Valeriana officinalis Extract Improves Sleep Quality in Primary Health Care in Brazil: A Controlled and Quasi-Experimental Study
Jorge Nei Borba Antunes1, Rebeca Vargas Antunes Schunck2, Caroline Dani1,2 and Ionara Rodrigues Siqueira1,2,*
1Programa de Pós-graduação em Ciências Biológicas: Farmacologia e Terapêutica, Universidade Federal do Rio Grande do Sul, Porto Alegre, RS, Brazil
2Departamento de Farmacologia, Instituto de Ciências Básicas da Saúde, Universidade Federal do Rio Grande do Sul, Porto Alegre, RS, Brazil
Received Date: 06/02/2025; Published Date: 11/03/2025
*Corresponding author: Ionara Rodrigues Siqueira, Laboratório de Neuropsicofarmacologia, Departamento de Farmacologia, Instituto de Ciências Básicas da Saúde, Universidade Federal do Rio Grande do Sul, Rua Ramiro Barcelos, 2600, prédio UFRGS 21116, sala 410. Campus Saúde. Bairro Santa Cecília, CEP 90035-003, Porto Alegre, RS, Brasil
Abstract
Background and Aim: Diazepam and clonazepam, although inappropriate, have been provided free of charge in the Primary Health Care in Brazil, to management of sleep quality, even with several side effects and potential dependence. Complementary and alternative medical treatments may be useful as an alternative. The aim was to evaluate the effects of capsules containing Valeriana officinalis extract (100 mg) in the treatment of self-reported insomnia, compared with a positive control, Diazepam.
Experimental Procedure: A quasi-experimental clinical trial was conducted in real-world clinical practices. The participants consisted of 68 people of both sexes, aged between 18 and 80 years old, who sought in Primary Health Care with self-reported insomnia that achieved a score at least of 5 in the Pittsburgh Sleep Quality Index and received the prescription of capsules containing Valeriana officinalis extract 100 mg or Diazepam 5 mg.
Key Results, Conclusions and Implications: The Pittsburgh Sleep Quality Index and the Sleep Hygiene Index questionaries were used to evaluate the quality of sleep before and at 28 follow up days. In addition, a Report of Adverse Effects was applied. Both groups showed improved scores in the Pittsburg Sleep Quality Index, in the total score, the primary outcome, and in the individual components, such as Duration, Subjective Quality, Efficiency and Sleep Disorders. The Valerian group also showed significantly lower rates of adverse effects compared to Diazepam. Valerian can improve the quality of sleep of individuals with self-reported insomnia in the context of Primary Health Care.
Keywords: Valeriana officinalis; Herbal medicine; Sleep disturbances; SUS; Primary Health Care
Introduction
Recently, Madruga et al (2019) reported a misuse of benzodiazepines in Brazil, since the prevalence of use is 9.8%1. It is relevant to point out benzodiazepine-induced side effects, such as psychomotor and cognitive impairments, daytime sleepiness, falls in the elderly, dependence and tolerance, what can raise a relevant concern [1]. In this context, clonazepam and diazepam are provided free of charge by the Unified Health System (SUS). Although cognitive behavioral therapy is the first-line treatment for management of sleep disturbances, integrative and complementary treatments can be considered an alternative to benzodiazepine.
The Brazilian Ministry of Health implemented the National Policy on Integrative and Complementary Practices (“Política Nacional de Práticas Integrativas e Complementares” - PNPIC) in 2008, what among several aims, we can highlight the rational use of medicinal plants and herbal medicines at Unified Health System (SUS – “Sistema Único de Saúde”) [2]. To achieve this aim, several approaches have been raised, such as the concept of “living pharmacy” was adopted (“Farmácias Vivas”, [3]) that supplies selected herbal medicines to communities [4-6]. Carmona and Pereira (2022) evaluated the prepared and dispensed herbal medicines at a Brazilian Living Pharmacy, they described that the top two most frequently prescribed single-constituent herbal medicine was Valeriana officinalis L. (Caprifoliaceae) [4].
Valerian roots (Valeriana officinalis L), a perennial herb, have been used traditionally as sedative and their extracts have been used for improvements on sleep quality around the world. In the meta-analysis conducted by Fernandez-San-Martin and colleagues (2010), valerian seems to improve sleep quality in controlled-placebo trials, especially in qualitative dichotomous outcomes (yes or not), what represents a subjective improvement of insomnia. In addition, they reported that studies had substantial differences, such as execution conditions, sample size, range of dosages, variable follow-up times [7].
It is important to note that official compendia recognized by the ANVISA (Brazilian Health Regulatory Agency), a responsible for health surveillance, indicates different ranges of dosages. Brazilian Herbal Formulary indicates dosages of valerian root extract ranging from 322 to 420 mg/day up to three times a day [8], while Herbal Memento of the Brazilian Pharmacopeia indicates 45 a 125 mg once to three times a day [9]. However, to the best of our knowledge, there is no clinical studies conducted in Brazil evaluating valerian effects and there are few clinical trials with dosage range suggested in the Herbal Memento of the Brazilian Pharmacopeia. Here our group performed a quasi-experimental clinical trial that can bring evidence-based practice on the use of medicinal and herbal plants to be applied in Primary Health Care, such as in the SUS-Brazil.
This work aimed to study the effect of Valeriana officinalis extract on several sleep quality and hygiene parameters comparing with positive control, Diazepam, in public Primary Health Care. Specifically, we conducted a controlled, open-label and pragmatic clinical trial whose subjects with sleep complaints who received Diazepam 5 mg or V. officinalis prescription 100 mg were eligible and the most widely relevant tools, the validated questionnaires (“Pittsburgh Sleep Quality Index” and “Sleep Hygiene Index”), were used.
Material and Methods
Study Design and Ethical Considerations
The project was approved by COMPESQ ICBS and CEP UFRGS (CAAE: 64721022.5.0000.5347). This study followed the routine of health service, patients received their medical prescription at Primary Healthcare Units in the Igrejinha town (Rio Grande do Sul, a southern Brazilian state; 29.5734° S, 50.7925° W). The public health service of this city has nine Family Health Strategies Units, a Psychosocial Care Center and a Philanthropic Hospital (Hospital Bom Pastor) and two Primary Care Teams. Individuals who have a prescription from doctors in the network can order their prescriptions at the public manipulation pharmacy of Igrejinha, where products are produced and dispensed according to the demand.
Participants, Criteria and Randomization
Patients of either gender aged between 18 and 80 years who received a routine prescription for Valeriana officinalis or Diazepam 5 mg by the medical team at Primary Health Care complaining of insomnia. Those patients that requested Valerian or Diazepam capsules at the Municipal Pharmacy of Igrejinha were potential participants. At this point, inclusion and exclusion criteria were assessed. Exclusion criteria were as follows: age <18 or >80 years, pregnant patients; lactation; patients using other Central Nervous System (CNS) depressant drugs and/or oral anticoagulants and antiplatelet agents; alcoholics; patients with severe and deep skin lesions; patients with circulatory disorders or heart failure. The concomitant or alternate use of Valeriana officinalis and Diazepam 5 mg was considered an exclusion criterion. In addition to self-reported conditions, description of exclusion criteria in the medical record or report by the medical team was considered, including of severe injuries, such as circulatory disorders and heart failure.
Eligible participants were invited; before agreeing to participate, they receive information on the study, such as confidentiality, aims, procedures, benefits, and risks. Each participant signed an informed consent form. At this point, 113 participants were enrolled in the study. The allocation was a medical decision into Valerian and Diazepam groups.
Preparation of dry extract capsules of Valeriana officinalis L
The dry extract of the roots of Valeriana officinalis L. was provided by Florien Fitoativos Ltda (SM Empreendimentos Farmacêuticos Ltda.), with a certificate of analysis and presenting at least 0.02% of Valeremic Acid content (Supplementary material). All the quality control analyses were performed according to Brazilian Health Regulatory Agency (in Portuguese: Agência Nacional de Vigilância Sanitária): Resolution of the Collegiate Board (RDC) 67/2007 and RDC 87/2008.
Assessment
The impact of Valerian and Diazepam capsules on sleep quality symptoms was assessed using translated to Portuguese and validated Pittsburgh Sleep Quality Indeex (PSQI) and Sleep Hygiene Index (SHI). The PSQI was validated in Portuguese 10. PSQI is a self-report questionnaire that assesses sleep quality. It consists of 19 individual items that create 7 components that produce one global score (0–21). These components (each one rated 0–3) are subjective sleep quality (component 1), sleep latency (component 2), sleep duration (component 3), habitual sleep efficiency (component 4), sleep disturbance (component 5), use of sleeping medication (component 6), and daytime dysfunction (component 7). Higher scores mean worst symptoms.
PSQI and SHI were applied before and after the treatment (28 days), since there is a recommendation for a short-term use of benzodiazepines (<2–4 weeks). The patients were contacted by the researchers during and at the end of the follow-up for evaluation of adherence and efficacy. At the end of the follow-up, a form of adverse effects was answered by the participants. All adverse reactions, including non-serious ones, were monitored by our team.
Sample Size and Statistical Analysis
The sample size was calculated using the G Power sample calculation software. Thus, for the sample size, independent variables were used, two proportions for alternative hypothesis, considering the criterion of coefficient of variation. The error was set as α as a unilateral significance level of 0.05 (α); sampling power set at 80% (Power); the difference in sleep time was considered the primary outcome (between day zero and day 30; Pittsburg question 4). The need for 40 patients per group was calculated. The sample size was predicted, considering a dropout rate of 30%.
The results were submitted to a homogeneity analysis; non-parametric data were analyzed using the Kruskal-Wallis test followed by Dunn's or Wilcoxon, and parametric data were analyzed using the two-way ANOVA, followed by Fisher's LSD post hoc test, considering p<0.05 as significant. Binary measurements were evaluated using Fischer's Exact test, nominal categorical variables were evaluated using the chi-square test (χ2). Clinical outcomes are expressed as medians (interquartile ranges 25/75). The SPSS statistical program (version 20.0) was used, with a significance level of P< 0.05.
Results
We collected data between March 10, 2023, and January 29, 2024. 113 patients were assessed for eligibility and agreed to participate. Although 36.5% and 20% of patients that belong, respectively, to Diazepam and Valerian dropped out, there was no significant difference on lost to follow-up between Valeriana officinalis 100 mg and Diazepam 5 mg groups (Fisher’s Exact Test, p = 0.119). 80 participants completed the study and returned 28 days after the inclusion (Figure 1).
The baseline characteristics of the participants are reported in Table 1. Both groups were comparable in terms of baseline clinical characteristics. In a real-world scenario, as observed in a routine clinical practice, there were slight differences in sociodemographic characteristics, such as age, gender and marital status distribution at baseline. Overall, the mean age of the participants in the study was 54.14 (±11.94) years, 71.25% were woman and 13.75% were current smokers (Table 1).
Two-way ANOVA indicated the effect of treatments on the primary endpoint, PSQI total score, considering the time factor, indicating a significant reduction in this total score (F(1,39)=7.22; p=0.012). Interestingly, post hoc Fisher's LSD showed a significant reduction on PSQI total score after 28 days only in the Valeriana officinallis group (Figure 2). As described in the method item, the total score was calculated by the sum of all the components (C1 to C7).
In addition, we analyzed the components individually (Table 2). PSQI Components 1, 3, 4 and 5, namely, subjective quality, duration, efficiency and disturbances, had a time factor effect as indicated by Two-Way ANOVA, because reduced scores were found after treatment. In addition, Valeriana officinallis group showed a significant decrease in these relevant components as indicated by post hoc Fisher's LSD after the treatment (Table 2). Two-Way ANOVA showed a significant effect of group factor on the PSQI C2, sleep latency, once the sleep latency score was improved compared with the positive control group, Diazepam. In order to figure out this result, Figure 2 shows the sleep latency in minutes (Panel A), the sleep duration in hours (Panel B) and sleep efficiency, percentage of total sleep time scored during time in bed (Panel C). Post hoc Fisher's LSD indicated a significant reduction in the sleep latency after the treatment only in the Valeriana officinalis group (Figure 2A). Figure 2B shows a difference between Valeriana and Diazepam groups at baseline in the sleep duration in hours, indicating a worst sleep condition in the Valeriana group before the treatment. Two-Way ANOVA indicated an effect of the time factor, while post hoc test showed a huge improvement induced by Valeriana officinalis (Fig 2B). In accordance, post hoc test indicated an improvement in the sleep efficiency (%) comparing before and after the treatment by Valeriana officinalis, as well as Valeriana group showed a better sleep efficiency compared with the positive control group, Diazepam (Figure 2C). Both groups equally showed reduced daytime dysfunction (excessive daytime sleepiness) after the treatment (Table 2).
Two-way ANOVA indicated the effect of treatments on the secondary endpoint, SHI total score, considering the time factor, indicating a significant reduction in total score (F(1,78)=5.870; p=0.0177)). Interestingly, post hoc Fisher's LSD showed a significant reduction on SHI total score after 28 days only in the Valerian group (Figure 3). As described in the method item, the total score was calculated by the sum of all the components (SHI 1 to 13), components were individually evaluated (Table 3).
Considering the indexes, at SHI-1 (daytime naps), we observed a statistical difference between the groups indicated by Two-way ANOVA (0.0072), and the post hoc test indicated a higher frequency of naps in the Diazepam group, compared with Valerian one. However, the Diazepam group showed a statistical reduction in this item, comparing before and after 28 days of treatment. In addition, at the end, the Valerian group showed lower scores than the Diazepam (Table 3).
At index SHI-3 (regular get-up time), we observed a statistical interaction between both factors, time and group (F(1,78)=7.723; p=0.0068)). The post hoc test indicated a higher frequency of this behavior at the end of the treatment in the Diazepam group (higher score values), meanwhile Valerian group showed lower score values (less frequent) than Diazepam group (Table 3).
At SHI-6 (use of stimulants or alcohol close do bedtime) and SHI-7 (performing activities that promote wakefulness prior to sleeping) scores, the Valerian group showed lower scores than the Diazepam group, considering the group factor (F(1,78)=5.302; p=0.023)). In the post hoc test in the SHI-6 index, we observed a significatively reduction in Valerian group compared with its baseline and Diazepam group at the end of the treatment (Table 3). Both groups had significant reductions at SHI-13 (worrying in bed/ nervousness in bed), since ANOVA indicated an effect of the time factor (F(1,78) =4.364; p=0.040)) (Table 3).
We did not observe any statistically differences between the groups, times or interactions, in the indexes SHI-2 (regular bedtime), SHI-4 (nighttime physical exercise), SHI-5 (prolonged time in bed), SHI-8 (distressed emotional states at bedtime), SHI-9 (use of bed for activities other than sleeping or sex), SHI-10 (uncomfortable bed), SHI-11 (inadequate room conditions) and SHI-12 (dealing with important matter at bedtime) (Table 3).
The Valerian group showed few side effects, since only 3 participants (8.1%) had mild reactions, such as sedation, dizziness, stimulant effects, and insomnia, and then 91.9% reported no adverse reactions, what was statistically different of the Diazepam group, because 11 participants (35.5%) reported several mild to moderate reactions, such as: fatigue, tiredness, insomnia, nausea, gastrointestinal upset, irritability, tremors, headache, mydriasis, and tachycardia (Fischer's exact test, p = 0.007).
Table 1: Sociodemographic and clinical characteristics at the baseline.
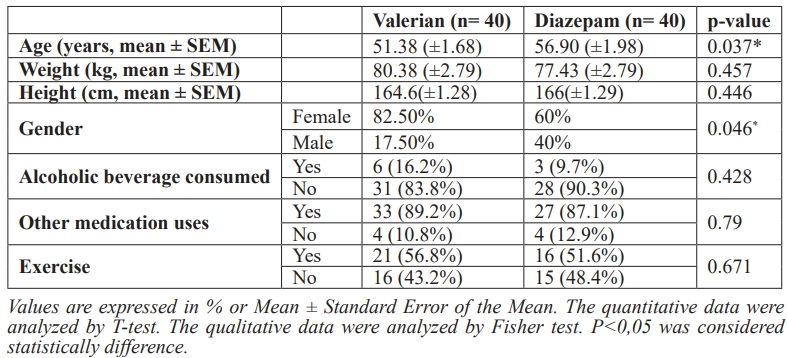
Table 2: Pittsburgh Sleep Quality Index (PSQI) Scores before and after 28 days receiving Diazepam 5 mg
or Valeriana officinalis 100 mg.
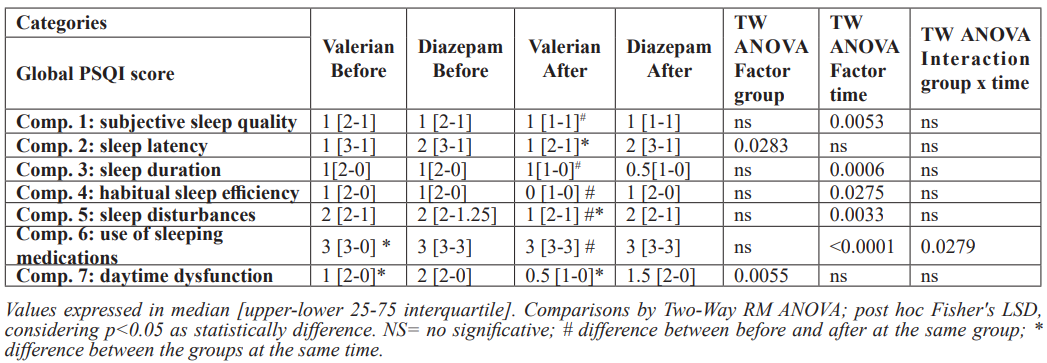
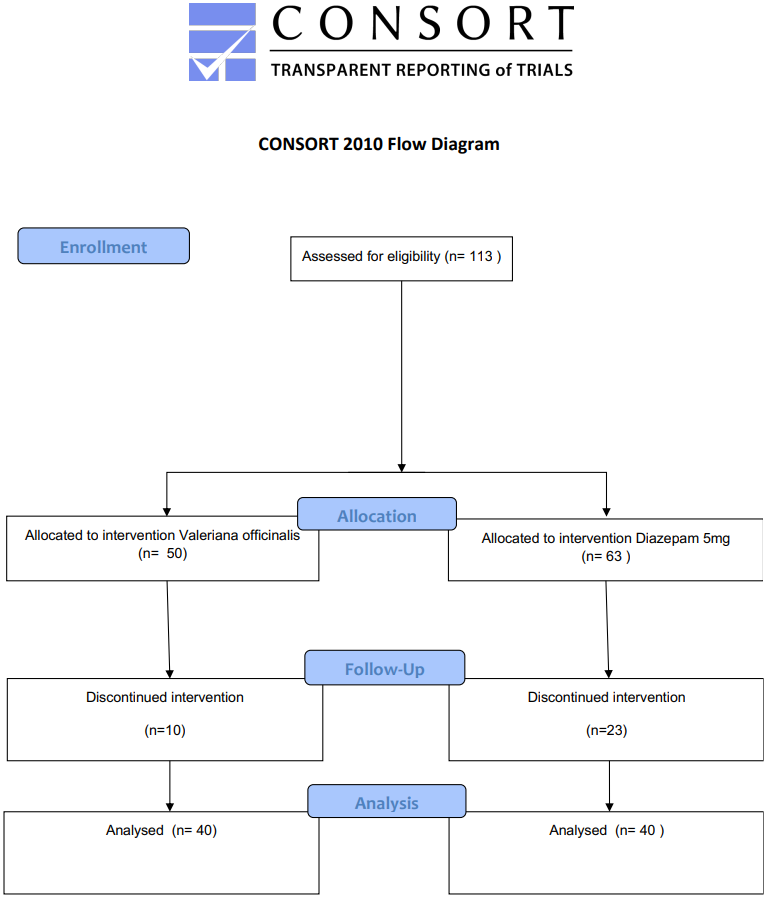
Figure 1: Consort Valerian.
Table 3: Sleep Hygiene Index (SHI) before and after 28 days receiving Diazepam 5 mg or Valeriana officinalis 100 mg.
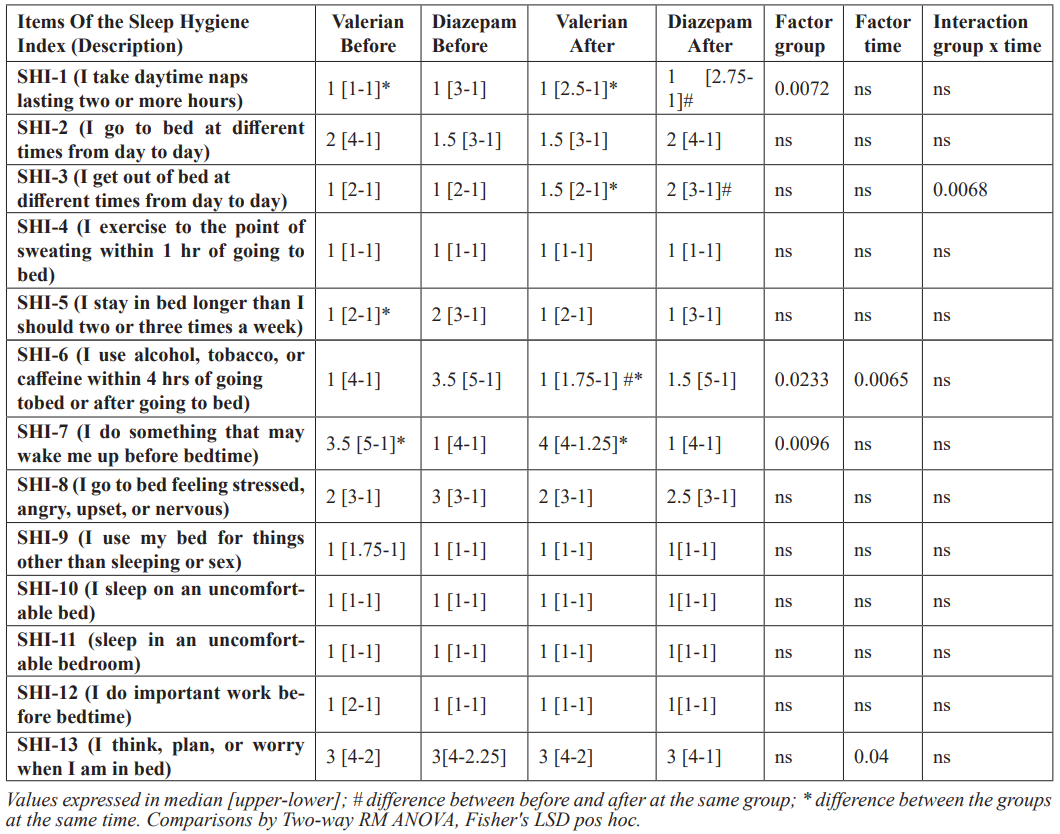
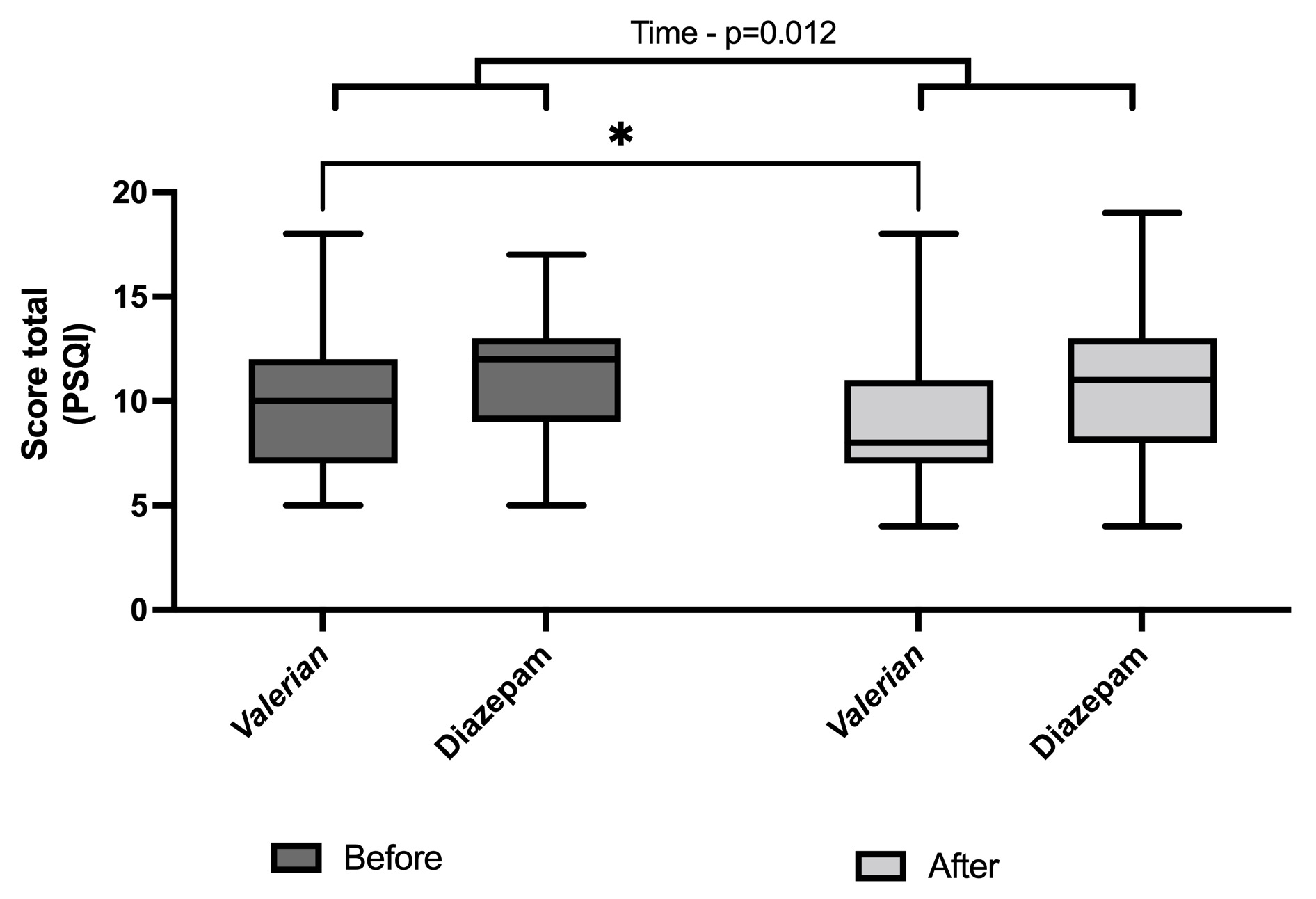
Figure 2: PSQI_total score
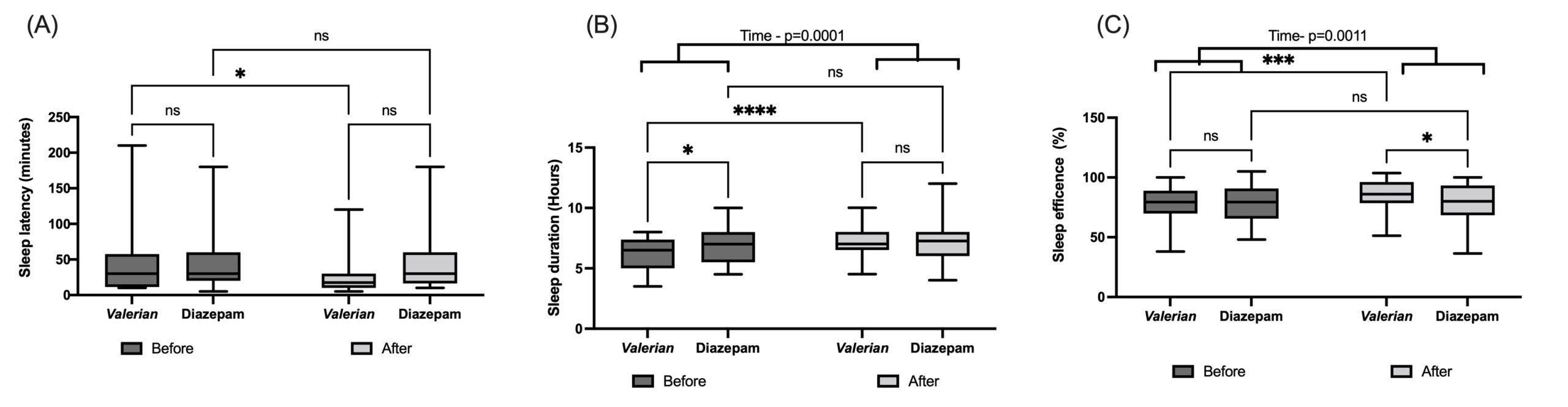
Figure 3: SPSQI individual component scores
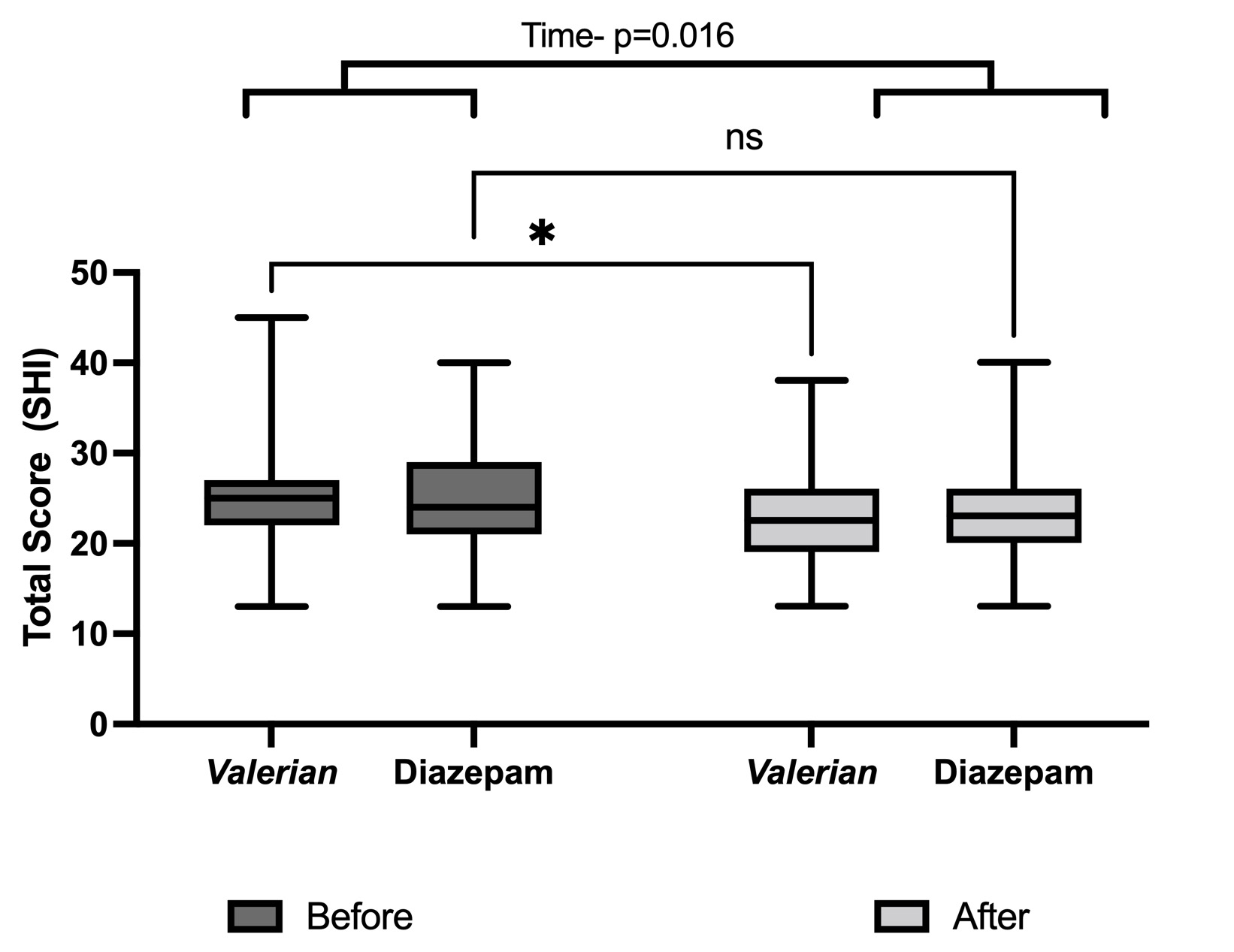
Figure 4: SHI score
Discussion
This is the first work, to the best of our knowledge, evaluating the effects of Valerian officinalis, in a dose suggested in the Herbal Memento of the Brazilian Pharmacopeia, comparing with a positive control, Diazepam, both produced/dispensed by a public compounding pharmacy that offers herbal medicines. Beyond the positive effects of Valerian officinalis 100 mg, similar to Diazepam 5 mg, on several sleep quality parameters in the context of Unified Health System - Brazil (SUS – “Sistema Único de Saúde”), our work brings evidence about the potential of explanatory evaluation, and generating further pragmatic clinical study, as tool for establishing scientific basis for approaches in Primary Health Care (Tosh et al., 2011), especially those available in the Unified Health System (“Sistema Único de Saúde – SUS, Brazil). In this context, a relevant strategy to verify efficacy might be involved with a National Registry Approach focused on medicinal plants/herbal medicines/phytotherapies produced/dispensed by a public compounding pharmacy.
Interestingly, as above described, Brazil has public policies of herbal medicines uses, such as PNPIC) and The National Policy and Program of Medicinal Plants and Herbal Medicines (“Política Nacional de Plantas Medicinais e Medicamentos Fitoterápicos”, PNPMF) [6,9]. However, most species included in Brazilian Official Pharmacopoeia and national formulary are native from Europe and North America. Paradoxically, unfortunately despite the diverse traditional knowledge and our higher biodiversity, rarely strong clinical evidence has been raised from 100% Brazilian phytomedicines, achieving local or global market [11]. Indeed, even widely worldwide used phytomedicines, such as Valerian extracts, have never been evaluated in Brazil or in the Brazilian public health system.
Our group have searched to add knowledge of evidence-based phytotherapy, including on native Brazilian species. A traditionally used species, infusions of Achyrocline satureioides inflorescence, was evaluated on the clinical outcomes of viral respiratory infections in a randomized, open-label, placebo-controlled clinical trial [12], bringing evidence-based validation for the traditional use. Besides, an ongoing broader project designed to evaluate the efficacy of Monteverdia ilicifolia (Maytenus ilicifolia, Celastraceae, popularly called as “espinheira-santa”) capsules has been conducted in a clinical trial. Firstly, we investigated the potential pharmacokinetic interaction and hepatotoxicity of an aqueous extract of this species. Considering the potential M. ilicifolia-induced hepatotoxicity, our group recommend that this medicinal plant must be avoided by patients with liver diseases [13]. This finding opened a new perspective on plant species released and available for use in the SUS [14,15], bringing serious concern about both efficacy and safety. In accordance, this work brings on rational use of Valerian in the context of SUS-Brazil.
Although there is a lack of randomization, which would reduce bias, our findings may indicate how (and whom) a valerian treatment works in a “real-world scenario”, for example in our sample, perimenopause and post menopause women seem to accept/adhere the valerian treatment instead of Diazepam. This phase of life is frequently related to sleep problems, what can be involved with comorbidities, such as depression. The effect of valerian on sleep quality in postmenopausal women has been reported [16]. Interestingly, greater attention must be focused on middle aged women health, a growing proportion of the population.
Our findings indicate that Diazepam and Valerian 100 mg improved total scores PSQI, since there are improved scores after 28 days in both treatment groups. PSQI components, subjective quality, duration, efficiency and disturbances, showed improved scores after both treatments, as indicated by Two-Way ANOVA. However, Valeriana officinallis group seems to achieve better outcomes in relevant components, sleep latency, sleep duration and sleep efficiency.
Besides the total score, Valerian 100 group improved specific items, such as daytime naps, and avoided unhealthy lifestyle factors, such as consumption of substances (i.e., caffeine, alcohol, and nicotine), and stay worrying and thinking at bed). Tonon et al (2023) reported that higher SHI score that means worse sleep hygiene are correlated with poor sleep-related outcomes, such as sleep quality, daytime sleepiness, in addition to depressive symptoms using the Brazilian-Portuguese version of the SHI [17]. In accordance with previous findings, valerian induced mild and few side effects (Shinjyo et al., 2020). This is relevant since sedative-hypnotic drugs, such as all BZDs, in general induce several side effects, including drowsiness, dizziness, lethargy, and fatigue [18]. Adverse effects related to long-term use, tolerance, dependence, and withdrawal are widely recognized, while dementia has been raised recently. Taken together, all findings may suggest that valerian can be an adjuvant for cognitive-behavior therapy, the first-line treatment for management of sleep disturbances, since improved the sleep hygiene and induced mild and few side effects.
Conclusion
Our data suggest the efficacy and safety of capsules containing 100 mg dry extract of Valeriana officinalis L. roots comparing to Diazepam 5 mg for improvement of sleep quality and brings evidence-based practice on this species to be applied in Primary Health Care in the SUS-Brazil. Our work supports the idea that quasi-experimental study, and further pragmatic clinical trial, are useful tools for studying safety and efficacy evidence for decision-making of medicinal plants and herbal medicines available in the Unified Health System (SUS, Brazil).
Acknowledgments:
Authors gratefully acknowledge the institutional and staff support provided by the Primary Healthcare Units of Igrejinha (Rio Grande do Sul, Brazil). Thanks to Maria Paz Loayza Hidalgo for the appreciate contributions to this manuscript. Additionally, the authors extend the thanks to all the participants of this trial, whose involvement was essential in advancing this research. I.R.S, C.D and R.V.A.S received the Researcher Productivity Grant from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Ethical Considerations: This study was registered and approved by the ethics committee of the Federal University of Rio Grande do Sul - CEP UFRGS (CAAE: 64721022.5.0000.5347) and registered at the Brazilian Registry of Clinical Trial (ReBEC). All the participants were informed, and they read and signed a written informed consent form.
Conflicts of interest: The authors have declared no conflicts of interest.
References
- Madruga CS, Paim TL, Palhares HN, et al. Prevalence of and pathways to benzodiazepine use in brazil: The role of depression, sleep, and sedentary lifestyle. Revista Brasileira de Psiquiatria, 2019; 41(1): 44-50. doi:10.1590/1516-4446-2018-0088.
- Castro Braga F. Brazilian traditional medicine: Historical basis, features and potentialities for pharmaceutical development. Journal of Traditional Chinese Medical Sciences, 2021; 8: S44-S50. doi:10.1016/j.jtcms.2020.06.005.
- BRAZIL. RESOLUÇÃO - RDC No 18, DE 3 DE ABRIL DE, 2013.
- Carmona F, Pereira AMS. Prescription patterns of herbal medicines at a Brazilian Living Pharmacy: The Farmácia da Natureza experience, 2013–2019. J Herb Med, 2022; 36: 100597. doi: 10.1016/J.HERMED.2022.100597.
- Carvalho ACB, Lana TN, Perfeito JPS, Silveira D. The Brazilian market of herbal medicinal products and the impacts of the new legislation on traditional medicines. J Ethnopharmacol, 2018; 212: 29-35. doi: 10.1016/J.JEP.2017.09.040.
- Carvalho ACB, Perfeito JPS, e Silva LVC, Ramalho LS, de Oliveira Marques RF, Silveira D. Regulation of herbal medicines in Brazil: advances and perspectives. Brazilian Journal of Pharmaceutical Sciences, 2011; 47(3): 467-473. doi: 10.1590/S1984-82502011000300004.
- Fernández-San-Martín MI, Masa-Font R, Palacios-Soler L, Sancho-Gómez P, Calbó-Caldentey C, Flores-Mateo G. Effectiveness of Valerian on insomnia: A meta-analysis of randomized placebo-controlled trials. Sleep Med, 2010; 11(6): 505-511. doi:10.1016/j.sleep.2009.12.009.
- BRAZIL. Formulário de Fitoterápicos Agência Nacional de Vigilância Sanitária-Anvisa 2a EDIÇÃO, 2021.
- BRAZIL. Farmacopeia Brasileira Memento Fitoterápico, 2016.
- Del Rio João KA, Becker NB, de Neves Jesus S, Isabel Santos Martins R. Validation of the Portuguese version of the Pittsburgh Sleep Quality Index (PSQI-PT). Psychiatry Res, 2017; 247: 225-229. doi: 10.1016/j.psychres.2016.11.042.
- Dutra RC, Campos MM, Santos ARS, Calixto JB. Medicinal plants in Brazil: Pharmacological studies, drug discovery, challenges and perspectives. Pharmacol Res, 2016; 112: 4-29. doi:10.1016/J.PHRS.2016.01.021.
- Bastos CIM, Dani C, Cechinel LR, et al. Achyrocline satureioides as an adjuvant therapy for the management of mild viral respiratory infections in the context of COVID-19: Preliminary results of a randomized, placebo-controlled, and open-label clinical trial. Phytother Res, 2023. doi: 10.1002/PTR.7976.
- Danilevicz CK, Pizzolato LS, Bianchi SE, Meirelles G, Bassani VL, Siqueira IR. Pharmacological evaluation of a traditional Brazilian medicinal plant, Monteverdia ilicifolia. Part I - Preclinical safety study. J Ethnopharmacol, 2024; 324. doi: 10.1016/J.JEP.2024.117806.
- Andrade SAL de, Tristão MI da S, Miguel MD, et al. Fitoterápicos da relação nacional de medicamentos essenciais no Brasil. Revista Cubana de Plantas Medicinales, 2017; 22(1).
- BRASIL. Resolução RDC No 10. Dispõe Sobre a Notificação de Drogas Vegetais Junto à Agência Nacional de Vigilância Sanitária (ANVISA) e Dá Outras Providências, 2010.
- Taavoni S, Ekbatani N, Kashaniyan M, Haghani H. Effect of valerian on sleep quality in postmenopausal women: a randomized placebo-controlled clinical trial. Menopause, 2011; 18(9): 951-955. doi: 10.1097/GME.0B013E31820E9ACF.
- Tonon AC, Amando GR, Carissimi A, et al. The Brazilian-Portuguese version of the Sleep Hygiene Index (SHI): validity, reliability and association with depressive symptoms and sleep-related outcomes. Sleep Sci, 2020; 13(1): 37-48. doi: 10.5935/1984-0063.20190130.
- Griffin CE, Kaye AM, Rivera Bueno F, Kaye AD. Benzodiazepine Pharmacology and Central Nervous System–Mediated Effects. Ochsner J, 2013; 13(2): 214.

