cord over maternal IgG immunoglobulin: evidence for an active placental IgG transport, Nature, 210 (1966) 1070-1071, doi: https://doi.org/10.1038/2101070a0
- A.B. Choo, H.L. Tan, S.N. Ang et al., Selection Against Undifferentiated Human Embryonic Stem Cells by a Cytotoxic Antibody Recognizing Podocalyxin-Like Protein-1, Stem Cells, 26 (2008) 1454–1463, doi: https://doi.org/10.1634/stemcells.2007-0576
- H. Zeng, C. Xu, J. Fan et al., Antibodies in infants born to mothers with COVID-19 pneumonia, JAMA, (2020), doi: https://doi.org/10.1001/jama.2020.4861
- L. Dong, J. Tian, S. He et al., Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn, JAMA, (2020), doi: https://doi.org/10.1001/jama.2020.4621
- S. Wang, L. Guo, L. Chen et al., A case report of neonatal COVID-19 infection in China, Clin. Infect. Dis., (2020), ciaa225, doi: https://doi.org/10.1093/cid/ciaa225
- C. C. Shek, P. C. Ng, G. P. Fung et al., Infants born to mothers with Severe Acute Respiratory Syndrome, Pediatrics, 112 (2003) 4, e254, doi: https://doi.org/10.1542/peds.112.4.e254
- C. Fan, D. Lei, C. Fang et al., Perinatal transmission of COVID-19 associated SARS-CoV-2: should we worry? Clin. Infect. Dis., (2020), ciaa226, doi: https://doi.org/10.1093/cid/ciaa226
- L. Wang, X. Li, H. Chen et al., Coronavirus Disease 19 Infection Does Not Result in Acute Kidney Injury: An Analysis of 116 Hospitalized Patients from Wuhan, China, Am J Nephrol, 51 (2020) 5, 343–348, 2020, doi: https://doi.org/10.1159/000507471
- D. Paoli, F. Pallotti, S. Colangelo et al., Study of SARS-CoV-2 in semen and urine samples of a volunteer with positive naso-pharyngeal swab, J Endocrinol Invest (2020), doi: https://doi.org/10.1007/s40618-020-01261-1
- The COVID-19 Investigation Team, Clinical and virologic characteristics of the first 12 patients with coronavirus disease 2019 (COVID-19) in the United States, Nat Med (2020), doi: https://doi.org/10.1038/s41591-020-0877-5
- S.W.X. Ong, Y.K. Tan, P.Y. Chia et al. Air, Surface Environmental, and Personal Protective Equipment Contamination by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) From a Symptomatic Patient, JAMA, 323 (2020) 1610, doi: https://doi.org/10.1001/jama.2020.3227
- L. Peng, J. Liu, W. Xu et al., SARS‐CoV‐2 can be detected in urine, blood, anal swabs, and oropharyngeal swabs specimens, J Med Virol, (2020) 25936, doi: https://doi.org/10.1002/jmv.25936
- J. Sun, A. Zhu, H. Li et al., Isolation of Infectious SARS-CoV-2 from Urine of a COVID-19 Patient, Emerging Microbes & Infections (2020) 1–8, doi: https://doi.org/10.1080/22221751.2020.1760144
- V. Nikhra, The Trans-zoonotic Virome interface: Measures to balance, control and treat epidemics, Ann Biomed Sci 4 (2020) 20–27, doi: https://doi.org/10.29328/journal.abse.1001009
- V. Avadhanula, Y. Wang, A. Portner, E. Adderson, Nontypeable Haemophilus influenzae and Streptococcus pneumoniae bind respiratory syncytial virus glycoprotein, Journal of Medical Microbiology, 56 (2007) 1133–1137, doi: https://doi.org/10.1099/jmm.0.47086-0
- M.E. Bustamante-Calvillo, F.R. Velázquez, L. Cabrera-Muñoz et al., Molecular detection of respiratory syncytial virus in postmortem lung tissue samples from Mexican children deceased with pneumonia, The Pediatric Infectious Disease Journal, 20 (2001) 495–501, doi: https://doi.org/10.1097/00006454-200105000-00005
- V. Avadhanula, C. A. Rodriguez, J. P. DeVincenzo et al., Respiratory Viruses Augment the Adhesion of Bacterial Pathogens to Respiratory Epithelium in a Viral Species- and Cell Type-Dependent Manner, JVI, 80 (2006) 1629–1636, doi: https://doi.org/10.1128/JVI.80.4.1629-1636.2006
- G. Zhang, C. Hu, L. Luo et al., Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan, China, Journal of Clinical Virology, 127 (2020) 104364, doi: https://doi.org/10.1016/j.jcv.2020.104364
- Z. Wang, B. Yang, Q. Li, L. Wen, R. Zhang, Clinical Features of 69 Cases With Coronavirus Disease 2019 in Wuhan, China, Clinical Infectious Diseases (2020) ciaa272, doi: https://doi.org/10.1093/cid/ciaa272
- N. Chen, M. Zhou, X. Dong et al., Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study, The Lancet, 395 (2020) 507–513, doi: https://doi.org/10.1016/S0140-6736(20)30211-7
- T.M. Rawson, L.S.P. Moore, N. Zhu et al., Bacterial and fungal co-infection in individuals with coronavirus: A rapid review to support COVID-19 antimicrobial prescribing, Clinical Infectious Diseases, (2020) ciaa530, doi: https://doi.org/10.1093/cid/ciaa530
- M.A. Meier, A. Branche, O.L. Neeser et al., Procalcitonin-guided Antibiotic Treatment in Patients With Positive Blood Cultures: A Patient-level Meta-analysis of Randomized Trials, Clinical Infectious Diseases, 69 (2019) 388–396, doi: https://doi.org/10.1093/cid/ciy917
- E. de Jong, J.A. Van Oers, A. Beishuizen et al., Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial, The Lancet Infectious Diseases, 16 (2016) 819–827, doi: https://doi.org/10.1016/S1473-3099(16)00053-0
- W. Lodder, A.M. de Roda Husman, SARS-CoV-2 in wastewater: potential health risk, but also data source, Lancet Gastroenterol. Hepatol., (2020) S246812532030087X, doi: https://doi.org/10.1016/S2468-1253(20)30087-X
- G. Medema, L. Heijnen, G. Elsinga et. al., Presence of SARS-Coronavirus-2 in sewage, medRxiv, (2020), doi: https://doi.org/10.1101/2020.03.29.20045880
- Y. C. Wu, C. S. Chen, Y. J. Chan, The outbreak of COVID-19: an overview, J. Chin. Med. Assoc., 83 (2020) 3, 217-220, doi: https://doi.org/10.1097/JCMA.0000000000000270
- S. Wurtzer, V. Marechal, J. M. Mouchel, L. Moulin, Time course quantitative detection of SARS-CoV-2 in Parisian wastewaters correlates with COVID-19 confirmed cases, medRxiv, (2020), doi: https://www.medrxiv.org/content/10.1101/2020.04.12.20062679v1.full.pdf
- W. Ahmed, N. Angel, J. Edson et al., First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community, Sci. Total Environ, 728 (2020) 138764, doi: https://doi.org/10.1016/j.scitotenv.2020.138764
- X. W. Wang, J. Li, T. Guo et al., Concentration and detection of SARS coronavirus in sewage from Xiao Tang Shan hospital and the 309th Hospital of the Chinese People’s Liberation Army, Water Sci. Technol., 52 (2005) 213-221, doi: https://doi.org/10.2166/wst.2005.0266
- K.R. McKinney, Y.Y. Gong, T.G. Lewis, Environmental transmission of SARS at Amoy Gardens, J. Environ. Health, 68 (2006) 26-30
- R. G. Sinclair, C. Y. Choi, M. R. Riley, C. P. Gerba, Pathogen surveillance through monitoring of sewer systems, Adv. Appl. Microbiol., 65 (2008) 249-269, doi: https://doi.org/10.1016/S0065-2164(08)00609-6
- I. Xagoraraki, E. O’Brien, Wastewater-Based Epidemiology for Early Detection of Viral Outbreaks, v Women in Water Quality, D.J. O’Bannon, Ur. Cham: Springer International Publishing, (2020) 75–97
- H. Asghar, O.M. Diop, G. Weldegebriel et. al., Environmental surveillance for polioviruses in the global polio eradication initiative, J. Infect. Dis., 210 (2014) S294-S303, doi: https://doi.org/10.1093/infdis/jiu384
- M. Hellmér, N. Paxéus, L. Magnius et al., Detection of Pathogenic Viruses in Sewage Provided Early Warnings of Hepatitis A Virus and Norovirus Outbreaks, Appl. Environ. Microbiol., 80 (2014) 6771–6781, doi: https://doi.org/10.1128/AEM.01981-14
- X.W. Wang, J. S. Li, M. Jin et. al., Study on the resistance of Severe Acute Respiratory Syndrome-associated Coronavirus, J. Virol. Methods, 126 (2005) 1-2, 171-177, doi: https://doi.org/10.1016/j.jviromet.2005.02.005
- P.M. Gundy, C.P. Gerba, I.L. Pepper, Survival of coronaviruses in water and wastewater, Food. Environ. Virol., 10 (2009) 10-14, doi: https://doi.org/10.1007/s12560-008-9001-6
- World Health Organization: Water, sanitation, hygiene, and waste managementfor the COVID-19 virus (2020). Technical brief. https://www.who.int/publications-detail/water-sanitation-hygiene-and-waste-management-for-the-covid-19-virus-interim-guidance, 08.05.2020
- World Health Organization: Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV). https://www.who.int/news-room/detail/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov), 08.05.2020
- Y. Ye, R.M. Ellenberg, K.E. Graham, K.R. Wigginton, Survivability, partitioning, and recovery of enveloped viruses in untreated municipal wastewater. Environ. Sci. Technol., 50 (2016) 5077–5085, doi: https://doi.org/10.1021/acs.est.6b00876
- O.E. Hart, R.U. Halden, Computational analysis of SARS-CoV-2/COVID-19 surveillance by wastewater-based epidemiology locally and globally: feasibility, economy, opportunities and challenges, Sci. Total Environ., 730 (2020) 138875, doi: https://doi.org/10.1016/j.scitotenv.2020.138875
- M. Kitajima, W. Ahmed, K. Bibby et al., SARS-CoV-2 in wastewater: state of the knowledge and research needs, Sci. Tot. Environ. (2020) 139076, doi: https://doi.org/10.1016/j.scitotenv.2020.139076
- EFSA. Coronavirus: no evidence that food is a source or transmission route. https://www.efsa.europa.eu/en/news/coronavirus-no-evidence-food-source-or-transmission-route, 11.4.2020
- M.L. Holshue, C. DeBolt, S. Lindquist et al., First case of 2019 novel coronavirus in the United States, N. Engl. J. Med., 382 (2020) 329-336, doi: https://www.nejm.org/doi/10.1056/NEJMoa2001191
- S.H. Wong, N. . Lui Rashid, J.Y. Joseph Sung, COVID‐19 and the digestive system, J. Gastroenterol. Hepatol., 35 (2020) 5, doi: https://doi.org/10.1111/jgh.15047
- R. Mao, J. Liang, J. Shen et al., Implications of COVID-19 for patients with pre-existing digestive diseases, Lancet Gastroenterol. Hepatol., 5 (2020) 5, doi: https://doi.org/10.1016/S2468-1253(20)30076-5
- F. D'Amico, D.C. Baumgart, S. Danese, L. Peyrin-Biroulet, Diarrhea during COVID-19 infection: pathogenesis, epidemiology, prevention and management, Clin. Gastroenterol. Hepatol., (2020), S1542-3565(20)30481-X, doi: https://doi.org/10.1016/j.cgh.2020.04.001
- W. Zhang, R.H. Du, B. Li et al., Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes, Emerg. Microb. Infect., 9 (2020) 386-389, doi: https://doi.org/10.1080/22221751.2020.1729071
- Y. Ling, S.B. Xu, Y.X. Lin et al., Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients, Chin. Med. J., 133 (2020) 1039-1043, doi: https://doi.org/10.1097/CM9.0000000000000774
- C. Han, C. Duan, S. Zhang et al., Digestive symptoms in COVID-19 patients with mild disease severity: clinical presentation, stool viral RNA testing, and outcomes, Am. J. Gastroenterol., (2020), doi: https://doi.org/10.14309/ajg.0000000000000664
- F. Xiao, M. Tang, X. Zheng et al., Evidence for Gastrointestinal Infection of SARS-CoV-2, Gastroenterology, 158 (2020) 1831-1833.e3, doi: https://doi.org/10.1053/j.gastro.2020.02.055
- I.C. Lee, T.I. Huo, Y.H. Huang, Gastrointestinal and liver manifestations in patients with COVID-19, J. Chin. Med. Assoc. (2020) 1, doi: https://doi.org/10.1097/JCMA.0000000000000319
- Y. Chen, L. Chen, Q. Deng et al., The presence of SARS‐CoV‐2 RNA in the feces of COVID‐19 patients, J Med Virol (2020), jmv.25825, doi: 10.1002/jmv.25825
- X. Ma, L. Su, Y. Zhang et al., Do children need a longer time to shen SARS-CoV-2 in stool than adults?, J. Microbiol. Immunol. Infect., (2020), in press, doi: https://doi.org/10.1016/j.jmii.2020.03.010
- A.D. Bowser: Coronavirus may cause environmental contamination through fecalshedding. Medscape Medical News, https://www.medscape.com/viewarticle/926390, 09.03.2020
- D.J. Weber, W.A. Rutala, W.A. Fischer et al., Emerging infectious diseases: Focus on infection control issues for novel coronaviruses (Severe Acute Respiratory Syndrome-CoV and Middle East Respiratory Syndrome-CoV), hemorrhagic fever viruses (Lassa and Ebola), and highly pathodenic avian influenza viruses, A(H5N1) and A(H7N9), Am. J. Infect. Control, 44 (2016), e91–e100, doi: https://doi.org/10.1016/j.ajic.2015.11.018
- L. Casanova, W.A. Rutala, D.J. Weber, M.D. Sobsey, Survival of surrogate coronaviruses in water, Water Res., 43 (2009), 1893-1898, doi: https://doi.org/10.1016/j.watres.2009.02.002
- J.S. Peiris, C.M. Chu, V.C.C. Cheng et al., Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. The Lancet, 361 (2003) 1767-1772, doi: https://doi.org/10.1016/s0140-6736(03)13412-5
- V. Naddeo, H. Liu, Editorial Perspectives: 2019 novel coronavirus (SARS-CoV-2): what is its fate in urban water cycle and how can the water research community respond? Environ. Sci.: Water Res. Technol, 6 (2020) 1213-1216, doi: https://doi.org/10.1039/D0EW90015J
- A. Oarga-Mulec, P. D. Jenssen, K. Klemen, T. Griessler Bulc, Zero-discharge solution for blackwater treatment at remote tourist facilities, J. Clean. Prod., 166 (2017) 798-805, doi: https://doi.org/10.1016/j.jclepro.2017.08.002
- M.R. Templeton, R.C. Andrews, R. Hofmann, Particle-associated viruses in water: impacts on disinfection processes, Crit. Rev. Environ. Sci. Technol., 38 (2008) 137–164, doi: https://doi.org/10.1080/10643380601174764
- M.E. Verbyla, J.R. Mihelcic, A review of virus removal in wastewater treatment pond systems, Water Res., 71 (2015) 107-124, doi: https://doi.org/10.1016/j.watres.2014.12.031
- A.T. Rachmadi, M. Kitajima, I.L. Pepper, C.P. Gerba, Enteric and indicator virus removal by surface flow wetlands, Sci. Tot. Environ., Part A, 542 (2016) 976–982, doi: https://doi.org/10.1016/j.scitotenv.2015.11.001
- K. Jalava, First respiratory transmitted foodborne outbreak?, Int. J. Hyg. Environ. Health., 226 (2020) 113490, doi: https://doi.org/10.1016/j.ijheh.2020.113490
- FAO/WHO, Viruses in food: scientific advice to support risk management activities: Meeting Report, https://www.who.int/foodsafety/publications/micro/Viruses_in_food_MRA.pdf?ua=1. 05.05.2020.
- Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19), 16–24, https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf, 08.05.2020
- S. Ramalingam, C. Graham, J. Dove et al., Hypertonic saline nasal irrigation and gargling should be considered as a treatment option for COVID-19, J Glob Health. 10 (2020), doi: https://doi.org/10. 10.7189/jogh.10.010332
- R. Patel, E. Babady, E. S. Theel et al., Report from the American Society for Microbiology COVID-19 International Summit, Mar 23, 2020: Value of Diagnostic Testing for SARS–CoV-2/COVID-19. mBio 11 (2020) e00722-20, doi: https://doi.org/10.1128/mBio.00722-20
- L. Mullis, L. Saif, Y. Zhang et al., Stability of bovine coronavirus on lettuce surfaces under household refrigeration conditions. Food Microbiol., 30 (2012) 180-186, doi: https://doi.org/10.1016/j.fm.2011.12.009
- S. Butot, T. Putallaz, G. Sanchez, Effects of sanitation, freezing and frozen storage on enteric viruses in berries and herbs. International Journal of Food Microbiology, 126 (2008) 30-35, doi: https://doi.org/10.1016/j.ijfoodmicro.2008.04.033
- M.E. Darnell, K. Subbarao, S.M. Feinstone, D.R. Taylor, Inactivation of the coronavirus that induces severe acute respiratory syndrome, SARS-CoV. J. Virol. Meth., 121 (2004) 85-91, doi: https://doi.org/10.1016/j.jviromet.2004.06.006
- A. Rismanbaf, S. Zarei, Letter to editor: Liver and Kidney Injuries in COVID-19 and Their Effects on Drug Therapy; a Letter to Editor, Archives of Academic Emergency Medicine, 8 (2020) e17
- A. Ferenczy, C. Bergeron, R. M. Richart, Human papillomavirus DNA in fomites on objects used for the management of patients with genital human papillomavirus infections, International Journal of Gynecology & Obstetrics, 32 (1990) 291–291, doi: https://doi.org/10.1016/0020-7292(90)90371-Q
- E. Duizer, P. Bijkerk, B. Rockx et al., Inactivation of Caliciviruses, Applied and Environmental Microbiology, 70 (2004) 4538–4543, doi: https://doi.org/10.1128/AEM.70.8.4538-4543.2004
- P.W. Brickner, The Application of Ultraviolet Germicidal Irradiation to Control Transmission of Airborne Disease: Bioterrorism Countermeasure, Public Health Reports, 118 (2003) 99–114, doi: https://doi.org/10.1093/phr/118.2.99
- C.P. Gerba, D.M. Gramos, N. Nwachuku, Comparative Inactivation of Enteroviruses and Adenovirus 2 by UV Light, AEM 68 (2002) 5167–5169, doi: https://doi.org/10.1128/AEM.68.10.5167-5169.2002
- Q.S. Meng, C.P. Gerba, Comparative inactivation of enteric adenoviruses, poliovirus and coliphages by ultraviolet irradiation, Water Research, 30 (1996) 2665–2668, doi: https://doi.org/10.1016/S0043-1354(96)00179-0
- J.A. Thurston-Enriquez, C.N. Haas, J. Jacangelo et al. Inactivation of Feline Calicivirus and Adenovirus Type 40 by UV Radiation, AEM, 69 (2003) 577–582, doi: https://doi.org/10.1128/AEM.69.1.577-582.2003
- C.C. Tseng, C.S. Li, Inactivation of Viruses on Surfaces by Ultraviolet Germicidal Irradiation, Journal of Occupational and Environmental Hygiene, 4 (2007) 400–405, doi: https://doi.org/10.1080/15459620701329012
- G. Lindsley, S.B. Martin, R.E. Thewlis et al., Effects of Ultraviolet Germicidal Irradiation (UVGI) on N95 Respirator Filtration Performance and Structural Integrity, Journal of Occupational and Environmental Hygiene, 12 (2015) 509–517, doi: https://doi.org/10.1080/15459624.2015.1018518
- H.F. Rabenau, G. Kampf, J. Cinatl, H.W. Doerr, Efficacy of various disinfectants against SARS coronavirus, Journal of Hospital Infection, 61 (2004) 107–111, doi: https://doi.org/10.1016/j.jhin.2004.12.023
- A.E. Torres, A.B. Lyons, S. Narla et al., Ultraviolet-C and other methods of decontamination of filtering facepiece N-95 respirators during the COVID-19 pandemic, Photochem. Photobiol. Sci., (2020), doi: https://doi.org/10.1039/D0PP00131G
- E. Livingston, A. Desai, M. Berkwits, Sourcing Personal Protective Equipment During the COVID-19 Pandemic, JAMA (2020), doi: https://doi.org/10.1001/jama.2020.5317
- A.W.H. Chin, J.T.S. Chu, M.R.A. Perera et al., Stability of SARS-CoV-2 in different environmental conditions, The Lancet Microbe (2020), S2666524720300033, doi: https://doi.org/10.1016/S2666-5247(20)30003-3
- L. Liao, W. Xiao, M. Zhao et al., Can N95 Respirators Be Reused after Disinfection? How Many Times?, ACS Nano (2020), doi: https://doi.org/10.1021/acsnano.0c03597
- Theory Division, Cleveland Clinic Lerner Research Institute et al., UV Sterilization of Personal Protective Equipment with Idle Laboratory Biosafety Cabinets During the Covid-19 Pandemic, Occupational and Environmental Health (2020), preprint, doi: https://doi.org/10.1101/2020.03.25.20043489
- J.J. Lowe, K.D. Paladino, J.D. Ferke et al, N95 filtering facemask respirator ultraviolet germicidal irradiation (UVGI) process for decontamination and reuse (2020), https://www.nebraskamed.com/sites/default/files/documents/covid-19/n-95-decon-process.pdf
- I.H. Hamzavi, A.B. Lyons, I. Kohli et al., Ultraviolet germicidal irradiation: possible method for respirator disinfection to facilitate reuse during COVID-19 pandemic, Journal of the American Academy of Dermatology (2020) S0190962220305089, doi: https://doi.org/10.1016/j.jaad.2020.03.085
- A. Cramer, D. Plana, H. L. Yang et al., Analysis of SteraMist ionized hydrogen peroxide technology as a method for sterilizing N95 respirators and other personal protective equipment, Occupational and Environmental Health (2020), preprint, doi: https://doi.org/10.1101/2020.04.19.20069997
- A. Schwartz, M. Stiegel, N. Greeson et al., Decontamination and Reuse of N95 Respirators with Hydrogen Peroxide Vapor to Address Worldwide Personal Protective Equipment Shortages During the SARS-CoV-2 (COVID-19) Pandemic, Appl Biosaf. (2020) 153567602091993, doi: https://doi.org/10.1177/1535676020919932
- J. Wong, Q.Y. Goh, Z. Tan et al., Preparing for a COVID-19 pandemic: a review of operating room outbreak response measures in a large tertiary hospital in Singapore, Can J Anesth/J Can Anesth (2020), doi: https://doi.org/10.1007/s12630-020-01620-9
- E. Oral, K.K. Wannomae, R.L. Connolly et al., Vapor H 2 O 2 sterilization as a decontamination method for the reuse of N95 respirators in the COVID-19 emergency, Occupational and Environmental Health (2020), preprint, doi: https://doi.org/10.1101/2020.04.11.20062026
- P. Kenney, B.K. Chan, K. Kortright et al., Hydrogen Peroxide Vapor sterilization of N95 respirators for reuse, Infectious Diseases (except HIV/AIDS) (2020), preprint, doi: https://doi.org/10.1101/2020.03.24.20041087
- L. Hao, J. Wu, E. Zhang et al., Disinfection efficiency of positive pressure respiratory protective hood using fumigation sterilization cabinet, Biosafety and Health, 1 (2019) 46–53, doi: https://doi.org/10.1016/j.bsheal.2019.02.006
- T.H. Derr, M.A. James, C.V. Kuny et al., Aerosolized Hydrogen Peroxide Decontamination of N95 Respirators, with Fit-Testing and Virologic Confirmation of Suitability for Re-Use During the COVID-19 Pandemic, Infectious Diseases (except HIV/AIDS) (2020), preprint, doi: https://doi.org/10.1101/2020.04.17.20068577
- V.C.C. Cheng, S.C. Wong, G.S. W. Kwan et al., Disinfection of N95 respirators by ionized hydrogen peroxide during pandemic coronavirus disease 2019 (COVID-19) due to SARS-CoV-2, Journal of Hospital Infection (2020) S019567012030178X, doi: https://doi.org/10.1016/j.jhin.2020.04.003
- G. Kampf, D. Todt, S. Pfaender, E. Steinmann, Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents, Journal of Hospital Infection, 104 (2020) 246–251, doi: https://doi.org/10.1016/j.jhin.2020.01.022
- Y. Goh, W. Chua, J.K.T. Lee et al., Operational Strategies to Prevent Coronavirus Disease 2019 (COVID-19) Spread in Radiology: Experience From a Singapore Radiology Department After Severe Acute Respiratory Syndrome, Journal of the American College of Radiology (2020) S1546144020303069, doi: https://doi.org/10.1016/j.jacr.2020.03.027
- A. Lizasoain, L.F.L. Tort, M. García et al., Human enteric viruses in a wastewater treatment plant: evaluation of activated sludge combined with UV disinfection process reveals different removal performances for viruses with different features, Lett. Appl. Microbiol., 66 (2018) 215-221, doi: https://doi.org/10.1111/lam.12839
- B. Yu, Y. Zhou, Z.W. Huang, Research on simple disinfection system for medical wastewater of township hospital, Asian J. Chem., 26 (2014) 11, 3243-3245, doi: https://doi.org/10.14233/ajchem.2014.17501
- J. Wang, J. Shen, D. Ye et al., Disinfection technology of hospital wastes and wastewater: suggestions for disinfection strategy during coronavirus Disease 2019 (COVID-19) pandemic in China, Environ. Pollut., 262 (2020), 114665, doi: https://doi.org/10.1016/j.envpol.2020.114665
- NHC, 2002: Technical Standard for Disinfection. https://scholar.google.com/scholar?q=NHC+Technical+Standard+for+Disinfection+2002+, 04.05.2020
- M.R. Braden: Chlorinator for wastewater treatment systems, US6627071B1 (United States patent). https://patentimages.storage.googleapis.com/e2/de/be/6705bc1c877136/US6627071.pdf, 02.04.2020
- L. Kong, G. Chu, Y. Wang et al., Study of wastewater advanced treatment and reclaimed water reuse for a large general hospital, Meteorolog. Environ. Res., 7 (2016) 37-39
- D. Fan, Y. Tan, L. Han et al., Catalytic electrolysis of sodium chlorite to prepare highly pure chlorine dioxide, J. Funct. Mater., 48 (2017) 9150-9156, doi: https://doi.org/10.3969/j.issn.1001-9731.2017.09.027
- L.W. Casson, J.W. Bess, On-site sodium hypochlorite generation, Proc. Water Environ. Fed., 2006 (2006) 6335-6352, doi: https://doi.org/10.2175/193864706783761374
- C.-F. Chiang, C.-T. Tsai, S.-T. Lin et al., Disinfection of hospital wastewater by continuous ozonization, J. Environ. Sci. Health, Part A, 38 (2003) 2895-2908, doi: https://doi.org/10.1081/ESE-120025839
- L.T. Kist, E.C. Rosa, Ê.L. Machado et al., Glutaraldehyde degradation in hospital wastewater by photoozonation, Environm. Technol., 34 (2013) 2579-2586, doi: https://doi.org/10.1080/09593330.2013.781200
- C. C. E. Meulemans, The basic principles of UV-disinfection of water, Ozone: Sci. Engin., 9 (1987) 299-313, doi: https://doi.org/10.1080/01919518708552146
- WHO 2020: Coronavirus disease (COVID-19) advice for the public. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public, 08.04.2020
- D. Pittet, B. Allegranzi, H. Sax et al., Evidence-based model for hand transmission during patient care and the role of improved practices, Lancet Inf. Dis., 6 (2006) 641-652, doi: 10.1016/S1473-3099(06)70600-4
- P. Mathur, Hand hygiene: back to the basics of infection control, Ind. J. Med. Res., 134 (2011) 611, doi: 10.4103/0971-5916.90985
- H. Carabin, T.W. Gyorkos, J.C. Soto et al., Effectiveness of a training program in reducing infections in toddlers attending day care centers, Epidemiology, 10 (1999) 219-227
- M.B. Ladegaard, V. Stage, Hand-hygiene and sickness among small children attending day care centers. An intervention study, Ugeskr. Laeg., 161 (1999) 4396-4400
- D.M. Conover, K.E. Gibson, A review of methods for the evaluation of handwashing efficacy, Food Contr., 63 (2016) 53-64, doi: 10.1016/j.foodcont.2015.11.020
- R. Montville, D. W. Schaffner, A meta-analysis of the published literature on the effectiveness of antimicrobial soaps, J. Food Protect., 74 (2011) 1875-1882, doi: 10.4315/0362-028X.JFP-11-122
- D.A. Jensen, D.R. Macinga, D.J. Shumaker et al., Quantifying the effects of water temperature, soap volume, lather time, and antimicrobial soap as variables in the removal of Escherichia coli ATCC 11229 from hands, J. Food Protect., 80 (2017) 1022-1031, doi: 10.4315/0362-028X.JFP-16-370
- WHO 2009: WHO guidelines on hand hygiene in health care: first global patient safety challenge: clean care is safer care, World Health Organization, Patient Safety, Geneva, Switzerland 2009, 262. https://apps.who.int/iris/bitstream/handle/10665/44102/9789241597906_eng.pdf?sequence=1, 01.04.2020
- A. Trampuz, A.F. Widmer, Hand hygiene: a frequently missed lifesaving opportunity during patient care, Mayo Clin. Proceed., 79 (2004) 109–116, doi: 10.4065/79.1.109
- D. Pires, H. Soule, F. Bellissimo-Rodrigues et al., Hand hygiene with alcohol-based hand rub: How long is long enough?, Inf. Contr. Hosp. Epidemiol., 38 (2017) 547-552, doi: 10.1017/ice.2017.25
- Q.-X. Ma, H. Shan, H.-L. Zhang et al., Potential utilities of mask-wearing and instant hand hygiene for fighting SARS-CoV-2, J. Med. Virol., (2020), doi: 10.1002/jmv.25805
- N. Ramesh, A. Siddaiah, B. Joseph, Tackling corona virus disease 2019 (COVID 19) in workplaces, Ind. J. Occupat. Environ. Med., 24 (2020) 16, doi: 10.4103/ijoem.IJOEM_49_20
- A. Wilder-Smith, D.O. Freedman, Isolation, quarantine, social distancing and community containment: pivotal role for old-style public health measures in the novel coronavirus (2019-nCoV) outbreak, J. Travel Med., 27 (2020) 2, doi: 10.1093/jtm/taaa020
- European centre for disease prevention and control: Considerations relating to social distancing measures in response to the COVID-19 epidemic. https://www.ecdc.europa.eu/en/publications-data/considerations-relating-social-distancing-measures-response-covid-19-second#no-link, 06.05.2020
- CDC 2020: Social distancing, quarantene and isolation. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/social-distancing.html, 22.04.2020
- Q.A. Ahmed, Z.A. Memish, The cancellation of mass gatherings (MGs)? Decision making in the time of COVID-19, Travel Med. Infect. Dis., 34 (2020) 101631, doi: 10.1016/j.tmaid.2020.101631
- M. Cetron, J. Landwirth, Public health and ethical considerations in planning for quarantine, Yale J. Biol. Med., 78 (2005) 329-334
- N.M. Linton, T. Kobayashi, Y. Yang et al., Incubation period and other epidemiological characteristics of 2019 Novel Coronavirus infections with right truncation: a statistical analysis of publicly available case data, J. Clin. Med., 9 (2020) 538, doi: 10.3390/jcm9020538
- Y. Wang, Y. Wang, Y. Chen et al., Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID‐19) implicate special control measures, J. Med. Virol., 92 (2020) 568-576, doi: 10.1002/jmv.25748
- K.-T. Goh, J. Cutter, B.-H. Heng et al., Epidemiology and control of SARS in Singapore, Ann. Acad. Med. Singap., 35 (2006) 301-316
- N. van Doremalen, T. Bushmaker, D. H. Morris et al., Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1, N. Engl. J. Med., 382 (2020) 1564-1567, doi: 10.1056/NEJMc2004973
- WHO 2020: Infection prevention and control guidance forlong-term care facilities in the context of COVID-19. Technical brief supplements on water, sanitation, hygiene and waste management for COVID-19. https://apps.who.int/iris/bitstream/handle/10665/331508/WHO-2019-nCoV-IPC_long_term_care-2020.1-eng.pdf, 08.04.2020
- WHO 216: Decontamination and reprocessing of medical devices for health-care facilities. Geneva: World Health Organization. https://www.who.int/infectionprevention/publications/decontamination/en/, 08.04.2020
- S.A. Sattar, V.S. Springthorpe, Y. Karim et al., Chemical disinfection of non-porous inanimate surfaces experimentally contaminated with four human pathogenic viruses, Epidemiol. Inf., 102 (1989) 493-505, doi: 10.1017/S0950268800030211
- WHO 2014: Annex G, Use of disinfectants: alcohol and bleach. Infection prevention and control of epidemic-and pandemic-prone acute respiratory infections in health care (65-66). https://apps.who.int/iris/bitstream/handle/10665/112656/9789241507134_eng.pdf?sequence=1, 08.04.2020
- EPA 2020: List N: Disinfectants for use against SARS-CoV-2. https://www.epa.gov/pesticide-registration/list-n-disinfectants-use-against-sars-cov-2, 04.22.2020
- V. Offeddu, C.F. Yung, M.S.F. Low et al., Effectiveness of masks and respirators against respiratory infections in healthcare workers: a systematic review and meta-analysis, Clin. Inf. Dis., 65 (2017) 1934-1942, doi: 10.1093/cid/cix681
- C.R. MacIntyre, Q. Wang, S. Cauchemez et al., A cluster randomized clinical trial comparing fit-tested and non-fit-tested N95 respirators to medical masks to prevent respiratory virus infection in health care workers: RCT of face masks in health workers, Influenza and other Respiratory Viruses, 5 (2011) 170–179, doi: https://doi.org/10.1111/j.1750-2659.2011.00198.x
- CDC 2020: Recommendation regarding the use of cloth face coverings, especially in areas of significant community-based transmission. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/cloth-face-cover.html, 04.22.2020
- R. Tellier, Y. Li, B.J. Cowling et al., Recognition of aerosol transmission of infectious agents: a commentary, BMC Infect. Diseas., 19 (2019) 1, doi: 10.1186/s12879-019-3707-y
- Modes of transmission of virus causing COVID-19: Implications for IPC precaution recommendations. https://www.who.int/publications-detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations, 07.05.2020
- GOV. UK: Transmission characteristics and principles of infection prevention and control. https://www.gov.uk/government/publications/wuhan-novel-coronavirus-infection-prevention-and-control/transmission-characteristics-and-principles-of-infection-prevention-and-control, 07.05.2020
- C. Zhao, A. Viana, Y. Wang et al., Otolaryngology during COVID-19: Preventive care and precautionary measures, Am. J. Otolaryng., (2020) 102508, doi: 10.1016/j.amjoto.2020.102508
- A. Lovato, C. de Filippis, Clinical presentation of COVID-19: a systematic review focusing on upper airway symptoms, Ear, Nose & Throat J., (2020) 014556132092076, doi: 10.1177/0145561320920762
- B. Fu, K. Qian, X. Fu, SARS-CoV-2-induced vomiting as onset symptom in a patient with COVID-19, Digest. Dis. Sci., (2020), doi: 10.1007/s10620-020-06285-4
- P. Raspor, Total food chain safety: how good practices can contribute?, Trends in Food Sci. Tec., 19 (2008) 405-412, doi: 10.1016/j.tifs.2007.08.009
- Codex Alimentarius Commission, Joint FAO WHO Food Standards Programme, Food hygiene: basic texts; Codex Alimentarius, 4th ed., FAO, Rome 2009, 125
- P. Raspor, M. Jevšnik, Good Nutritional practice from producer to consumer, Crit. Rev. Food Sci. Nutrit., 48 (2008) 276-292, doi: 10.1080/10408390701326219
- Codex Alimentarius: Protecting the food supply chain from COVID-19. http://www.fao.org/fao-who-codexalimentarius/news-and-events/news-details/en/c/1270223/, 08.04.2020
- Y. Wu, C. Guo, L. Tang et al., Prolonged presence of SARS-CoV-2 viral RNA in faecal samples, Lancet Gastroent. Hepatol., 5 (2020) 434-435, doi: 10.1016/S2468-1253(20)30083-2
- L. Zhang, Y. Liu, Potential interventions for novel coronavirus in China: a systematic review, J. Med. Virol., 92 (2020) 479-490, doi: 10.1002/jmv.25707
- R. D. Semba, Vitamin A and immunity to viral, bacterial and protozoan infections, Proceed. Nutrit. Soc., 58 (1999) 719-727, doi: 10.1017/S0029665199000944
- E. Villamor, R. Mbise, D. Spiegelman et al., Vitamin A supplements ameliorate the adverse effect of HIV-1, malaria, and diarrheal infections on child growth, Pediatrics, 109 (2002) E6, doi: 10.1542/peds.109.1.e6
- U.S. National Library of Medicine: ClinicalTrials.gov. https://clinicaltrials.gov, 01.05.2020
- J. Xu, J. Yang, J. Chen et al., Vitamin D alleviates lipopolysaccharide-induced acute lung injury via regulation of the renin-angiotensin system, Molec. Med. Rep., 16 (2017) 7432-7438, doi: 10.3892/mmr.2017.7546
- Y. Zhao, Z. Zhao, Y. Wang et al., Single-cell RNA expression profiling of ACE2, the receptor of SARS-CoV-2, Bioinformatics (preprint), (2020), doi: 10.1101/2020.01.26.919985
- W. Li, M.J. Moore, N. Vasilieva et al., Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus, Nature, 426 (2003) 6965, 450-454, doi: 10.1038/nature02145
- D.S. Dimitrov, The Secret Life of ACE2 as a Receptor for the SARS Virus, Cell, 115 (2003) 652-653, doi: 10.1016/S0092-8674(03)00976-0
- S. Ajabshir, A. Asif, A. Nayer, The effects of vitamin D on the renin-angiotensin system, J Nephropathol., 3 (2014) 41-43, doi: 10.12860/jnp.2014.09
- Y.C.Li, Vitamin D and the renin-angiotensin system, Vitamin D, 3rd ed., Elsevier, 2011, 707-723. doi: https://doi.org/10.1016/B978-0-12-381978-9.10040-X
- M. Laplana, J. L. Royo, J. Fibla, Vitamin D receptor polymorphisms and risk of enveloped virus infection: a meta-analysis, Gene, 678 (2018) 384-394, doi: 10.1016/j.gene.2018.08.017
- WHO 2017: Vitamin D for prevention of respiratory tract infections. https://www.who.int/elena/titles/commentary/vitamind_pneumonia_children/en/, 02.05.2020
- C.L. Greiller, A.R. Martineau, Modulation of the immune response to respiratory viruses by vitamin D, Nutrients, 7 (2015) 4240-4270, doi: 10.3390/nu7064240
- T.-T. Wang, B. Dabbas, D. Laperriere et al., Direct and indirect induction by 1,25-dihydroxyvitamin D3 of the NOD2/CARD15-defensin β2 innate immune pathway defective in Crohn disease, J. Biol. Chem., 285 (2010) 2227–2231, doi: 10.1074/jbc.C109.071225
- A.F. Gombart, N. Borregaard, H.P. Koeffler, Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3, FASEB J., 19 (2005) 1067-1077, doi: 10.1096/fj.04-3284com
- M.Y. Yakoob, R.A. Salam, F.R. Khan et al., Vitamin D supplementation for preventing infections in children under five years of age, Cochrane Database Syst. Rev., 11 (2016) CD008824, doi: 10.1002/14651858.CD008824.pub2
- P. Bergman, Å.U. Lindh, L. Björkhem-Bergman et al., Vitamin D and respiratory tract infections: a systematic review and meta-analysis of randomized controlled trials, PLoS ONE, 8 (2013) e65835, doi: 10.1371/journal.pone.0065835
- J. Charan, J. Goyal, D. Saxena et al., Vitamin D for prevention of respiratory tract infections: a systematic review and meta-analysis, J. Pharmacol. Pharmacother., 3 (2012) 300, doi: 10.4103/0976-500X.103685
- A. R. Martineau, D. A. Jolliffe, R. L. Hooper et al., Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data, Brit. Med. J., (2017) i6583, doi: 10.1136/bmj.i6583
- H. Hemila, Vitamin C and SARS coronavirus, J. Antimicrob. Chemother., 52 (2003) 1049-1050, doi: 10.1093/jac/dkh002
- J.G. Atherton, C.C. Kratzing, A. Fisher, The effect of ascorbic acid on infection of chick-embryo ciliated tracheal organ cultures by coronavirus, Arch. Virol., 56 (1978) 195-199, doi: 10.1007/BF01317848
- M. Maares, H. Haase, Zinc and immunity: an essential interrelation, Arch. Biochem. Biophys., 611 (2016) 58-65, doi: 10.1016/j.abb.2016.03.022
- A.J.W. te Velthuis, S.H.E. van den Worm, A.C. Sims et al., Zn2+ inhibits Coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture, PLoS Pathog., 6 (2010) e1001176, doi: 10.1371/journal.ppat.1001176
- D. Schatz, A. Vardi, Extracellular vesicles - new players in cell-cell communication in aquatic environments, Curr. Opin. Microbiol., 43 (2018) 148-154, doi: 10.1016/j.mib.2018.01.014
- K.D.A. Mojica, C.P.D. Brussaard, Factors affecting virus dynamics and microbial host - virus interactions in marine environments, FEMS Microbiol. Ecol., 89 (2014) 495-515, doi: 10.1111/1574-6941.12343
- T. Wurdinger, N.N. Gatson, L. Balaj et al., Extracellular vesicles and their convergence with viral pathways, Adv. Virol., 2012 (2012) 1-12, doi: 10.1155/2012/767694
- S. Gill, R. Catchpole, P. Forterre, Extracellular membrane vesicles in the three domains of life and beyond, FEMS Microbiol. Rev., 43 (2019) 273-303, doi: 10.1093/femsre/fuy042
- W. Lösche, T. Scholz, U. Temmler et al., Platelet-derived microvesicles transfer tissue factor to monocytes but not to neutrophils, Platelets, 15 (2004) 109-115, doi: 10.1080/09537100310001649885
- N. Raab-Traub, D.P. Dittmer, Viral effects on the content and function of extracellular vesicles, Nat. Rev. Microbiol., 15 (2017) 559-572, doi: 10.1038/nrmicro.2017.60
- E. Nolte-‘t Hoen, T. Cremer, R.C. Gallo et al., Extracellular vesicles and viruses: are they close relatives? Proc. Natl. Acad. Sci. USA, 113 (2016) 9155-9161, doi: 10.1073/pnas.1605146113
- M. Gaudin, M. Krupovic, E. Marguet et al., Extracellular membrane vesicles harbouring viral genomes: viral vesicles, Environ. Microbio.l, 16 (2014) 1167-1175, doi: 10.1111/1462-2920.12235
- N. Altan-Bonnet, Extracellular vesicles are the Trojan horses of viral infection, Curr. Opin. Microbiol., 32 (2016) 77–81, doi: 10.1016/j.mib.2016.05.004
- D. Schatz, S. Rosenwasser, S. Malitsky, et al., Communication via extracellular vesicles enhances viral infection of a cosmopolitan alga, Nat. Microbiol., 2 (2017) 1485-1492, doi: 10.1038/s41564-017-0024-3
- S.J. Biller, F. Schubotz, S.E. Roggensack et al., Bacterial vesicles in marine ecosystems, Science, 343 (2014) 183-186, doi: 10.1126/science.1243457
- S. Kumar, K. Zhi, A. Mukherji et al., Repurposing antiviral protease inhibitors using extracellular vesicles for potential therapy of COVID-19, Viruses, 12 (2020) 486, doi: 10.3390/v12050486
- K. Lee, A. Mazare, P. Schmuki, One-dimensional titanium dioxide nanomaterials: nanotubes, Chem. Rev., 114 (2014) 9385-9454, doi: 10.1021/cr500061m
- A. Iglič, M. Kulkarni, A. Flasker et al., Binding of plasma proteins to titanium dioxide nanotubes with different diameters, Int. J. Nanomed., (2015) 1359, doi: 10.2147/IJN.S77492
- M. Kulkarni, A. Mazare, E. Gongadze et al., Titanium nanostructures for biomedical applications, Nanotechnology, 26 (2015) 062002, doi: 10.1088/0957-4484/26/6/062002
- T. Mavrič, M. Benčina, R. Imani et al., Electrochemical biosensor based on TiO2 nanomaterials for cancer diagnostics, Adv. Biomemb. Lipid Self-Assembly, Elsevier, 2018, 63-105, doi: 10.1016/bs.abl.2017.12.003
- M. Benčina, I. Junkar, T. Mavrič et al., Performance of annealed TiO2 nanotubes in interactions with blood platelets, Mater. Technol., 53 (2019) 791-795, doi: 10.17222/mit.2018.249
- M. Kulkarni, J. Šepitka, I. Junkar et al., Mechanical properties of anodic titanium dioxide nanostructures, Mater. Technol. (in print), (2020).
- T. Lampe, M. Benčina, V. Šuštar et al., Interaction of blood cells and extracellular vesicles with nanostructured surfaces, (in preparation), (2020).

