Considering Costs of Complications in Bariatric Surgery for the Evaluation of New Technologies based on the Example of Surgical Robotics
Monika EH1*, Jonathan D1, Gilles C2, Minoa KJ1, Nicolas CB1 and Christian T1
1Division of Digestive and Transplant Surgery, Department of Surgery, University Hospital Geneva, 4 Rue Gabrielle-Perret-Gentil, 1211 Geneva, Switzerland
2Medical controlling, University Hospital Geneva, 4 Rue Gabrielle-Perret-Gentil, 1211 Geneva, Switzerland
Received Date: 27/07/2020; Published Date: 14/08/2020
*Corresponding author: Monika E Hagen, Department of Surgery, Division of Digestive and Transplant Surgery, University Hospital Geneva, 4 Rue Gabrielle-Perret-Gentil, 1211 Geneva, Switzerland. Tel: +41 76 615 9447; E-mail: monikahagen@aol.com
Abstract
Purpose:The purpose of this analysis is to analyze the cost of complications of bariatric surgery to support the economic evaluation of new surgical technologies based on the example of robotic surgery.
Methods: Patients who underwent robotic bariatric surgery from 2014 to 2015 with complete economic data were included. The itemized and treatment costs were derived using the REKOLE method and stratified using the Clavien-Dindo classification.
Results: A total of 195 patients were included. 88.7% of patients underwent primary Roux-en-Y gastric bypass surgery, 6.2% underwent gastric sleeve resection, and 5.1% underwent revisional surgery. 136 patients had no complication, 42 were classified with a Clavien I complication, 6 with a Clavien II complication, and 11 with a Clavien III complication. The mean treatment costs were USD 19,857 for patients without complication, USD 20,575 for patients with a Clavien I complication, USD 29,069 for a Clavien II complication, and USD 52,473 for a Clavien III complication.
Conclusion: Complications are important cost drivers with an incremental correlation to the Clavien-Dindo classification. While minor complications have a clear impact, major complications have an exponential effect on overall costs. As such, a decrease in complications might justify higher procedural costs due to the use of new technologies.
Keywords: Cost; Complications; Robotic Surgery; Gastric Bypass; RYGB; Bariatric Surgery
Introduction
New surgical techniques like minimally invasive surgeries, including robotics provide patient benefits such as shorter hospital stay and reduced surgical trauma when compared to the traditional open approach [1]. However, several studies have shown higher costs for robotic surgery when compared with laparoscopy or open surgery [2-4]. Although new technologies are integral to the advancement of surgical care, they often have – sometimes obstructive - incremental costs that are typically associated with significant upfront investments for the initial purchase as well as procedure-related costs, which is also the case for surgical robotics [5, 6].
Since the first published robotic procedures more than two decades ago, the adoption of this technology has increased across surgical specialties with a clear uptake in recent years [7]. However, adoption of robotic surgery still limited to the minority of overall world-wide performed surgical procedures [7]. An important factor that contributes to the limited adoption are the high costs of these systems while the clinical value is still under evaluation as independent high impact factor. As an example, the da Vinci Surgical System – the currently most widely used robotic system - accrues capital costs ranging from USD 0.5M to 2.5M, the annual service costs range from USD 80K to 190K, and the per procedure instruments and accessories’ costs range from USD 700 to 3,500 [7]. These costs are – depending on the structure of the respective healthcare environment – at the burden of either the healthcare provider, the insurer, the patient, or another third party [5]. If these upfront costs cannot be balanced with other factors, they might become prohibitive, particularly in healthcare systems that utilize a flat-fee reimbursement. In addition, high-quality and industry-independent research supporting clinical superiority of robotics over conventional approaches is missing at present. As such, technologies should not only be evaluated for its safety and efficacy, but also its clinical impact and cost efficiency.
Potential options for reducing the cost of surgeries using expensive equipment include savings on other material and the improvement of surgical quality, resulting in fewer complications. This concept has been demonstrated for robotic gastric bypass surgeries [8]. However, detailed research is complex, and it seems intriguing to understand the cost structures of surgical complications to estimate the impact of clinical improvements on the cost of surgery.
To date, there is no available reliable model that estimates the specific costs of complications, which could facilitate modeling the cost-effectiveness of new technologies. This study aims to analyze the costs of complications during bariatric surgery and discuss these costs in light of incremental costs for new technologies using the example of robotics.
Materials and Methods
Patients
This is a retrospective single center analysis of patients who underwent bariatric surgery between January 2014 and December 2015 at the University Hospital Geneva (Switzerland). Patients with missing economic data were excluded.
Patients were operated with either a fully robotic or hybrid approach using the da Vinci Si or Xi Surgical System (Intuitive Surgical Inc., Sunnyvale, CA, USA). The procedures have been previously described [8-11].
Patients routinely entered the hospital the morning of the procedure and stayed in the intermediate care unit until released from anesthesia to the standard ward. Routine blood testing was performed on day 1 and patients were discharged after successful re-alimentation, pain control with oral medication, and a willingness to return home. If medically necessary, deviations from this path were planned and executed.
Data Collection
Clinical data was derived from our prospective database and individual cases’ charts were reviewed is information was missing. Patients were stratified using the Clavien-Dindo Classification as per the original publication [12]. In case patients underwent additional imaging or other testing outside the previously described clinical pathway, they were classified as a Clavien-Dindo I case, even if imaging or other testing was negative and not strictly indicated (for example ordered by a resident without approval).
The medical cost data included in this study was obtained using the REKOLE method [13]. The REKOLE method is the Swiss national cost accounting system used in hospitals in Switzerland. The REKOLE system was introduced because the Swiss government opted for comparable and transparent hospital costs. Under the REKOLE method, costs are defined as total direct costs of inpatient care and all hospitals adhere to a minimum standard. The REKOLE system was established at the same time as the change of the reimbursement system from the per-diem payment system to the Diagnose Related Groups (DRG) payment system after January 2012, and it has been used since then for academic cost analyses [5].
Statistical Analysis
Statistical analysis was carried with Stata 15.0 software (Stata Corp., College Station, USA). P-value lower than 0.05 was considered statistically significant. Descriptive statistics included means and standard deviation for continuous data, or frequency and percentage for discrete data. Considering more than 2 groups of complications, costs comparisons were carried through one-way analysis of variances (ANOVA) assuming the normal distribution of costs within groups, independence of observations and normal distribution of variances. Tukey’s honest significant difference (Tukey HSD) test was chosen as post-hoc test to explore differences between groups and determine their relative statistical significance.
Results
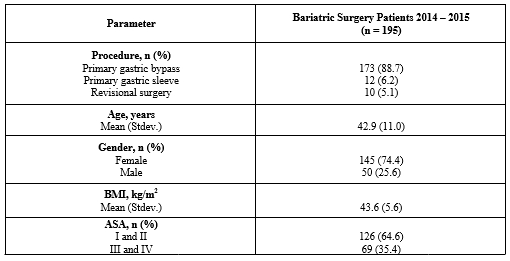
195 patients were included in this analysis. 173 (88.7%) underwent primary gastric bypass, 12 (6.2%) underwent gastric sleeve resection, and 10 (5.1%) underwent a revisional procedure. The mean age in this cohort was 42.9 (+/-11) years and 145 (74.4%) were female. The mean BMI was 43.6 (+/- 5.6) kg/m2. 126 (64.6%) were classified as American Society of Anesthesiologists (ASA) 1 or 2 and 69 (53.4%) as ASA 3 or 4. The details regarding demographic parameters can be found in Table 1.
Table 1: Demographic parameters.
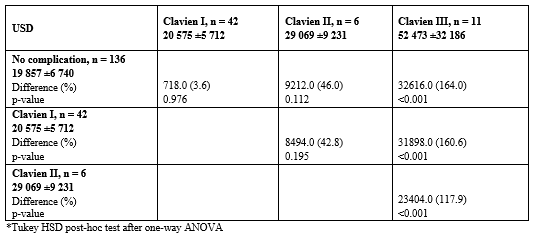
136 patients had no complication, 42 a Clavien I, 6 a Clavien 2 and 11 a Clavien 3. The mean 18 days treatment costs for patients without complications were USD 19,857 (+/- 6,740), USD 20,575 (+/- 5,712) for patients with a Clavien I, USD 29,069 (+/- 9,231) for patients with a Clavien II, and USD 52,473 (+/- 32,185.97) for patients with a Clavien III complication. There was a statistically significant difference between groups as determined by one-way ANOVA (F(3,191) = 39.132, p <0.001). The details regarding costs per complication and incremental costs can be found in Table 2.
Table 2: Treatment costs stratified by Clavien-Dindo classification.
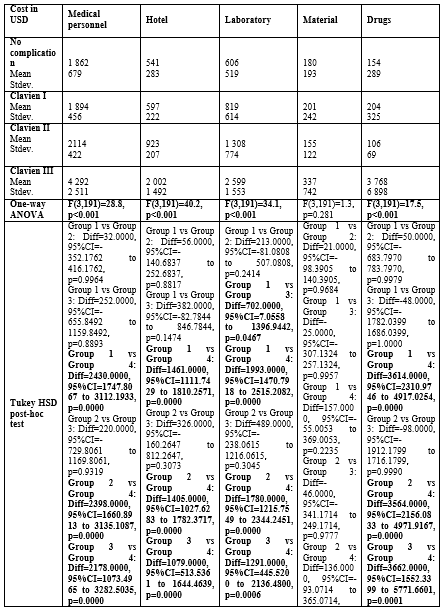
The costs were itemized into operating room, anesthesia, intensive care unit, nursing, imaging, medical personnel, hotel, laboratory, material, and drugs. No statistically significant differences were observed for all patient groups for materials. All other categories showed significantly higher costs for patients with a Calvien III complication versus patients without complications and patients with a Clavien I complication. Differences for all categories were non-significant between a Clavien I complication and no complications. A significant difference between a Clavien II complication and no complications were only observed for laboratory. The itemized costs are listed in Table 3 and Table 4.
Table 3: Itemized costs stratified by Clavien-Dindo classification: Operating Room, Anesthesia, Intensive Care Unit, Nursing, Imaging.

Table 4: Itemized costs stratified by Clavien-Dindo classification: Medical Personnel, Hotel, Laboratory, Material, Drugs.
Discussion
To our knowledge, this is the first attempt to systematically evaluate the specific cost of complications in bariatric surgery. While it appears logical that more significant complications generate higher perioperative treatment costs, more detailed insights are essential to allow more precise economic modeling when evaluating the financial impact of postoperative complications. A detailed cost data of complications may also offer insights into the source of expenses when perioperative complications occur. In addition, if the structure of the cost data is well understood in the clinical context, it may be used as a tool to minimize the financial impact of complications.
Our data show the baseline cost outcomes of patients without complications, which are in line with previous publications from our group [5, 8]. Interesting observations can be found when closely evaluating the itemized costs as outlined in Table 3: operating room and anesthesia costs are similar for patients with and without a Clavien I complication, but this post is slightly higher for patients with a Clavien II and significantly higher for Clavien III complication. It appears logical that a Clavien III complication incurs higher costs for the operating room on average because, as per its definition, this class of complication requires a re-intervention. However, this should not be the case for patients suffering from a Clavien II complication. While not statistically significant – also due to the relatively small cohort of patients with a Clavien II complication - we found higher OR costs for patients with a Clavien II complication when compared to patients with or without a Clavien I complication. In this cohort, the cost increases most likely stem from the initial surgery as no re-intervention was conducted. This can be caused either by a prolonged procedure or by higher usage of instruments during the initial surgery. It seems reasonable to assume that a deviation from the standard surgical path (standard length case and/or standard set of instruments) might put the patients at a higher risk of perioperative complications. Still, this dataset does not allow the exact identification of cost drivers in each itemized segment and this comparison is not adequately powered. As such, there might also be a bias since longer procedures can be more complex in patients with high BMIs and other conditions that may lead to longer OR times or higher risk of postoperative complications. It would be interesting to explore further if cost-driving events in the OR can be used as predictors for perioperative complications and as such, if warning systems could be developed depending on the course of the surgical procedures. However, this dataset is not sufficiently deep for such analyses.
A closer look at the itemized costs of the patients with a Clavien I complication show comparable costs to the cohort without complications. However, we found an increase - although not statistically significant - in imaging and laboratory costs. This observation seems reasonable in the clinical context as a Clavien I complication is a deviation from the standard clinical path not requiring specific treatment. As a matter of fact, most of these patients did not have a real medical problem, bur received additional imaging or laboratory work prescribed by an inexperienced resident. As a teaching institution, residents are primarily responsible for the post-operative patients at night and over weekends and have the freedom to order these tests. Under these circumstances, we often observe a rather generous use sometimes without clear medical indication. However, as per definition these patients need to be classified as a Clavien I and it easily explains increased costs for imaging and laboratory. Another logical observation is the absence of intensive care unit costs for patients with or without a Clavien I and Clavien II complication, but the presence of intensive care unit costs when a re-intervention is needed. All the above mentioned clinically relevant consistencies suggest that this set of cost data is robust and can be used as a solid baseline for economic modeling.
Overall, Clavien I complications within the 18 days of robotic bariatric surgery seem to incur a moderate cost increase of about 3.6%, but the further increase of about 36% and 164% for Clavien II and III complications are rather exponential. It appears evident that any complication requiring treatment is not only devastating for the patient, but also an economic challenge to the healthcare system. As such, there is room for investments to avoid complications and from an economic perspective. As this is a first dataset quantifying the economic impact of complications in bariatric surgery, it can be used as a guide to show the extent of upfront investments that can be afforded to improve outcomes. While these investments might include a wide range of measures including surgeon training, patient preparation, and others, we want to focus on the investment in new surgical technologies. Albeit the absence of high quality and high level of evidence supporting robotics for a widespread application in bariatric surgery, this technology finds an increasing adoption, also driven by the desire to reduce perioperative complications of bariatric surgery. As an example, a few groups have published the positive impact of surgical robotics for gastric bypass surgery regarding procedure times per BMI and a reduction in severe complications such as intestinal leaks [8,14-16]. One study showed a cost reduction with a robotic approach versus laparoscopy for gastric bypass surgery due to a reduction in complications and the use of fewer surgical staplers [8]. This dataset shows that a Clavien III complication costs about USD 33,000. Therefore, a 1% reduction in a postoperative re-intervention rate would save USD 33,000 in 100 patients, and this money can be spent on new surgical technologies such as robotics.
Currently, the da Vinci Surgical System is the most widely used robot and its published costs have been listed above [7]. While these costs may fluctuate depending on the exact surgical approach and the instruments required, this post has been calculated to be USD 1,582.91 in a previous academic publication for gastric bypass surgery in 2011 [8]. Applying this cost structure, it becomes obvious, that a 1% reduction in re-interventions (saving about USD 33,000) would not be sufficient to counterbalance this upfront investment, but a significantly greater reduction of Clavien III complications would be required to financially justify the use of the da Vinci Surgical System. While this is a very simplified model, it outlines the practical applicability of this dataset and the extent of financial considerations that have to be made before widespread clinical adoption.
Still, several shortcomings of this analysis have to be mentioned: first, this dataset is mostly based on economic data and many conclusions require more data breadth and depth. In addition, this analysis includes a limited number of subjects in the healthcare environment of Switzerland. As such, the data might not be applicable in other geographic regions or might not be the same when looking at larger cohorts. Also, this is a retrospective analysis with all commonly known sources of bias. Lastly, the cost of robotic surgery is currently under development: Intuitive Surgical as well as new competitors address the cost issue with alternative models including flat-fee payments and lease options [17, 18]. Still, this is a unique approach for analyzing the bariatric cohort and might be a decent source of reflection for both clinicians and economists.
Conclusion
Complications are important cost drivers and correlate to the Clavien-Dindo classification with an exponential increase for more significant complications. While this is a good rationale to justify higher procedural costs if complications can be avoided, major investments including current surgical robotics might not be offset by a clinically realistic reduction of complications. More systematic research, including the analyses of broader and deeper datasets, is needed to generate more detailed insights into the correlation of perioperative complications and costs.
Conflict of Interest
Dr. Hagen reports grants from Intuitive Surgical Inc., personal fees and non-financial support from Quantgene Inc., personal fees and non-financial support from Verb Surgical, personal fees and non-financial support from Johnson & Johnson, outside the submitted work.
Dr. Jung reports personal fees and non-financial support from Intuitive Surgical Inc., personal fees and non-financial support from Ethicon Endosurgery, outside the submitted work.
Dr. Douissard reports grants from Intuitive Surgical Inc., personal fees and non-financial support from Verb Surgical, outside the submitted work.
Dr. Cohen has nothing to disclose.
Dr. Buchs has nothing to disclose.
Dr. Toso reports grants from Intuitive Surgical Inc, personal fees and non-financial support from Ethicon Endosurgery Inc., outside the submitted work.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Acknowledgements
We thank Francoise Bernardi (University Hospital Geneva) for data collection.
References
-
-
-
1. Fuchs KH, Minimally invasive surgery. Endoscopy. 2002;34(2):154-9.
2. Abdelmoaty WF, et al. Robotic-assisted versus laparoscopic unilateral inguinal hernia repair: a comprehensive cost analysis. Surg Endosc. 2018.
3. Hagen ME, et al. Robotic single-site versus multiport laparoscopic cholecystectomy: a case-matched analysis of short- and long-term costs. Surg Endosc. 2018. 32(3):1550-1555.
4. Trehan A. and TJ. Dunn, The robotic surgery monopoly is a poor deal. BMJ, 2013;347:7470.
5. Hagen, ME. et al, Robotic Gastric Bypass Surgery in the Swiss Health Care System: Analysis of Hospital Costs and Reimbursement. Obes Surg. 2017.27(8):2099-2105.
6. Niklas, C., et al., da Vinci and Open Radical Prostatectomy: Comparison of Clinical Outcomes and Analysis of Insurance Costs. Urol Int. 2016;96(3):287-94.
7. Inc., I.S., Investor Presentation Q4 2019. 2019, Accessed 11-19-2019: https://isrg.intuitive.com/static-files/0fc01a59-8d32-481f-9872-b262fd1f87b2.
8. Hagen, M.E., et al., Reducing cost of surgery by avoiding complications: the model of robotic Roux-en-Y gastric bypass. Obes Surg. 2012;22(1):52-61.
9. Buchs, N.C., et al., Intra-operative fluorescent cholangiography using indocyanin green during robotic single site cholecystectomy. Int J Med Robot. 2012;8(4):436-40.
10. Buchs, N.C., et al., Robot-assisted Roux-en-Y gastric bypass for super obese patients: a comparative study. Obes Surg, 2013;23(3):353-7.
11. Hagen, M.E., et al., Robotic versus laparoscopic stapling during robotic Roux-en-Y gastric bypass surgery: a case-matched analysis of costs and clinical outcomes. Surg Endosc, 2018;32(1):472-477.
12. Dindo, D., N. Demartines, and P.A. Clavien, Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg, 2004;240(2):205-13.
13. Besson, P., REKOLE® - Betriebliches Rechnungswesen im Spital, (Comptabilité analytique à l’hôpital) 2013: Die Spitäler der Schweiz H+, Bern.
14. Sanchez BR, et al., Comparison of totally robotic laparoscopic Roux-en-Y gastric bypass and traditional laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2005;1(6):549-54.
15. Mohr, C.J., et al., Totally robotic laparoscopic Roux-en-Y Gastric bypass: results from 75 patients. Obes Surg. 2006;16(6):690-6.
16. Tieu K, et al. Robotic-assisted Roux-en-Y gastric bypass: update from 2 high-volume centers. Surg Obes Relat Dis. 2013;9(2):284-8.
17. Guthard G. JP Morgan Healtcare Conference 2020. 2020.
18. CMR. accessed 02-16-2020: https://cmrsurgical.com/versius/.
-
-

