Clinical Pharmacology of Felodipine
Gian Maria Pacifici*
Professor of Pharmacology, Via Sant’Andrea 32, 56127 Pisa, Italy
Received Date: 04/03/2025; Published Date: 09/04/2025
*Corresponding author: Gian Maria Pacifici, Professor of Pharmacology, Via Sant’Andrea 32, 56127 Pisa, Italy. E-mail: prof.pacifici.gianmaria@gmail.com
Abstract
Felodipine, a dihydropyridine, is a multiple Ca2+ channel blocker approved for clinical use. Felodipine was found to be efficacy and safe in hypertensive patients, effectively treated hypertensive patients, and six studies have been reported on the trials conducted with felodipine in hypertensive patients. The pharmacokinetics of felodipine have been studied in healthy subjects and in hypertensive patients following oral administration. Felodipine is rapidly absorbed as the time to reach the peak plasma is about 1.5 hours and is rapidly eliminated as the elimination half-life ranges from 18.4 to 28.7 hours and increases with the age of subjects and patients. Felodipine undergoes a presystemic first-pass metabolism being metabolized by CYP3A4 and the bioavailability of felodipine is about 15%. The bioavailability of felodipine is reduced by inducers of CYP3A4 and is increased by inhibitors of CYP3A4. Felodipine is rapidly cleared and the clearance of felodipine ranges from 448 to 821 ml/min and decreases with the age of subjects and patients. Four studies have been reported on the interaction of felodipine with drugs or with grapefruit juice and a study showed that an overdose of 250 mg of felodipine kills a man. The aim of this study is to review the efficacy and safely of felodipine, the treatment of hypertensive patients with felodipine, and the trials conducted with felodipine in hypertensive patients. Furthermore, the metabolisms of felodipine, the pharmacokinetics of felodipine, the interaction of felodipine with drugs or with grapefruit juice, and the toxicity induced by felodipine have been reviewed.
Keywords: Drug-interaction; Efficacy-safely; Felodipine; Metabolism; Pharmacokinetics; Toxicity; Treatment; Trials
Introduction
Mechanisms of action of felodipine
Felodipine, a dihydropyridine, is a multiple Ca2+ channel blocker approved for clinical use. An increased concentration of cytosolic Ca2+ causes increased concentration in both cardiac and vascular smooth muscle cells. In cardiac myocytes, the entry of extracellular Ca2+ causes a larger Ca2+ release from intracellular stores (Ca2+-induced Ca2+release) and thereby initiates the contraction twitch. In smooth muscle cells, entry of Ca2+ plays a dominant role, but the release of Ca2+ from the intracellular storage sites also contributes to contraction of vascular smooth muscle, particularly in some vascular beds. Cytosolic Ca2+ concentrations can be increased by diverse contractile stimuli in vascular smooth cells. Many hormones and autacoids increase Ca2+ influx through so-called receptor-operated channels, whereas increases in external concentration of K+ and depolarizing electrical stimuli increase Ca2+ influx through voltage-gated or “potential operated” channels. Felodipine produces its effects by binding to the α1 subunit of the L-type voltage-gated Ca2+ channels and lowering Ca2+ flux through the channel [1].
Pharmacological actions of felodipine
Depolarization of vascular smooth muscle cells depends primarily on the influx of Ca2+. At least three distinct mechanisms may be responsible for contraction of vascular smooth cells. First, voltage-gated Ca2+ channels open in response to depolarisation of the membrane, and extracellular Ca2+ moves down its electrochemical gradient into the cell. After closure of Ca2+ channels, a finite period of time is required before the channels open again in response to a stimulus. Second, agonist-induced contractions that occur with-out depolarization of the membrane result from stimulation of the Gq-phospholipase C (inositol 1,4,5-triphosphate) pathway, resulting in the release of intracellular Ca2+ from the sarcoplasmic reticulum. Emptying of intracellular Ca2+ stores may trigger further influx of extracellular Ca2+ (store-operated Ca2+ entry), but its relevance in smooth muscle is unresolved. Third, receptor-operated Ca2+ channels allow the entry of extracellular Ca2+ in response to receptor occupancy. An increase in cytosolic Ca2+ results in enhanced binding of Ca2+ to calmodulin. The Ca2+-calmodulin complex in turn activates myosin light-chain kinase, with resulting phosphorylation of the myosin light chain. Such phosphorylation promotes interaction between actin and myosin and leads to sustained contraction of smooth muscle. Ca2+ channel blockers inhibit the voltage-dependent Ca2+ channels in vascular smooth muscle and decreases Ca2+ entry. Felodipine relaxes arterial smooth muscle and thereby decrease arterial resistance, blood pressure, and cardiac afterload [1].
Absorption, distribution, metabolism, and elimination of felodipine
Felodipine undergoes a presystemic first-pass metabolism being metabolized by CYP3A4 and the bioavailability of felodipine is about 15% and is increased by inhibitors of CYP3A4 such as macrolide and imidazole antibiotics, antiretroviral agents, and grapefruit juice and is reduced by inducers of CYP3A4 such as rifampin, carbamazepine, and hypericum. Felodipine is rapidly eliminated; the elimination half-life ranges from 18.4 to 28.7 hours and increases with the age of patients. In patients with hepatic cirrhosis, the bioavailability and the half-life of felodipine is increased and felodipine dosage should be decreased accordingly [1].
Material and Method
A dOFD is used for PINPT. To create a dOFD, the distal end of the gastric channel of a triluminal tube (Freka®Trelumina, CH/Fr 16/9, 150 cm, Fresenius, Germany) is covered with a 25 cm long, 3-4 cm wide strip of a thin, transparent, open-pore, double-layered film (DF) (Suprasorb® CNP drainage film, Lohmann & Rauscher International GmbH, Germany) [2,5-8] (Figure 1). Negative pressure can be applied to the film-covered drainage element (DE). Enteral nutrition can be provided simultaneously via the integrated feeding tube (iT).
The DF was originally developed for use in the open abdomen for peritonitis. The DF consists of two multi-perforated membranes separated by a small gap. When negative pressure is applied to the DF, fluids can be aspirated over the entire surface of the space and through the numerous pores. By wrapping the DF around a drain, small lumen open pore film drainages (OFD) can be created for ENPT. These are only 4-6 mm in diameter. OFDs can be placed transnasally using the same technique as a nasogastric tube (NGT) [5-8].
For PINPT, the dOFD is inserted into the duodenum. The film-coated portion is placed in the duodenal lumen to cover the anastomotic area. The iT is placed in the jejunum distal to the anastomosis. A continuous negative pressure of 125 mmHg is applied. This will permanently aspirate the duodenal secretions and keep them away from the anastomosis. Digestive secretions should have little or no effect at this vulnerable point. Feeding can take place through the iT at the same time as negative pressure is applied.
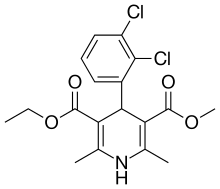
Felodipine molecular structure (molecular weight = 384.25 grams/mole)
Literature search
The literature search was performed electronically using PubMed database as search engine and the following key words were used: “felodipine efficacy, safety”, “felodipine treatment”, “felodipine trials”, “felodipine metabolism”, “felodipine pharmacokinetics”, “felodipine drug interactions”, and “felodipine toxicity”. In addition, the book: Goodman@Gilman’s. The Pharmacological basis of Therapeutics [1] has been consulted.
Results
Efficacy and safely of felodipine
Eleven studies have been reported on the efficacy and safely of felodipine. Felodipine was administered at the daily dose of 5 to 10 mg for 5 weeks to 56 hypertensive patients. This treatment effectively lowered the systolic and diastolic blood pressures and was found to be safe and well-tolerated [2]. Amlodipine and felodipine were administered at the daily dose of 5 to 10 mg, respectively, for 6 months to 115 hypertensive patients. The blood pressure was decreased to < 140/90 mmHg in 87% of patients treated with amlodipine and in 33% of patients treated with felodipine. Amlodipine and felodipine effectively and safely lowered the blood pressure in hypertensive patents but amlodipine was more efficacious than felodipine in lowering the blood pressure [3]. Nifedipine and felodipine were administered at the daily dose of 30 mg and 5 mg, respectively, for 6 weeks to 122 hypertensive patients who did not react adequately to 5 mg of nifedipine as the diastolic blood pressure was about 115 mmHg. Sixty-three patients were diagnosed as hypertensive emergencies and 59 were diagnosed as hypertensive crisis. Nifedipine and felodipine effectively and safely lowered the blood pressure in more than 90% of patients who did not respond adequately to 5 mg of nifedipine [4]. Felodipine was administered at the daily dose of 10 mg for 8 weeks to 23 patients with congestive heart failure. This treatment effectively increased the cardiac output and the stroke volume, decreased the systemic vascular resistance and the oxygen consummation and was found to be safe and well-tolerated [5]. Felodipine was administered at the daily dose of 5 to 10 mg for 8 weeks to 13 patients with heart failure. One hour after felodipine administration, the echocardiographic ejection fraction (%), the cardiac index, and the pulmonary wedge pressure (mmHg) significantly changed from 21+2 to 26+2, from 2,350+150 to 2,790+160, and from 24+4 to 17+4, respectively. Therefore, felodipine increases the echocardiographic ejection fraction and the cardiac index and decreases the pulmonary wedge pressure [6]. Felodipine was administered at the dose of 10 mg twice-daily for 3 weeks to 18 patients with chronic congestive heart failure. Felodipine lowered the mean arterial pressure by 9%, the systemic vascular resistance by 24% and increased the stroke volume by 25% and the cardiac index by 23%. The heart rate and the right and left ventricular filling pressures were unchanged. Therefore three weeks of treatment with felodipine at the dose of 10 mg twice-daily effectively and safely improved haemodynamic function in patients with chronic congestive heart failure [7]. Patients with mild to moderate hypertension received either felodipine extended-release (N = 59) or amlodipine (N = 59). The starting dose of both drugs was 5 mg daily and after 2 weeks of treatment the dose of both drugs was increased to 10 mg daily in patients whose diastolic blood pressure was > 90 mmHg. After 2 weeks of treatment with felodipine extended-release or with amlodipine, the seated systolic and diastolic blood pressures were lowered by 18 and 13 mmHg, respectively, and after 6 weeks of treatment the systolic and diastolic blood pressures were lowered by 25 and 18 mmHg, respectively. Felodipine extended-release lowered the systolic and diastolic blood pressures in hypertensive patients as amlodipine and both treatments were found to be safe and well-tolerated [8]. A total of 502 hypertensive patients received the combination of 5 mg daily of enalapril plus 2.5 mg daily of felodipine extended-release. If the diastolic blood pressure was > 90 mmHg after 4 weeks of treatment the dose of enalapril and felodipine extended-release was increased to 10 mg daily. The combination of enalapril plus felodipine extended-release resulted in mean diastolic blood pressure of 85 to 89 mmHg (decrease of 13 to 16 mmHg from baseline) and the systolic blood pressure was 137 to 140 mmHg (decrease of 13 to 21 mmHg from baseline). Overall, 407 of 502 patients (81%) achieved a diastolic blood pressure < 90 mmHg or a reduction from baseline ≥ 10 mmHg. The combination of enalapril plus felodipine extended-release effectively lowered the diastolic and systolic blood pressures and was found to be safe and well-tolerated [9]. A total of 130 hypertensive patients received either felodipine or nitrendipine at the initial dose of 10 mg once-daily and the dose of both drugs was increased to 20 mg once-daily or 20 mg twice-daily if the seating diastolic blood pressure was > 90 mmHg 24 hours after the previous dose. Felodipine was found more efficacious than nitrendipine in lowering the blood pressure and both drugs were found to be safe and well-tolerated [10]. The efficacy and safely of felodipine and barnidipine were assessed in 59 patients with mild to moderate hypertension. Patients received either barnidipine or felodipine at the dose of 5 mg once-daily and the dose of both drugs was increased to 10 or 15 mg once-daily if the blood pressure was not properly lowered. Barnidipine and felodipine lowered the blood pressure in ≥ 68% of patients. The mean reduction in systolic and diastolic blood pressures was 23.7+13.5 and 12.7+7.9 mmHg, respectively, in patients treated with barnidipine and was 24.3+8.4 and 14.5+10.0 mmHg, respectively, in patients treated with felodipine. Felodipine and barnidipine were similarly efficacious in lowering the blood pressure and were found to be safe and well-tolerated [11].
Treatment of hypertensive patients with felodipine
Ten studies have been reported on the treatment of hypertensive patients with felodipine. Elmfeldt D, Hedner T, and Westerling S reviewed the dosing and the adverse-effects of felodipine in hypertensive patients. The antihypertensive effect of felodipine is dose related. In patients with moderate hypertension a dose regimen of 5 mg twice-daily is usually sufficient and doses greater than 10 mg twice-daily are not often required. Felodipine is generally well-tolerated and the most common adverse-effects are ankle swelling, headache, and dizziness which are usually transient or diminish in intensity with continued treatment [12]. In 56 patients with mild to moderate hypertension the mean supine and standing blood pressures were significantly reduced by felodipine administered at the dose of 5 mg once-daily. This treatment reduced the supine blood pressure from 27 to 21 mmHg (P-value < 0.0001) and the standing blood pressure from 25 to 19 mmHg (P-value < 0.0001). Of 56 treated patients 54 (96%) achieved the diastolic blood pressure of < 90 mmHg. The treatment was discontinued in 6 patients because of headaches and this adverse-effect was not reported in the remaining 50 patients who completed the study. Felodipine administered at the dose of 5 mg once-daily effectively lowered the blood pressure in patients with mild to moderate hypertension [13]. The effects of felodipine on blood pressure and on heart activity were studied in 10 male hypertensive patients, aged 25 to 62 years, and felodipine was administered at the dose of 10 mg twice-daily for 8 weeks. The diastolic blood pressure was reduced in supine and in upright positions whereas the systolic blood pressure was reduced only in upright position. During dynamic exercise the blood pressure was reduced. The heart rate was unchanged in supine position and it was decreased in upright position. [14]. The effects of felodipine on blood pressure and on heart activity were studied in 600 hypertensive patients who received felodipine at the daily dose of 10 mg. The diastolic blood pressure was decreased and ranged from 90 to 94 mmHg in 86 patients (11%), from 80 to 89 mmHg in 186 patients (31%), from 80 to 84 mmHg in 180 patients (30%) and it was < 80 mmHg in 148 patients (25%). The overall blood pressure fell from 175/103 to 137/83 mmHg (P-value < 0.001). Felodipine decreased the dimension of the left ventricle from 0.4% to 0.8% (P-value < 0.0001) and the greatest decrease was observed in patients with lower diastolic blood pressure. The function of the left ventricle improved by 0.8% (P-value < 0.0001) and the improvement was depending on diastolic blood pressure fall and the cardiac output decreased by 2.4% (P-value < 0.0001). The wall of the left ventricle and the total peripheral resistance fell by 18% and 14%, respectively (P-value < 0.0001). The aortic root distensibility was reduced by 55% (P-value < 0.0001). It is concluded that felodipine, administered at the daily dose of 10 mg, reduces the systolic and diastolic blood pressures and improves the cardiovascular structure and function which are directly related to the diastolic blood pressure levels [15]. The effects of felodipine on blood pressure and on heart activity were studied in 450 hypertensive male patients who received felodipine at the dose of 5 mg twice-daily (N = 250) or placebo (N = 200) and the treatments lasted 18 months. Patients had cardiac dysfunction and impaired exercise performance. Felodipine significantly lowered the blood pressure (P-value < 0.001), and after 3 months of treatment, the ejection fraction was increased by 2.1% with felodipine and by 0.1% with placebo Therefore felodipine increased the ejection fraction more effectively than placebo (P-value = 0.001). Felodipine, administered at the dose of 5 mg twice-daily, is more efficacious than placebo in reducing the blood pressure and in improving the cardiac function [16]. It was compared the antihypertensive efficacy of felodipine to that of the diuretic combination hydrochlorothiazide/triamterene in a group of 65 elderly hypertensive patients aged ≥ 70 years with a blood pressure ≥ 160/95 mmHg. It was also assessed the ambulatory blood pressure at morning and the changes in the metabolic parameters caused by felodipine or by hydrochlorothiazide/triamterene. After 6 months of treatment, the ambulatory blood pressure was controlled in 62% of patients who received felodipine and in 74% of patients who received hydrochlorothiazide/triamterene (P-value = 0.4). Felodipine reduced the episodes of ischemic type ST-segment depression from 49 to 9 (P-value < 0.001) whereas hydrochlorothiazide/triamterene reduced these episodes from 24 to only 21 (P-value = 0.5). Both felodipine and hydrochlorothiazide/triamterene decreased the left ventricle wall thickness (P-value < 0.05) whereas the decline in the mass of the left ventricle muscle was significant (P-value < 0.05) only with felodipine. Felodipine did not induce any change in metabolic parameters whereas hydrochlorothiazide/triamterene significantly (P-value < 0.05) increased the concentration of serum creatinine and uric acid, the plasma concentration of prorenin, and the plasma activity of renin. Therefore felodipine and hydrochlorothiazide/triamterene have similar antihypertensive efficacy, both felodipine and hydrochlorothiazide/triamterene decrease the left ventricular wall thickness, felodipine but not hydrochlorothiazide/triamterene reduces the episodes of ischemic type ST-segment depression, and hydrochlorothiazide/triamterene but not felodipine alters metabolic parameters [17]. It was compared the efficacy of felodipine to that of minoxidil in management of severe hypertension in a group of 17 men. Satisfactory control of blood pressure was achieved in patients who received felodipine or minoxidil. The blood systolic and diastolic blood pressures were 150+19 and 88+8 mmHg, respectively, in patients who received felodipine and they were 148+23 and 87+11 mmHg, respectively, in patients who received minoxidil. The supine heart rate was lower (P-value < 0.05) in patients who received felodipine than in patients who received minoxidil. Therefore, felodipine raises the plasma enzymes (P-value < 0.001) whereas minoxidil does not. Felodipine is effective as minoxidil in lowering the systolic and diastolic blood pressures whereas felodipine is more efficacious than minoxidil in reducing the supine heart rate. Furthermore, felodipine but not minoxidil raises plasma enzymes [18]. It was compared the antihypertensive efficacy of felodipine-metoprolol to that of enalapril in 32 hypertensive patients. After 8 weeks of treatment, the average reduction of diastolic blood pressure was significantly greater (P-value < 0.05) in patients who received felodipine-metoprolol than in patients who received enalapril. The cost of treatment (costs of drugs and physician visits) was somewhat higher (P-value < 0.05) in patients who received felodipine-metoprolol. After 16 weeks of treatment, the diastolic blood pressure was decreased by an extra 4.8 mmHg in patients treated with felodipine-metoprolol and an additional 22% of patients treated with felodipine-metoprolol reached target diastolic blood pressure. In conclusion, felodipine-metoprolol is more efficacious than enalapril in lowering the diastolic blood pressure and the cost of treatment is higher in patients who received felodipine-metoprolol [19]. It was investigated the effects of amlodipine or those of felodipine or those of isradipine on the blood pressure in 73 males and 45 females aged 45+10 years and weighing 67+10 kg with diastolic blood pressure of 95 to 115 mmHg. Amlodipine was administered to 41 patients, felodipine was administered to 38 patients, and isradipine was administered to 39 patients and the treatment with these drugs lasted 8 weeks. The mean seated systolic and diastolic blood pressures lowered by 23 and 17 mmHg, respectively, in patients treated with amlodipine, they lowered by 30 and 17 mmHg, respectively, in patients treated with felodipine, and they lowered by 20 and 15 mmHg, respectively, in patients treated with isradipine and these reductions were statistically significant. The blood pressure was controlled (defined as diastolic pressure < 90 mmHg or decreased from baseline of ≥ 10 mmHg) in 85%, 74%, and 74% of patients who received amlodipine, felodipine, and isradipine, respectively. These drugs did not change the heart rate but mild adverse-effects including headaches, flushing, tachycardia, dizziness, and oedema, were reported in 1 patient (2%) who received amlodipine, in 6 patients (16%) who received felodipine, and in 5 patients (13%) who received isradipine. Therefore amlodipine, felodipine, and isradipine effectively lower the blood pressure in patients with mild to moderate hypertension and the adverse-effects occur in higher frequency in patients treated with felodipine and isradipine than in patients treated with amlodipine [20]. It was compared the efficacy of isradipine to that of felodipine in lowering the systolic and diastolic blood pressures in 143 patients with mild to moderate hypertension. Patients were randomized to receive either isradipine (N = 72) or felodipine (N = 71) and both drugs were administered at the dose of 2.5 mg twice-daily for 12 weeks. The systolic and diastolic blood pressures were 165+13 and 104+6 mmHg, respectively, at baseline. Isradipine lowered the systolic and diastolic blood pressures to 144+13 and 88+8 mmHg (P-value < 0.001), respectively, and felodipine lowered the systolic and diastolic blood pressures to 150+19 and 92+9 mmHg (P-value < 0.001), respectively. The adverse-effects such as headache, flushing, dizziness and tachycardia occurred with similar rate in patients treated with these two drugs. However, the ankle oedema occurred in 14% of patients treated with isradipine and in 30% of patients treated with felodipine (P-value < 0.028). Therefore isradipine and felodipine effectively lower the systolic and diastolic blood pressures, the rate of adverse-effects is similar with these drugs, but the rate of ankle oedema is lower in patients treated with isradipine than in patients treated with felodipine [21].
Trials conducted with felodipine in hypertensive patients
Six studies have been reported on the trials conducted with felodipine in hypertensive patients. A prospective, multicentre, double-blind, randomized, placebo-controlled trial was conducted in 9,800 hypertensive patients of either sex, aged 50-79 years, with one or two additional cardiovascular risk factors or disease. In these patients, the systolic blood pressure ranged from 140 to 180 mmHg and the diastolic blood pressure ranged from 90 to 100 mmHg. A 57.7% of patients received a low dose of hydrochlorothiazide-felodipine and 42.3% of patients received a low dose hydrochlorothiazide-placebo and patients were followed at 3 month interval for an average of 40 months. In patients treated with hydrochlorothiazide-felodipine, the blood pressure lowered from 154.2/91.0 to 137.3/82.5 mmHg (P-value < 0.01) and in patients treated with hydrochlorothiazide-placebo the blood pressure lowered from 154.4/91.3 to 142.5/85.0 mmHg (P-value < 0.05). In patients treated with hydrochlorothiazide-felodipine, the primary endpoint (fatal and non-fatal stroke) was reduced by 27% (P-value = 0.001). Among secondary endpoints, the rate of all cardiovascular events was reduced by 27% (P-value < 0.001), that of death by any cause was reduced by 31% (P-value = 0.006), that of coronary events was reduced by 32% (P-value = 0.024), that of heart failure was reduced by 30% (P-value = 0.239), that of cardiovascular death was reduced by 33% (P-value = 0.019), and that of cancer was reduced by 36% (P-value = 0.017). The treatments with hydrochlorothiazide-felodipine and with hydrochlorothiazide-placebo were well-tolerated. Therefore hydrochlorothiazide-felodipine is associated with substantial reduction in the incidence of most types of cardiovascular events and lowers the blood pressure more effectively than hydrochlorothiazide-placebo. Furthermore hydrochlorothiazide-felodipine reduces the rate of cardiovascular death, that of death by any cause, and that of cancer [22]. A randomized, double-blind, parallel-group, multicentre, clinical trial compared the efficacy of extended-release felodipine administered at the daily dose of 2.5, or 5, or 10 mg to that of placebo in lowering the blood pressure and in assessing the effects on heart. The trial included 171 patients with average age of 66.7 years and the treatments lasted 52 weeks. The systolic and diastolic blood pressures were 137+11.7 and 80+6 mmHg, respectively, in patients treated with extended-release felodipine and they were 147+51 and 83+7 mmHg, respectively, in patients treated with placebo and both the systolic and diastolic blood pressures were higher (P-value = 0.01) in patients treated with placebo. The incidence of left ventricle hypertrophy was 7% with extended-release felodipine and was 24% with placebo (P-value < 0.04) and the quality of life was higher (P-value < 0.001) in patients treated with extended-release felodipine. There were no clinically significant differences between treatments in tolerability or adverse-effects. Therefore extended-release felodipine reduces the systolic and diastolic blood pressures, reduces the incidence of left ventricular hypertrophy, and improves the quality of life more effectively than placebo [23]. A clinical trial compared the efficacy of felodipine extended-release to that of the diuretic combination triamterene/hydrochlorothiazide in lowering the blood pressure in 216 hypertensive patients (86 men and 130 women), aged 60 to 85 years, with a systolic blood pressure of 160 mmHg or a systolic and diastolic blood pressures > 140 and > 90 mmHg, respectively. Felodipine extended-release was administered at the daily dose of 2.5 mg and triamterene/hydrochlorothiazide was administered at the daily dose of 25/12.5 mg and the treatments lasted 8 weeks. The mean seated blood pressure lowered from 168/91 to 151/84 mmHg (P-value < 0.05) in patients treated with felodipine extended-release and it was lowered from 168/92 to 147/84 mmHg (P-value < 0.05) in patients treated with triamterene/hydrochlorothiazide. The clinical chemistry measurements taken before treatment and at the end of the study showed that more patients who received triamterene/hydrochlorothiazide developed abnormal values of blood urea nitrogen, uric acid, and creatinine. The incidence of adverse-effects was similar in both treatments, but 9 patients treated with felodipine extended-release and 3 patients treated with triamterene/hydrochlorothiazide discontinued treatment owing to an adverse-effect. Therefore felodipine extended-release is effective as triamterene/hydrochlorothiazide in lowering the blood pressure but more patients treated with felodipine extended-release discontinue the treatment owing an adverse-effect. Furthermore triamterene/hydrochlorothiazide alters clinical chemistry tests values [24]. A randomised, double-blind trial compared the efficacy and tolerability of felodipine administered at the daily dose of 5 to 20 mg to those of metoprolol administered at the daily dose of 50 to 200 mg or to their combination in 21 patients (13 men and 8 women) with a median age of 71 years (range, 29 to 85). Felodipine lowered the systolic and diastolic blood pressures by 40 and 20 mmHg, respectively, and metoprolol lowered the systolic and diastolic blood pressures by 15 and 9 mmHg, respectively. At 12 hours postdose, felodipine lowered the supine systolic blood pressure by 17 mmHg (P-value < 0.001) whereas no significant effect was observed with metoprolol and the combination of two drugs had an additive effect on lowering the systolic and diastolic blood pressures. Four patients treated with felodipine discontinued the treatment because the adverse-effects and 6 patients treated with felodipine or with felodipine plus metoprolol required dosage reductions. Therefore in hypertensive elderly patients felodipine effectively lowers the blood pressure but causes some adverse-effects whereas metoprolol is better tolerated than felodipine but is less efficacious than felodipine in lowering the blood pressure. The combination of felodipine plus metoprolol has an additive effect in lowering the blood pressure without causing adverse-effects [25]. In an open trial, the antihypertensive efficacy of felodipine and its effects on lipid metabolism were investigated in 117 Nordic patients with mild to moderate hypertension and hyperlipidaemia. After a treatment of 24 weeks, the mean blood pressure of 157/100 mmHg dropped to 145/92 mmHg in supine position (P-value < 0.01) and to 145/96 mmHg in erect position (P-value < 0.01). No relevant differences were seen in the pulse rate. The concentration of total cholesterol and triglycerides remained unchanged, whereas the concentration of HDL-cholesterol increased from 1.30 mmol/l to 1.33 mmol/l (P-value < 0.02). The concentration of LDL and VLDL-cholesterol, apolipoprotein A1, and apolipoprotein B remained unchanged during the treatment. Therefore felodipine lowers the blood pressure in hypertensive patients and proves to possess positive effects on lipid metabolism [26]. In an open trial, the antihypertensive efficacy of felodipine and its effects on lipid metabolism were investigated in 117 Nordic patients with mild to moderate hypertension and hyperlipidaemia. After 24 weeks of treatment, the mean blood pressure of 157/100 mmHg dropped to 145/92 mmHg in supine position (P-value < 0.01) and to 145/96 mmHg in erect position (P-value < 0.01). No relevant differences were seen in the pulse rate. The concentration of total cholesterol and triglycerides remained unchanged, whereas the concentration of HDL-cholesterol increased significantly from 1.30 mmol/l to 1.33 mmol/l (P-value < 0.02). The concentration of LDL- and VLDL-cholesterol, apolipoprotein A1 and apolipoprotein B remained unchanged during the 24-weeks of treatment. Therefore, felodipine lowers the blood pressure in hypertensive patients and proves to possess positive effects on lipid metabolism [27].
Metabolism of felodipine
Eriksson et al. [28] studied the metabolism of felodipine in liver microsomes from rats, dogs, and humans. The bioavailability of an oral dose of felodipine is about 15% because of high presystemic first-pass metabolism. Oxidation of the dihydropyridine ring to the corresponding achiral, pharmacologically inactive, pyridine metabolite is the predominant metabolic step. The metabolism of felodipine is catalysed by the cytochrome CYP3A4. The metabolic rate of (R)-felodipine and (S)-felodipine was compared in human liver microsomes and the (R)-enantiomer is metabolized more readily than (S)-felodipine. The mean value of Km is lower for (R)-felodipine, while the Vmax values of two enantiomers are similar. The intrinsic clearance, defined as the ratio of Vmax to Km, is about two-times higher for (R)-felodipine. In humans, the bioavailability of (S)-felodipine is about two-times higher than that of (R)-felodipine.
Pharmacokinetics of felodipine
Blychert et al. [29] studied the pharmacokinetics of felodipine in 140 individuals, aged 20 to 80 years, of which 67 were hypertensive patients and 73 were healthy subjects. The number of men was 126 and the number of women was 14. The healthy subjects did not receive any medication whereas the majority of hypertensive patients received other antihypertensive drugs. Twelve patients took a diuretic, 11 patients took a β-adrenoceptor antagonist, and 21 patients took a combination of diuretic plus a β-adrenoceptor antagonist. Forty-four of subjects/patients received felodipine orally at the dose of 5 mg twice-daily and the remaining 96 subjects/patients received felodipine orally at the dose of 10 mg twice-daily and the duration of treatment varied from 6 to 30 days. Table 1 provides the characteristics of the subjects and patients included in the study and table 2 summarizes the pharmacokinetic parameters of felodipine.
Table 1: Characteristics of the subjects and patients included in the study. Values are the mean+SD by Blychert et al. [29].
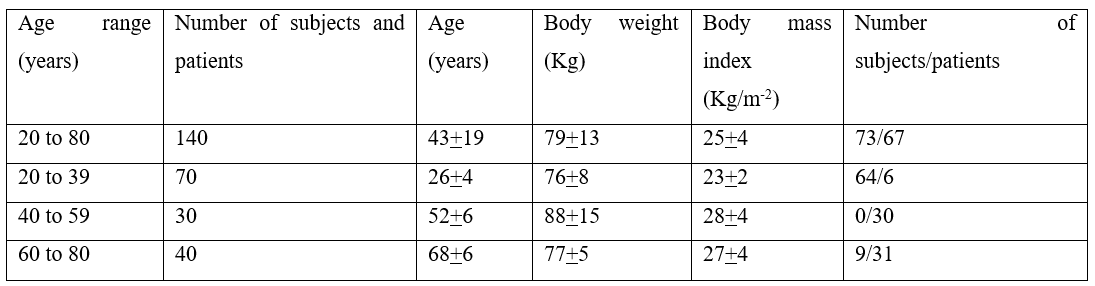
Table 2: Pharmacokinetic parameters of felodipine which have been obtained in subjects and patients included in the study. Values are the mean+SD, by Blychert et al. [29].
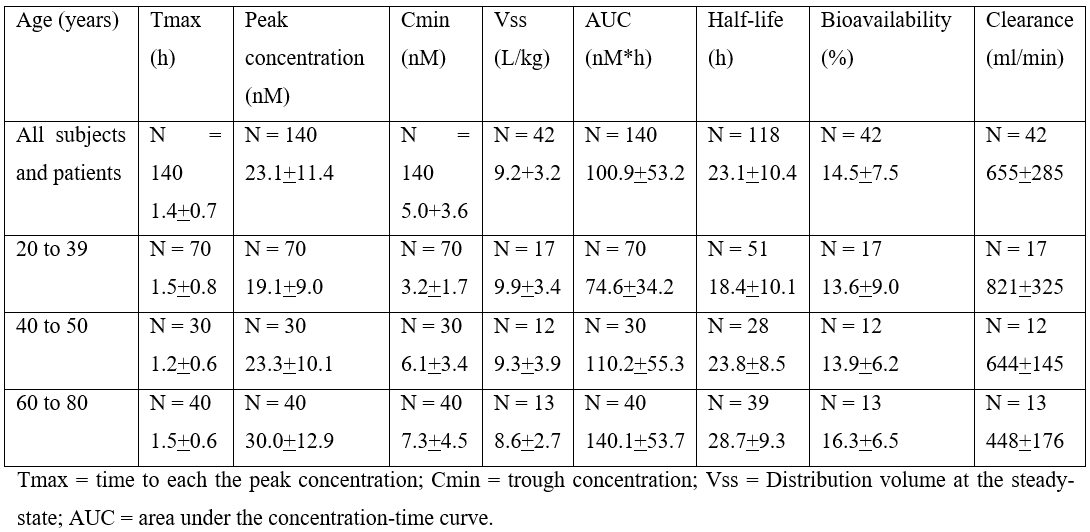
This table shows that felodipine is rapidly absorbed as the time to reach the peak concentration is about 1.5 hours and is similar in subjects and patients with different age intervals. The peak concentration of felodipine ranges from 19.1 to 30.0 nM and increases with the age interval of subjects and patients included in the study. The trough concentration of felodipine ranges from 3.2 to 7.3 nM and increases with the age interval of subjects and patents included in the study. The distribution volume of felodipine ranges from 8.6 to 9.9 L/kg suggesting that the distribution volume of felodipine is lower than the water volume and the distribution volume of felodipine lowers with the age interval of subjects and patients included in the study. The area under the concentration-time curve of felodipine ranges from 74.6 to 140.1 nM*h and increases with the age interval of subjects and patients included in the study. The elimination half-life of felodipine ranges from 18.4 to 28.7 hours suggesting that felodipine is rapidly eliminated and the elimination half-life of felodipine increases with the age interval of subjects and patients included in the study. The bioavailability of felodipine ranges from 13.6% to 16.3% suggesting that felodipine undergoes a presystemic first-pass metabolism and the bioavailability of felodipine increases with the age interval of subjects and patients included in the study. The clearance of felodipine ranges from 448 to 821 ml/min suggesting that felodipine is rapidly cleared from the body and the clearance of felodipine lowers with the age interval of subjects and patients included in the study. These results indicate that the elimination and the clearance of felodipine decrease with the age of subjects and patients. Furthermore, there is a remarkable interindividual variability in the pharmacokinetic parameters and this variability is accounted by a wide variation in the vital data of subjects and patients included in the study, by the disease of patients, and by the drugs token by patients.
Interaction of felodipine with drugs or with grapefruit juice
Four studies have been reported on the interaction of felodipine with drugs or with grapefruit juice. A 13-year-old boy with renal dysplasia received tacrolimus at the dose of 4 mg twice-daily and the plasma trough concentration of tacrolimus was 16.2 ng/ml. After receiving felodipine at the daily dose of 5 mg the plasma trough concentration of tacrolimus became higher than 30 ng/ml [30]. Enalapril and felodipine were co-administered at the dose of 5 mg twice-daily for 6 days to 12 healthy subjects. Enalapril increased the area under the concentration-time curve and the plasma peak concentration of felodipine whereas felodipine did not alter the pharmacokinetic parameters of enalapril. Therefore, enalapril co-administered with felodipine affects the pharmacokinetics of felodipine whereas felodipine does not alter the pharmacokinetics of enalapril [31]. Coffee (2×300ml) was co-administered with felodipine at the daily dose of 10 mg to middle-age normotensive subjects and this co-administration lasted 2 days. After ingestion of coffee the diastolic pressure increased (P-value < 0.001) and the systolic pressure increased (P-value < 0.001). Therefore, coffee hinders the antihypertensive effect of felodipine [32]. Felodipine extended-release was administered at the daily dose of 10 mg and 240 ml of Seville orange juice or grapefruit juice were ingested. The area under the concentration-time curve of felodipine increased by 76% and by 93% after the ingestion of Seville orange juice or grapefruit juice, respectively. The increase of the area under the concentration-time curve of felodipine caused by Seville orange juice or by grapefruit juice was due to the inhibition of CYP3A4. Therefore, Seville orange juice and grapefruit juice interact with felodipine by a common mechanism which is the inactivation of intestinal and hepatic CYP3A4 [33]. Felodipine is completely absorbed from the gastrointestinal tract following oral administration. However, felodipine undergoes a high presystemic first-pass metabolism resulting in low absolute bioavailability averaging to 15%. Both the gut wall and the liver appear responsible for presystemic elimination of felodipine and CYP3A4 is the cytochrome which metabolizes felodipine. Grapefruit juice selectively inactivates CYP3A4 in apical enterocytes and elevates the peak plasma concentration of felodipine. The interaction of felodipine with grapefruit juice results from the inactivation of CYP3A4 with consequent inhibition of presystemic metabolism of felodipine Therefore grapefruit juice increases the peak plasma concentration of felodipine [34].
Toxicity induced by felodipine
Felodipine is a safe drug and only one study has been reported on the toxicity induced by felodipine and the toxicity caused by felodipine was due to an overdose of felodipine. A 54-year-old-man was admitted to hospital within 4 hours of taking an overdose of felodipine tablets of approximately 250 mg. The initial management comprised fluid resuscitation, calcium chloride and glucagon. He remained hypotensive and the hypotension persisted with the development of progressive metabolic acidosis despite increasing inotropic support, hemofiltration, and high dose insulin-dextrose infusions but the patient died 60 hours after the ingestion of the overdose [35].
Discussion
Felodipine, a dihydropyridine, is a multiple Ca2+ channel blocker approved for clinical use and is generally administered by oral route [1]. The efficacy and safely of felodipine have been reviewed. Felodipine administered at the daily dose of 5 to 10 mg for 5 weeks to hypertensive patients effectively lowered the systolic and diastolic blood pressures and was found to be safe and well-tolerated [2], amlodipine and felodipine were administered at the daily dose of 5 and 10 mg, respectively, for 6 months to hypertensive patients and the blood pressure was decreased in 87% of patients treated with amlodipine and in 33% of patients treated with felodipine. Amlodipine and felodipine effectively and safely lowered the blood pressure but amlodipine was more efficacious than felodipine in lowering the blood pressure [3], nifedipine and felodipine were administered at the daily dose of 30 mg and 5 mg, respectively, for 6 weeks to hypertensive patients who did not react adequately to 5 mg of nifedipine. Nifedipine and felodipine effectively and safely lowered the blood pressure in more than 90% of patients who did not respond adequately to 5 mg of nifedipine [4], felodipine administered at the daily dose of 10 mg for 8 weeks to patients with congestive heart failure effectively increased the cardiac output and the stroke volume, decreased the systemic vascular resistance and the oxygen consummation and this treatment was found to be safe and well-tolerated [5], felodipine was administered at the daily dose of 5 to 10 mg for 8 weeks to patients with heart failure. One hour after felodipine administration, the echocardiographic ejection fraction and the cardiac index were increased whereas the pulmonary wedge pressure was decreased. Therefore, felodipine effectively and safely treated patients with heart failure [6], felodipine was administered at the dose of 10 mg twice-daily for 3 weeks to patients with chronic congestive heart failure and this treatment lowered the arterial pressure and the systemic vascular resistance and increased the stroke volume and the cardiac index. Therefore felodipine effectively and safely improves the haemodynamic function in patients with chronic congestive heart failure [7], patients with mild to moderate hypertension received either felodipine extended-release or amlodipine and the initial dose of both drugs was 5 mg daily. After 2 weeks of treatment the dose of both drugs was increased to 10 mg daily in patients who’s the diastolic blood pressure was > 90 mmHg. Felodipine extended-release and amlodipine lowered the systolic and diastolic blood pressures and the treatment with these drugs was found to be safe and well-tolerated [8], hypertensive patients received the combination of 5 mg daily of enalapril plus 2.5 mg daily of felodipine extended-release and the dose of enalapril and felodipine extended-release was increased to 10 mg daily if the diastolic blood pressure was > 90 mmHg. The combination of enalapril plus felodipine extended-release lowered the systolic and diastolic blood pressures in 81% of patients and this combination was found to be safe and well-tolerated [9], hypertensive patients received either felodipine or nitrendipine at the initial dose of 10 mg once-daily and the dose of both drugs was increased to 20 mg once-daily or to 20 mg twice-daily if the seating diastolic blood pressure was > 90 mmHg 24 hours after the previous dose. Felodipine was found more efficacious than nitrendipine in lowering the blood pressure and booth drugs were found safe and well-tolerated [10], patients with mild to moderate hypertension received either barnidipine or felodipine at the dose of 5 mg once-daily and the dose of both drugs was increases to 10 or 15 mg once-daily if the blood pressure was not properly lowered. Barnidipine and felodipine lowered the systolic and diastolic blood pressures in ≥ 68% of patients. Felodipine and barnidipine were similarly efficacious in lowering the systolic and diastolic blood pressures [11]. The treatment of hypertensive patients with felodipine has been reviewed. Emfeldt D, Hedner T, and Westerling S reviewed the dosing and the adverse-effects of felodipine in hypertensive patients. The antihypertensive effect of felodipine is dose related. A dose of felodipine of 5 mg twice-daily is usually sufficient to treat patients with mild to moderate hypertension and a dose of 10 mg twice-daily is not often required. Felodipine is generally well-tolerated and the most common adverse-effects are ankle swelling, headache, and dizziness and these adverse-effects are transient or diminish in intensity with continued treatment [12], a dose of felodipine of 5 mg once-daily reduced significantly the supine and standing blood pressures in patients with mild to moderate hypertension. Of 56 treated patients 54 (96%) achieved the diastolic blood pressure < 90 mmHg. This treatment was discontinued in only 6 of 56 patients (11%) because headache. Felodipine administered at the dose of 5 mg once-daily effectively lowered the blood pressure in patients with mild to moderate hypertension [13], the effects of felodipine on blood pressure and on heart activity were studied in male hypertensive patients. A dose of felodipine of 10 mg twice-daily was administered for 8 weeks to hypertensive patients with heart failure. The diastolic blood pressure was lowered in supine and upright positions whereas the systolic blood pressure was lowered only in upright position. During dynamic exercise the blood pressure was lowered. The heart rate was unchanged in supine position and it was decreased in upright position [14], the effects of felodipine on blood pressure and on heart activity were studied in hypertensive patients who received felodipine at the daily dose of 10 mg. The diastolic blood pressure lowered and the overall blood pressure fell from 175/103 to 137/83 mmHg (P-value < 0.001). This treatment significantly decreased the dimension of the left ventricle and the greatest decrease was observed in patients with lower diastolic blood pressure. The function of the left ventricle significantly improved, the improvement was depending on diastolic blood pressure fall, and the cardiac output significantly decreased. The wall of the left ventricle, the total peripheral resistance, and the aortic root distensibility were significantly reduced. Felodipine administered at the daily dose of 10 mg lowers the systolic and diastolic blood pressures and improves the cardiovascular structure and function which are directly related to the diastolic blood pressure [15], The effects of felodipine on blood pressure and on heart activity were studied in hypertensive male patients who received felodipine at the dose of 5 mg twice-daily or placebo and the treatments lasted 18 months. Patients had cardiac dysfunction and impaired exercise performance. Felodipine significantly lowered the blood pressure, and after 3 months of treatment, the ejection fraction was increased by 2.1% in patients treated with felodipine and by 0.1% in patients who received placebo Therefore felodipine increased the ejection fraction more effectively than placebo (P-value = 0.001). Felodipine administered at the dose of 5 mg twice-daily is more effective than placebo in lowering the blood pressure and in improving the cardiac function [16], the antihypertensive efficacy of felodipine was compared to that of diuretic combination hydrochlorothiazide/triamterene in elderly hypertensive patients whose blood pressure was ≥ 160/95 mmHg. It was also assessed the ambulatory blood pressure at morning and the changes in metabolic parameters caused by felodipine or by hydrochlorothiazide/triamterene. After 6 months of treatment, the control of ambulatory blood pressure was similar in patients who received felodipine and in those who received hydrochlorothiazide/triamterene. Felodipine significantly reduced the episodes of ischemic type ST-segment depression whereas hydrochlorothiazide/triamterene did not. The decrease of the left ventricular wall thickness was significantly reduced by both felodipine and hydrochlorothiazide/triamterene whereas the decline in the ventricular mass was reduced by only by felodipine. Felodipine did not induce any change in metabolic parameters whereas hydrochlorothiazide/triamterene significantly increased the concentration of serum creatinine and uric acid, the plasma concentration of prorenin, and the plasma activity of renin. Therefore felodipine and hydrochlorothiazide/triamterene have similar antihypertensive efficacy, both felodipine and hydrochlorothiazide/triamterene decrease of the left ventricular wall thickness, felodipine but hydrochlorothiazide/triamterene reduces the episodes of ischemic type ST-segment depression, and hydrochlorothiazide/triamterene but not felodipine alters metabolic parameters [17], it was compared the efficacy of felodipine to that of minoxidil in management of severe hypertension in 17 men. Felodipine and minoxidil were similarly efficacious in controlling the blood pressure in hypertensive male patients and the supine heart rate was significantly lowered in patients who received felodipine than in patients who received minoxidil. Felodipine raised the plasma enzymes whereas minoxidil did not. Therefore, felodipine is effective as minoxidil in lowering the blood pressure, felodipine is more efficacious than minoxidil in reducing the supine heart rate, and felodipine but not minoxidil rises plasma enzymes [18], it was compared the antihypertensive efficacy of felodipine-metoprolol to that of enalapril in hypertensive patients. After 8 weeks of treatment, the reduction of diastolic blood pressure was significantly greater in patients who received felodipine-metoprolol and the cost of treatment was significantly higher in patients who received felodipine-metoprolol. After 16 weeks of treatment, the diastolic blood pressure was decreased by an extra 4.8 mmHg in patients treated with felodipine-metoprolol and an additional 22% of patients treated with felodipine-metoprolol reached target diastolic blood pressure. Therefore felodipine-metoprolol is more efficacious than enalapril in lowering the diastolic blood pressure and the cost of treatment is higher in patients who received felodipine-metoprolol [19], it was investigated the effects of amlodipine or those of felodipine or those of isradipine in patients with diastolic blood pressure of 95 to 115 mmHg. The seated systolic and diastolic blood pressures were significantly lowered with these three drugs. The treatment with amlodipine or with felodipine or with isradipine was well-tolerated, did not change the heart rate, but mild adverse-effects were reported in 1 patient treated with amlodipine, in 6 patients treated with felodipine, and in 5 patients treated with isradipine. Therefore amlodipine, felodipine, and isradipine effectively lower the blood pressure but felodipine and isradipine induce more adverse-effects than amlodipine [20], it was compared the efficacy of isradipine to that of felodipine in lowering the systolic and diastolic blood pressures in patients with mild to moderate hypertension. Isradipine and felodipine were administered at the dose of 2.5 mg twice-daily and the systolic and diastolic blood pressures were significantly lowered with these drugs. Similar incidence of headache, flushing, dizziness, and tachycardia was reported with both drugs. However, the incidence of ankle oedema is lower in patients treated with isradipine than in patients treated with felodipine. Therefore isradipine and felodipine lower the systolic and diastolic blood pressures, both drugs cause similar adverse-effects, but the incidence of ankle oedema is lower in patients treated with isradipine than in patients treated with felodipine [21]. The trials conducted with felodipine in hypertensive patients have been reviewed. A prospective, multicentre, double-blind, randomized, placebo-controlled trial was conducted in hypertensive patients with one or two additional cardiovascular risk factors or disease whose systolic blood pressure ranged from 140 to 180 mmHg and the diastolic blood pressure ranged from 90 to 100 mmHg. A 57.7% of patients received a low dose of hydrochlorothiazide-felodipine and 42.3% of patients received a low dose hydrochlorothiazide-placebo. The blood pressure lowered from 154.2/91.0 to 137.3/82.5 mmHg (P-value < 0.01) in patients treated with hydrochlorothiazide-felodipine and lowered from 154.4/91.3 to 142.5/85.0 mmHg (P-value < 0.05) in patients treated with hydrochlorothiazide-placebo. The rate of fatal and non-fatal stroke, that of all cardiovascular events, that of death by any cause, that of coronary events, that of heart failure, that of cardiovascular death, and that of cancer significantly lowered in patients treated with hydrochlorothiazide-felodipine. The treatments with hydrochlorothiazide-felodipine and with hydrochlorothiazide-placebo were well-tolerated. Therefore hydrochlorothiazide-felodipine causes a substantial reduction in the incidence of most types of cardiovascular events and hydrochlorothiazide-felodipine lowers the blood pressure more effectively than hydrochlorothiazide-placebo. Furthermore, hydrochlorothiazide-felodipine reduces the rate of cardiovascular death, that of death by any cause, and that of cancer [22], a randomized, double-blind, parallel-group, multicentre, clinical trial compared the efficacy of extended-release felodipine administered at the daily dose of 2.5, or 5, or 10 mg to that of placebo in lowering the blood pressure and assessing the effects on heart and the treatments lasted 52 weeks. The systolic and diastolic blood pressures were 137+11.7 and 80+6 mmHg, respectively, in patients treated with extended-release felodipine and they were 147+51 and 83+7 mmHg, respectively, in patients treated with placebo and both the systolic and diastolic blood pressures were higher (P-value = 0.01) in patients treated with placebo. The incidence of left ventricle hypertrophy was significantly lower and the quality of life was significantly higher in patients treated with extended-release felodipine and the treatments with extended-release felodipine and with placebo were well-tolerated. Therefore extended-release felodipine lowers the systolic and diastolic blood pressures, reduces the incidence of ventricle hypertrophy, and improves the quality more effectively than placebo [23], a clinical trial compared the efficacy of felodipine extended-release to that of diuretic combination triamterene/hydrochlorothiazide in lowering the blood pressure in hypertensive patients who had the systolic blood pressure of 160 mmHg or the systolic and diastolic blood pressures were > 140 and > 90 mmHg, respectively. Felodipine extended-release was administered at the daily dose of 2.5 mg and triamterene/hydrochlorothiazide was administered at the daily dose of 25/12.5 mg and the treatments lasted 8 weeks. Felodipine extended-release lowered the mean seated blood pressure from 168/91 to 151/84 mmHg (P-value < 0.05) and triamterene/hydrochlorothiazide lowered the blood pressure from 168/92 to 147/84 mmHg (P-value < 0.05). More patients treated with triamterene/hydrochlorothiazide developed abnormal values of blood urea nitrogen, uric acid, and creatinine. The treatments with felodipine extended-release and with triamterene/hydrochlorothiazide were well-tolerated but 9 patients treated with felodipine extended-release and 3 patients treated with triamterene/hydrochlorothiazide discontinued the treatment owing to adverse-effects. Therefore felodipine extended-release and triamterene/hydrochlorothiazide effectively lower the blood pressure, are well-tolerated, but more patients treated with felodipine discontinued the treatment owing to adverse-effects [24], a randomised, double-blind trial compared the efficacy and tolerability of felodipine administered at the daily dose of 5 to 20 mg to those of metoprolol administered at the daily dose of 50 to 200 mg or to their combination. Felodipine lowered the systolic and diastolic blood pressures by 40 and 20 mmHg, respectively, and metoprolol lowered the systolic and diastolic blood pressures by 15 and 9 mmHg, respectively. At 12 hours postdose, felodipine reduced the supine systolic blood pressure by 17 mmHg (P-value < 0.001) whereas no significant effect was observed with metoprolol or with the combination of two drugs. Four patients treated with felodipine discontinued the treatment because the adverse-effects and 6 patients treated with felodipine or with felodipine plus metoprolol required reduction of dosage. Therefore felodipine effectively lowers the blood pressure but causes some adverse-effects whereas metoprolol is better tolerated but is less efficacious than felodipine in lowering the blood pressure. The combination of felodipine plus metoprolol has an additive effect in lowering the blood pressure without causing adverse-effects [25], an open trial assessed the antihypertensive efficacy of felodipine and its effects on lipid metabolism in Nordic patients with mild to moderate hypertension and hyperlipidaemia. After a treatment of 24 weeks, the mean blood pressure of 157/100 mmHg dropped to 145/92 mmHg in supine position (P-value < 0.01) and to 145/96 mmHg in erect position (P-value < 0.01). The concentration of total cholesterol and triglycerides remained unchanged, whereas the concentration of HDL-cholesterol increased significantly from 1.30 mmol/l to 1.33 mmol/l (P-value < 0.02). The concentration of LDL and VLDL-cholesterol, apolipoprotein A1, and apolipoprotein B remained unchanged during the treatment. Therefore felodipine lowers the blood pressure in hypertensive patients and proves to possess positive effects on lipid metabolism [26], an open trial assessed the antihypertensive efficacy of felodipine and its effects on lipid metabolism in Nordic patients with mild to moderate hypertension and hyperlipidaemia. After 24 weeks of treatment, the mean blood pressure of 157/100 mmHg dropped to 145/92 mmHg in supine position (P-value < 0.01) and to 145/96 mmHg in erect position (P-value < 0.01). The concentration of total cholesterol and triglycerides remained unchanged, whereas the concentration of HDL-cholesterol increased significantly from 1.30 mmol/l to 1.33 mmol/l (P-value < 0.02). The concentration of LDL- and VLDL-cholesterol, apolipoprotein A1 and apolipoprotein B remained unchanged during the 24-week treatment period. Therefore felodipine lowers the blood pressure in hypertensive patients and proves to possess positive effects on lipid metabolism [27]. Eriksson et al. [28] studied the metabolism of felodipine in liver microsomes from rats, dogs, and humans and after oral dosing the bioavailability is about 15% because of high presystemic first-pass metabolism caused by CYP3A4. Oxidation of the dihydropyridine ring to the corresponding achiral, pharmacologically inactive, pyridine metabolite is the predominant metabolic step. In human liver microsomes the (R)-enantiomer is metabolized more readily than (S)-felodipine. The mean value of Km is lower for (R)-felodipine, while the Vmax values of two enantiomers are similar. The intrinsic clearance, defined as the ratio of Vmax to Km, is about two-times higher for (R)-felodipine. In humans, the bioavailability of (S)-felodipine is about two-times higher than that of (R)-felodipine. Blychert et al. [29] studied the pharmacokinetics of felodipine in 140 subjects 67 were hypertensive patients and 73 were healthy subjects and the patients token a diuretic (N = 12) or a β-adrenoceptor antagonist (N = 11) or a combination of a diuretic plus a β-adrenoceptor antagonist (N = 21). Felodipine was administered orally at the dose of 5 mg twice-daily to 44 subjects and patients or at a dose of 10 mg twice-daily to 96 subjects and patients and the treatment lasted 6 to 30 days. Felodipine is rapidly absorbed as the time to reach the peak concentration is about 1.5 hours and the bioavailability of felodipine ranges from 13.6+9.0% to 16.3+6.5% and increases with the age interval of the subjects and patients included in the study Therefore felodipine undergoes a presystemic first-pass metabolism. The elimination half-life of felodipine is 18.4+10.1 hours in subjects and patients aged 29 to 39 years, is 23.8+8.5 hours in subjects and patients aged 40 to 50 years, and is 28.7+9.3 hours in subjects and patients aged 60 to 80 years Therefore the elimination half-life of felodipine increases with the age interval of the subjects and patients included in the study. The distribution volume of felodipine at the steady state ranges from 9.9+3.4 to 8.6+2.7 L/kg and decreases with the age interval of the subjects and patients included in the study suggesting that the distribution volume of felodipine is lower than the water volume. The clearance of felodipine ranges from 448+176 to 821+325 ml/min and decreases with the age interval of the subjects and patients included in the study suggesting that felodipine is rapidly cleared. These results indicate that there is a remarkable interindividual variability in the pharmacokinetic parameters of felodipine and this variability is accounted by the wide variation in vital data of the patients and subjects included in the study, by the diseases of patients, and by the drugs token by patients. The interaction of felodipine with drugs or with grapefruit juice has been reviewed. A 13 year-old boy with renal dysplasia received tacrolimus at the dose of 4 mg twice-daily and the trough plasma concentration of tacrolimus was 16.2 ng/ml and after receiving felodipine at the daily dose of 5 mg the plasma trough concentration of tacrolimus became higher than 30 ng/ml [30], enalapril and felodipine were co-administered at the dose of 5 mg twice-daily and enalapril increased the area under the concentration-time curve and the peak plasma concentration of felodipine whereas felodipine did not alter the pharmacokinetic parameters of enalapril [31], coffee (2x300 ml) was co-administered with felodipine at the daily dose of 10 mg and after ingestion of coffee the diastolic and systolic blood pressures significantly increased Therefore coffee hinders the antihypertensive effect of felodipine [32], felodipine extended-release was administered at the daily dose of 10 mg and 240 ml of Seville orange juice or grapefruit juice were ingested. After the ingestion of Seville orange juice or grapefruit juice the area under the concentration-time curve of felodipine increase by 76% and by 93%, respectively. The increase of the area under the concentration-time curve of felodipine is due to the inhibition of cytochrome CYP3A4 [33], felodipine administered orally undergoes a high presystemic first-pass metabolism and both the gut wall and the liver are responsible for the presystemic elimination of felodipine and the CYP3A4 is the cytochrome that metabolizes felodipine. The interaction of felodipine with grapefruit juice results from the inactivation of CYP3A4 with consequent inhibition of the presystemic metabolism of felodipine [34]. Felodipine is a safe drug and only one study has been reported on the toxicity induced by felodipine and the toxicity caused by felodipine was due to a felodipine overdose. A 54 year-old-man ingested approximately 250 mg of felodipine and he was admitted to hospital within 4 hours after taking the overdose. This man underwent resuscitation but remained hypotensive and 60 hours after the ingestion of felodipine overdose he died [35].
Conclusion
Felodipine, a dihydropyridine, is a multiple Ca2+ channel blocker approved for clinical use and felodipine is generally administered by oral route. Following oral administration, felodipine undergoes a presystemic first-pass metabolism being metabolized by CYP3A4. The bioavailability of felodipine is about 15% and is reduced by inducers of CYP3A4 and is increased by inhibitors of CYP3A4. Felodipine has been found to be efficacy and safe in hypertensive patients, felodipine effectively treats hypertensive patients, and six trials conducted with felodipine have been reported in hypertensive patients. Felodipine consists in two enantiomers and in humans and the (R)-enantiomer is metabolized more rapidly than the (S)-enantiomer. The pharmacokinetics of felodipine have been studied in healthy subjects and in hypertensive patients following oral administration and felodipine is rapidly absorbed as the time to reach the peak concentration is about 1.5 hours and felodipine is rapidly eliminated as the felodipine elimination half-life ranges from 18.4 to 28.7 hours and increases with the age of subjects and patients and felodipine is rapidly cleared as the felodipine clearance ranges from 448 to 821 ml/min and decreases with age of subjects and patients. Therefore, the elimination and the clearance of felodipine reduce with the age of subjects and patients. Four studies on the interaction of felodipine with drugs or with grapefruit juice have been reported and one study showed that an overdose of 250 mg of felodipine killed a man. The aim of this manuscript is to review the clinical pharmacology of felodipine.
Conflict of interests: The authors declare no conflicts of financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employments, gifts, and honoraria.
This article is a review and drugs have not been administered to men or animals.
Acknowledgments: The author thanks Dr. Patrizia Ciucci and Dr. Francesco Varricchio, of the Medical Library of the University of Pisa, for retrieving the scientific literature.
References
- Escenhagen T. Treatment of Ischemic Heart Disease. In Goodman@Gilman’s. The Pharmacological Basis of Therapeutics. Brunton LL, Knollmann BC editors. Mc Graw Hill. 14th Edition, 2023; pp. 604-624.
- DeQuattro V. Efficacy and safety of felodipine, a new dihydropyridine calcium channel blocker, in elderly hypertensive patients. Clin Exp Hypertens A, 1992; 14(6): 965-987.
- Blivin SJ, Pippins J, Annis LG, Lyons F. A Comparative Analysis of Amlodipine and Felodipine in a Military Outpatient Population: Efficacy, Outcomes, and Cost Considerations. Military Medicine, 2003; 168(7): 530-535.
- Risler T, Bohm R, Wetzchewald D, Nast HP, Koch HH, Stein G, et al. A comparison of the antihypertensive efficacy and safety of felodipine IV and nifedipine IV in patients with hypertensive crisis or emergency not responding to oral nifedipine. Eur J Clin Pharmacol, 1998; 54(4): 295-298.
- Dunselman PHJM, Kuntze CEE, Van Bruggen A, Hamer JPM, Scafj AHJ, Wesseling H, et al. Efficacy of felodipine in congestive heart failure. Eur Heart J, 1989; 10(3): 354-364.
- Agostoni P, Doria E, Riva S, Palese A. Acute and chronic efficacy of felodipine in congestive heart failure. Int J Cardiol, 1991; 30(1): 89-95.
- Kassis E, Amtorp O, Waldorff S, Fritz-Hansen P. Efficacy of felodipine in chronic congestive heart failure: a placebo controlled haemodynamic study at rest and during exercise and orthostatic stress. Br Heart J, 1987; 58(5): 505-511.
- Koenig W. Efficacy and Tolerability of Felodipine and Amlodipine in the Treatment of Mild to Moderate Hypertension. Drug Invest, 1993; 5(4): 200–205.
- Gradman AH, Cutler NR, Davis PJ, Robbins JA, Weiss RJ, Wood BC, et al. Long-term efficacy, tolerability, and safety of the combination of enalapril and felodipine ER in the treatment of hypertension. Enalapril-Felodipine ER Factorial Study Group. Clin Ther, 1998; 20(3): 527-538.
- Lassen E, Frimodt-Møller J, Ahlstrøm F, Bøgeskov-Jensen I, Nielsen MR, Wickers-Nielsen N, et al. Antihypertensive efficacy and safety of felodipine compared with nitrendipine in mild to moderate hypertension. Curr Ther Res, 1993; 53(6): 354-364.
- Liau CS, Chien KL, Chao CL, Lee TM. Efficacy and safety of barnidipine compared with felodipine in the treatment of hypertension in Chinese patients. J Int Med Res, 2002; 30(3): 330-336.
- Elmfeldt D, Hedner T, Westerling S. Felodipine in hypertension--a review. J Cardiovasc Pharmacol, 1987; 10(Suppl 1): S154-160.
- Kon SP, Tan HW, Chua CT, Ong ML, Kamsiah J, Maheendran KK, et al. Once daily felodipine monotherapy in mild to moderate hypertension. Med J Malaysia, 1992; 47(4): 290-296.
- Katzman PL, Hulthén UL, Hökfelt B. The effect of 8 weeks treatment with the calcium antagonist felodipine on blood pressure, heart rate, working capacity, plasma renin activity, plasma angiotensin II, urinary catecholamines and aldosterone in patients with essential hypertension. Br J Clin Pharmacol, 1986; 21(6): 633-640.
- Vyssoulis GP, Trikas AG, Paleologos AA, Toutouza MG, Toutouzas PK. Significance of blood pressure levels achieved with felodipine anti-hypertensive treatment on cardiovascular structure and function changes. J Hum Hypertens, 1998; 12(7): 427-432.
- Cohn JN, Ziesche S, Smith R, Anand I, Dunkman WB, Loeb H, et al. Effect of the calcium antagonist felodipine as supplementary vasodilator therapy in patients with chronic heart failure treated with enalapril: V-HeFT III. Vasodilator-Heart Failure Trial (V-HeFT) Study Group. Circulation, 1997; 96(3): 856-863.
- Trenkwalder P, Plaschke M, Aulehner R, Lydtin H. Felodipine or hydrochlorothiazide/triamterene for treatment of hypertension in the elderly: effects on blood pressure, hypertensive heart disease, metabolic and hormonal parameters. Blood Press, 1996: 5(3): 154-163.
- Wathen CG, MacLeod D, Tucker L, Muir AL. Felodipine as a replacement for minoxidil in the treatment of severe hypertension. Eur Heart J, 1986; 7(10): 893-897.
- Andersson F, Kartman B, Andersson OK. Cost-Effectiveness of Felodipine-Metoprolol (Logimax®) and Enalapril in the Treatment of Hypertension. Clin Exper Hyperten, 1998; 20(8): 833-846.
- Cheung BM, Lau CP, Wu BZ. Amlodipine, felodipine, and isradipine in the treatment of Chinese patients with mild-to-moderate hypertension. Clin Ther, 1998; 20(6): 1159-1169.
- Cutler SA, Hammond JJ. A multicenter comparison of isradipine and felodipine in the treatment of mild-to-moderate hypertension. The Physician's Study Group. Am J Hypertens, 1993; 6(3 Pt 2): 44-48.
- Liu L, Zhang Y, Liu G, Li W, Zhang X, Zanchetti A. FEVER Study Group. The Felodipine Event Reduction (FEVER) Study: a randomized long-term placebo-controlled trial in Chinese hypertensive patients. J Hypertens, 2005; 23(12): 2157-2172.
- Black HR, Elliott WJ, Weber MA, Frishman WH, Strom JA, Liebson PR, et al. One-Year Study of Felodipine or Placebo for Stage 1 Isolated Systolic Hypertension. Hypertension, 2001; 38(6): 1118-1123.
- McClennen W, Wilson T. Felodipine extended release versus conventional diuretic therapy for the treatment of systolic hypertension in elderly patients. The National Trial Group. Clin Invest Med, 1998; 21(3): 142-150.
- Russell AE, Tonkin AL, Bune AJ, West MJ, Chalmers JP. Felodipine, metoprolol and their combination compared with placebo in isolated systolic hypertension in the elderly. Blood Press, 1994; 3(1-2): 82-89.
- Hosie J, Langan JJ, D’Silva R, Murphy GA, Sinclair WA. Unchanged blood lipids and thyroid function after treatment with felodipine. A 12-month placebo-controlled trial. Am J Hypert, 1995; 8(4): 193-196.
- Reuter MK, Lorenz H, Verho P, Smith N, Degen A, Verho M. Effects of felodipine ER, a dihydropyridine calcium antagonist, on blood pressure and serum lipids. Curr Med Res Opin, 1998; 14(2): 193-196.
- Eriksson UG, Lundahl J, Bäärnhielm C, Regårdh CG. Stereoselective metabolism of felodipine in liver microsomes from rat, dog, and human. Drug Metab Dispos, 1991; 19(5): 889-894.
- Blychert E, Edgar B, Elmfeldt D, Hedner T. A population study of the pharmacokinetics of felodipine. Br J Clin Pharmacol, 1991; 31(5): 15-24.
- Butani L, Berg G, Makker SP. Effect of felodipine on tacrolimus pharmacokinetics in a renal transplant recipient. Transplantation, 2002; 73(1): 159-160.
- Li D, Xu S, Wang Y, Li D, Li X, Pan J, et al. Pharmacokinetics and drug-drug interaction between enalapril, enalaprilat and felodipine extended release (ER) in healthy subjects. Oncotarget, 2017; 8(41): 70752–70760.
- Bailey DG, Dresser GK, Urquhart BL, Freeman DJ, Arnold JM. Coffee-Antihypertensive Drug Interaction: A Hemodynamic and Pharmacokinetic Study with Felodipine. Am J Hypertens, 2016; 19(12): 1386-1393.
- Malhotra S, Bailey DG, Paine MF, Watkins PB. Seville orange juice-felodipine interaction: comparison with dilute grapefruit juice and involvement of furocoumarins. Clin Pharmacol Ther, 2001; 69(1): 14-23.
- Bailey DG, Malcolm J, Arnold O, Spence JD. Grapefruit juice–drug interactions. Br J Clin Pharmacol, 1998; 46(3): 101–110.
- Lota HK, Powell N, Negus R, Leonard R, Manikon M. A Case of Fatal Felodipine Overdose. Acute Med J, 2008; 7(1): 39-42.

