Comprehaem Registry - Safety and Performance of a Special Five Layers Bandaging Compression Haemostasis System After Coronary – Femoral Interventions. E Retrospective Analysis in Real World Patients in Kosovo Republic
Lirza Gorani, Daut Gorani, Lulzim Kamberi, Xhevdet Krasniqi, Jetmir Sejdiu, Artan Ahmeti, Artan Krasniqi, Taulant Sadriu, Luan Keka, Lulzim Bashota, Fisnik Basholli, Agim Krasniqi, Agon Nuhiu and Eleftherios Malakoudis*
Department of cardiology clinical Center Pristina, Kosovo, Greece
Received Date: 05/12/2024; Published Date: 21/01/2025
*Corresponding author: Eleftherios Malakoudis, Department of cardiology clinical Center Pristina, Kosovo, Greece
Abstract
Background: Percutaneous coronary vascular interventions in the last two decades managed to reach the old and burning goal for minimal or less trauma. The majority of minimal invasive techniques and materials are on a constant and perpetual development. The progress of overall PCI was definitive and of course still ongoing. However, the final and respectively crucial part of the PCI procedure- despite the usage of closure devices (VCD)- do not always meet the final goal which is to maintain the compression, securing the haemostasis without major complications or side effects. Ready to use femoral bandaging compression solutions were not available to maintain the haemostasis effect till the current era.
Objectives: This post market retrospective registry’s goal is to assess the visibility and short term outcomes of securing the manual compression haemostasis throughout a special bandaging compression system after femoral/coronary or vascular interventions.
Methods: At this retrospective registry patients enrolled from January 2022 to December 2022, two thousand patients underwent femoral coronary intervention receiving dual antiplatelet therapy post procedural. Clinical data, procedural outcome and clinical follow up to twelve months were obtained; hematoma/swelling was the principal study endpoint. Secondary endpoints were puncture side pain, coldness in the right or the left foot, pain while walking and surgical reconstruction after aneurysm or pseudoaneurysm.
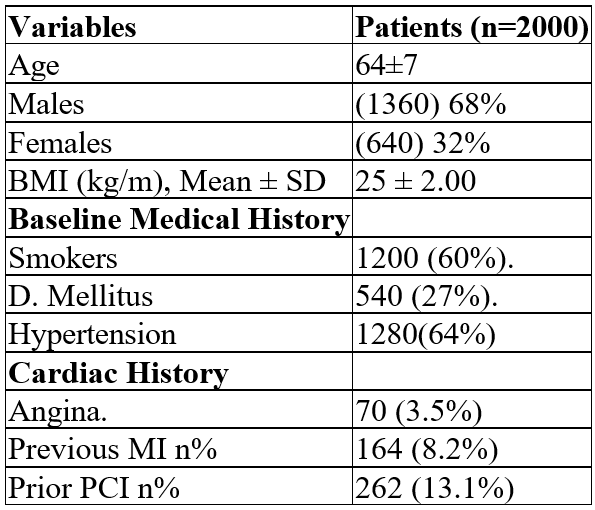
Results: Among the two thousand patients enrolled 68% were men and 32% were women with mean age of 64 years and 71 years old respectively. The BMI was 27 for the males and 25 for the females. 29% and 42% respectively had previous PCI and diabetes mellitus was 27% for both groups. The registry shows that: 1.8%/36 patients had minor hematoma/ swelling and 3.6%/72 patients had minor pain at the puncture site for 5 to 10 days after the procedure. No coldness in the right or left foot, no pain while walking. No surgical reconstruction of the site area (aneurysm or not related) needed or occurred.
Conclusion: The use of a dedicated special, ready for use femoral bandaging compression system (DFCD) is feasible, safe and effective for the maintenance of the haemostasis after femoral percutaneous intervention.
Keywords: Five layers bandaging compression system; Premofix uno
Introduction
Cardiovascular disease is one of the leading mortality causes in the world. According to the world health demography, the global deaths from CVD (coronary vascular disease) will increase from 17.1 million by 2030 (32% of all fatalities). PCI procedures are the main way of treating CVD in Kosovo Republic. Despite the usage of Vascular Closure Devices (VCD) the manual compression is the golden standard for decades (*) [1-5].
The limitation of the manual compression is the lack of the continuous pressure to the access site of the artery. Additionally, the absence of a ready for use system to maintain the pressure to the access site in a persistent and effective way remains a big obstacle. Both of them can lead to access site bleeding. The majority of the access site bleeding are large and small hematomas. Thus, compression maintenance system (ready to use) premofix uno (CE approved) has been developed (Andanza International, Eswenge Germany) with an objective to create a sufficient and effective compression maintenance system (ready to use).
In the real-world patients, there was no system that could deliver the pressure of the excess site and maintain the achieved haemostasis with an atraumatic, antiallergic and patient friendly way. The primary aim of this retrospective study is to evaluate the clinical performance of premofix uno in treatment of the patients enrolled in everyday practice at the clinical centre of Pristrina, Republic of Kosovo.
The primary endpoint was access site bleeding – hematomas and swelling. In addition, secondary endpoints such as puncture side pain, coldness in the right or left foot, pain while walking and surgical reconstruction after aneurysm and pseudoaneurysm formation, were analysed in the retrospective study [6-10].
Study Design and Patient Population
This is a retrospective non randomised single centre study with the main purpose to evaluate the safety and the efficacy of the premofix uno bandaging system for consecutive unselected patients treated in daily practice including those with dual antiplatelet drug therapy. These patients underwent PCI and have the puncture site securely compressed by premofix uno system (Andanza international, Eswenge Germany). The above patients have been enrolled from January 2022 to December 2022 at the clinical centre of Pristina, Kosovo Republic [11-15].
Description of the study compression bandaging system
The Premofix uno, Compression Bandaging System (CBS) is a five layers cotton bandaging system (CE approved). The premofix uno can be applied in seconds, can be opened and resealed within seconds for fast puncture side access and has a perfect fit with high stability. Premofix uno is a noninvasive, low cost, very convenient, safe and protective closure support system.
Interventional Procedure and Adjunctive Medications
All patients received a loading dose of 325 mg of aspirin and 300 mg of clopidogrel or 60 mg of prasugrel or 90 mg of ticagrelor. The procedure was performed according to the standard guidelines of the participating center. All patients received dual antiplatelet therapy (DAPT) aspirin 75-300 mg/day indefinitely and clopidogrel 75 mg/day or prasugrel 10 mg/day or ticagrelor 90 mg twice daily for at least 12 months after the PCI [16-20].
Definition and Endpoints
The primary endpoint of this study was to determine the rate and the nature of hematomas/swelling which is defined as bleeding and swelling at the coronary intervention puncture site, during the follow up period following the index procedure. Clinical evaluation of puncture site pain, bilateral coldness of the feet and reconstruction of aneurysm or pseudoaneurysms formation was obtained as secondary endpoints [21-25].
Statistical Analysis
Categorical data were presented as counts and percentages.
Results
Patients characteristics
Table 1: Baseline characteristics of the study population.
Procedural Characteristics
Premofix uno compression bandaging system treated 2000 puncturing sites (1 per puncturing). The greater percentage was the right site (84%) and the lower percentage was the left (14%).
Clinical Outcomes
At the mean follow up of 12 months the primary endpoint occurred in 36 (1.8% patients). From the secondary endpoints minor pain at the puncturing site occurred in 72 patients (3.6%) from 5 to 10 days after the index procedure. No coldness or any pain while walking in both legs and non-surgical reconstruction of the site (aneurysm or not related) needed or occurred [26-30].
Table 2: Cumulative clinical events for mean follow up of 12 months for patients receiving *Premofix uno (CBS).
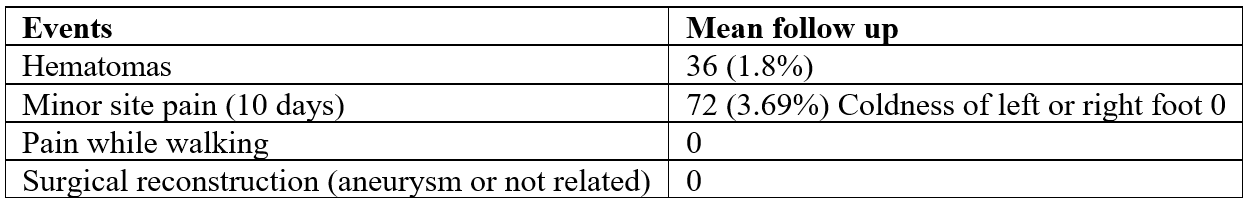
*CBS: Compression Bandaging System
Discussion
Premofix uno treated a total of 2000 patients included in this retrospective study. Construction of the premofix uno consists of a thin, five layers cotton bandage and utilizes a very strong Velcro at the edges. The system contains a polystyrene zeppelin shaped plug. Asset of this structure is that the longitudinal strength can be transferred and delivered from the bandage to the zeppelin structure. This feature leads to a precise, persistent and long-lasting pressure to the puncture site. Thischaracteristic features to be extremely effective and safe for the real-world consecutive patients of this retrospective study [31-35].
Demographic data showed that 64% of the patients had hypertension, 27% had diabetes mellitus and all of them received dual antiplatelet therapy. This plays a significant role in the reduction of the puncture site bleeding after PCI.
Comprehensive studies report bleeding, itching, swelling of the puncture site, coldness of the feet, pain while walking and aneurysm or pseudoaneurysm formation while using the traditional manual compression techniques (despite the usage of intravascular system or agents) [36-40].
On the other hand, there are not plenty of clinical studies having the aim to compare the manual compression with the ready to use Compression Bandaging Systems (CBS).
Several studies show a higher rate of access site bleeding after manual compression after femoral coronary (FC)-43% [41-55].
Despite the use of Vascular Closure Devices (VCD) the most common type of access site bleeding consisted of large and smaller hematomas.
This leads to the conclusion that the transfemoral arterial access is still associated with higher access site bleeding rates.
These consisted mostly of hematomas in 30 days follow up (OASIS, OASIS 2 and CURE studies). At FERARI study the access site bleeding in FC was 45% of the patient’s population [56-74].
Conclusion
The access site bleeding and the relevant hematomas for femoral closure (FC) after PCI is still a significant side effect while performing traditional manual compression.
In conclusion this retrospective study demonstrates a significantly (dramatically) lower access site bleeding, hematomas and swelling of the access site.
In this study performed at an uncontrolled, real world patient population with a long term follow up the PREMOFIX UNO ready to use femoral compression bandaging system demonstrated prolonged safety and effectiveness in both the primary and secondary endpoints.
Therefore, PREMOFIX UNO femoral compression bandaging system would be the compression system of choice for the reduction of the hematomas/swelling of the puncture site after PCI and femoral closure (FC) additionally assisted from the ready to use nature and the very low complication rate.
Limitations
This is a non-randomized study of a retrospective analysis in a single center. Thus, all the non-randomized studies’ limitations are involved.
For example: selection bias of the access sites by individual choices, sheath diameters, techniques and materials may be involved.
Further analysis and randomization will be needed.
Acknowledgments: The authors of this study would like to thank all the patients who participated in this retrospective study.
Monitoring&data management: Eleftherios Malakoudis
References
- JACC Cardiovasc Interv, 2013; 6(7): 698-706.
- Curr Probl Diagn Radiol, 2009; 38(1): 33-43.
- Eur Heart J, 2003; 24(20): 1815-1823.
- Heart Vessels, 2016; 31(6): 897-906.
- Circulation, 2011; 123(23): 2736-2747.
- Bauer Hypercoagulable States. In: Hoffman R, Benz EJ, Shattil SJ, Furie B, Silberstein LE, McGlave P, editors. Hematology Basic Principles and Practice. 3. Churchill Livingstone; New York, 2000; pp. 2009–2039.
- Bauer KA. Fondaparinux sodium: a selective inhibitor of factor Xa. Am J Health Syst Pharm, 2001; 58(Suppl 2): S14–S17. doi: 1093/ajhp/58.suppl2.S14.
- Bennett Hereditary disorders of platelet function. In: Hoffman R, Benz EJ, Shattil SJ, Furie B, Silberstein LE, McGlave P, editors. Hematology Basic Principles and Practice. 3. Churchill Livingstone; New York, 2000; pp 2154–2172.
- Bouma BN, von dem Borne PA, Meijers Factor XI and protection of the fibrin clot against lysis--a role for the intrinsic pathway of coagulation in fibrinolysis. Thromb Haemost, 1998; 80: 24–27.
- Brass The molecular basis for platelet activation. In: Hoffman R, Benz EJ, Shattil SJ, Furie B, Cohen HJ, Silberstein LE, McGlave P, editors. Hematology Basic Principles and Practice 3. Churchill Livingstone; New York, 2000; pp. 1753–1770.
- Broze GJ Jr, Warren LA, Novotny WF, Higuchi DA, Girard JJ, Miletich The lipoprotein-associated coagulation inhibitor that inhibits the factor VII-tissue factor complex also inhibits factor Xa: insight into its possible mechanism of action. Blood, 1988; 71: 335–343.
- Burch JW, Stanford N, Majerus Inhibition of platelet prostaglandin synthetase by oral aspirin. J Clin Invest, 1978; 61: 314–319. doi: 10.1172/JCI108941.
- Cohen Antiplatelet therapy in percutaneous coronary intervention: a critical review of the 2007 AHA/ACC/SCAI guidelines and beyond. Catheter Cardiovasc Interv, 2009; 74: 579–597. doi: 10.1002/ccd.22021.
- Cramer TJ, Griffin JH, Gale AJ. Factor V. Is an Anticoagulant Cofactor for Activated Protein C during Inactivation of Factor Pathophysiol Haemost Thromb, 2010; 37: 17–23. doi: 10.1159/000315141. Dahlback B. Blood coagulation. Lancet, 2000; 355: 1627–1632. doi: 10.1016/S0140-6736(00)02225-X.
- Damus PS, Hicks M, Rosenberg Anticoagulant action of heparin. Nature, 1973; 246: 355–357. doi: 10.1038/246355a0.
- Egeberg Inherited antithrombin deficiency causing thrombophilia. Thromb Diath Haemorrh, 1965; 13: 516–530.
- Emsley J, Knight CG, Farndale RW, Barnes MJ, Liddington RC. Structural basis of collagen recognition by integrin Cell, 2000; 101: 47–56. doi: 10.1016/S0092-8674(00)80622-4.
- Esmon CT, Owen Identification of an endothelial cell cofactor for thrombin-catalyzed activation of protein C. Proc Natl Acad Sci USA, 1981; 78: 2249–2252. doi: 10.1073/pnas.78.4.2249.
- Falati S, Gross P, Merrill-Skoloff G, Furie BC, Furie B. Real-time in vivo imaging of platelets, tissue factor and fibrin during arterial thrombus formation in the Nat Med, 2002; 8: 1175–1181. doi: 10.1038/nm782.
- Federici Prophylaxis of bleeding episodes in patients with von Willebrand’s disease. Blood Transfus, 2008; 6(Suppl 2): s26–s32. doi: 10.2450/2008.0034-08.
- Franchini M, Mannucci Venous and arterial thrombosis: different sides of the same coin? Eur J Intern Med, 2008; 19: 476–481. doi: 10.1016/j.ejim.2007.10.019.
- Fulcher CA, Gardiner JE, Griffin JH, Zimmerman TS. Proteolytic inactivation of activated human factor VIII procoagulant protein by activated protein C and its analogy to factor V. Blood, 1984; 63: 486–489.
- Furie B. Pathogenesis of thrombosis. Hematology Am Soc Hematol Educ Program, 2009: 255–258. doi: 1182/asheducation-2009.1.255.
- Furie B, Furie Molecular basis of vitamin K-dependent gamma-carboxylation. Blood, 1990; 75: 1753–1762.
- Giossi A, Pezzini A, Del ZE, Volonghi I, Costa P, Ferrari D, Padovani Advances in antiplatelet therapy for stroke prevention: the new P2Y12 antagonists. Curr Drug Targets, 2010; 11: 380–391. doi: 10.2174/138945010790711987.
- Guinto ER, Esmon CT. Loss of prothrombin and of factor Xa-factor Va interactions upon inactivation of factor Va by activated protein J Biol Chem, 1984; 259: 13986–13992.
- Hagen I, Nurden A, Bjerrum OJ, Solum NO, Caen J. Immunochemical evidence for protein abnormalities in platelets from patients with Glanzmann’s thrombasthenia and Bernard-Soulier J Clin Invest, 1980; 65: 722–731. doi: 10.1172/JCI109719.
- Han X, Fiehler R, Broze GJ Characterization of the protein Z-dependent protease inhibitor. Blood, 2000; 96: 3049–3055.
- Hanasaki K, Arita H. Characterization of thromboxane A2/prostaglandin H2 (TXA2/PGH2) receptors of rat platelets and their interaction with TXA2/PGH2 receptor antagonists. Biochem Pharmacol, 1988; 37: 3923–3929. doi: 10.1016/0006-2952(88)90075-5.
- Harenberg Development of idraparinux and idrabiotaparinux for anticoagulant therapy. Thromb Haemost, 2009; 102: 811–815. doi: 10.1160/TH09-08-0555.
- Hoffman M, Monroe , III A cell-based model of hemostasis. Thromb Haemost, 2001; 85: 958–965.
- Jackson The growing complexity of platelet aggregation. Blood, 2007; 109: 5087–5095. doi: 10.1182/blood-2006-12-027698.
- James K, Taylor FB, Jr, Fudenberg The effect of alpha-2-macroglobulin in human serum on trypsin, plasmin, and thrombin activities. Biochim Biophys Acta, 1967; 133: 374–376. doi: 10.1016/0005-2795(67)90080-3.
- Kahn ML, Zheng YW, Huang W, Bigornia V, Zeng D, Moff S, et al. A dual thrombin receptor system for platelet Nature, 1998; 394: 690–694. doi: 10.1038/29325.
- Kehrel B, Wierwille S, Clemetson KJ, Anders O, Steiner M, Knight CG, et al. Glycoprotein VI is a major collagen receptor for platelet activation: it recognizes the platelet-activating quaternary structure of collagen, whereas CD36, glycoprotein IIb/IIIa, and von Willebrand factor do not. Lood, 1998; 91: 491–499.
- Kirchhofer D, Nemerson Y. Initiation of blood coagulation: the tissue factor/factor VIIa Curr Opin Biotechnol, 1996; 7: 386–391. doi: 10.1016/s0958-1669(96)80112-1.
- Lam SC, Plow EF, D’Souza SE, Cheresh DA, Frelinger AL, III, Ginsberg Isolation and characterization of a platelet membrane protein related to the vitronectin receptor. J Biol Chem, 1989; 64: 3742–3749.
- Lane DA, Philippou H, Huntington Directing thrombin. Blood, 2005; 106: 2605–2612. doi: 10.1182/blood-2005-04-1710.
- Laux V, Perzborn E, Heitmeier S, von DG, Dittrich-Wengenroth E, Buchmuller A, et al. Direct inhibitors of coagulation proteins - the end of the heparin and low-molecular-weight heparin era for anticoagulant therapy? Thromb Haemost, 2009; 102: 892–899. doi: 1160/TH09-02-0134.
- Lijnen HR, Collen D. Molecular and cellular basis of fibrinolysis. In: Hoffman R, Benz EJ, Shattil SJ, Furie B, Silberstein LE, McGlave P, Hematology Basic Principles and Practice. 3. Churchill Livingstone; New York, 2000; pp. 1804–1814.
- Luo BH, Springer Integrin structures and conformational signaling. Curr Opin Cell Biol, 2006; 18: 579–586. doi: 10.1016/j.ceb.2006.08.005.
- Mann KG, Nesheim ME, Church WR, Haley P, Krishnaswamy Surface-dependent reactions of the vitamin K-dependent enzyme complexes. Blood, 1990; 76: 1–16.
- Mannucci Back to the future: a recent history of haemophilia treatment. Haemophilia, 2008; 14(Suppl 3): 10–18. doi: 10.1111/j.1365-2516.2008.01708.x.
- Mannucci PM, Tuddenham The hemophilias--from royal genes to gene therapy. N Engl J Med, 2001; 344: 1773–1779. doi: 10.1056/NEJM200106073442307.
- Marlar RA, Griffin Deficiency of protein C inhibitor in combined factor V/VIII deficiency disease. J Clin Invest, 1980; 66: 1186–1189. doi: 10.1172/JCI109952.
- Mills ADP receptors on platelets. Thromb Haemost, 1996; 76: 835–856.
- Mills DC, Puri R, Hu CJ, Minniti C, Grana G, Freedman MD, et al. Clopidogrel inhibits the binding of ADP analogues to the receptor mediating inhibition of platelet adenylate cyclase. Arterioscler Thromb, 1992; 12: 430–436. doi: 10.1161/01.atv.12.4.430.
- Nieswandt B, Brakebusch C, Bergmeier W, Schulte V, Bouvard D, Mokhtari-Nejad R, et al. Glycoprotein VI but not alpha2beta1 integrin is essential for platelet interaction with collagen. EMBO J, 2001; 20: 2120–2130. doi: 10.1093/emboj/20.9.2120.
- Phillips DR, Agin PP. Platelet membrane defects in Glanzmann’s thrombasthenia. Evidence for decreased amounts of two major glycoproteins. J Clin Invest, 1977; 60: 535–545. doi: 1172/JCI108805.
- Pipe Recombinant clotting factors. Thromb Haemost, 2008; 99: 840–850. doi: 10.1160/TH07-10-0593.
- Quinsey NS, Greedy AL, Bottomley SP, Whisstock JC, Pike Antithrombin: in control of coagulation. Int J Biochem Cell Biol, 2004; 36: 386–389. doi: 10.1016/s1357-2725(03)00244-9.
- Rau JC, Beaulieu LM, Huntington JA, Church FC. Serpins in thrombosis, hemostasis and J Thromb Haemost, 2007; 5(Suppl 1): 102–115. doi: 10.1111/j.1538-7836.2007.02516.x.
- Rodeghiero F, Castaman G, Tosetto How I treat von Willebrand disease. Blood, 2009; 114: 1158–1165. doi: 10.1182/blood-2009-01-153296.
- Ruggeri The role of von Willebrand factor in thrombus formation. Thromb Res, 2007; 120(Suppl 1): S5–S9. doi: 10.1016/j.thromres.2007.03.011.
- Ruggeri ZM, Mendolicchio Adhesion mechanisms in platelet function. Circ Res, 2007; 100: 1673–1685. doi: 10.1161/01.RES.0000267878.97021.ab.
- Schulze H, Shivdasani Mechanisms of thrombopoiesis. J Thromb Haemost, 2005; 3: 1717–1724. doi: 10.1111/j.1538-7836.2005.01426.x.
- Shen L, Dahlback Factor V and protein S as synergistic cofactors to activated protein C in degradation of factor VIIIa. J Biol Chem, 1994; 269: 18735–18738.
- Shimada K, Kobayashi M, Kimura S, Nishinaga M, Takeuchi K, Ozawa T. Anticoagulant heparin-like glycosaminoglycans on endothelial cell Jpn Circ J, 1991; 55: 1016–1021. doi: 10.1253/jcj.55.1016.
- Siedlecki CA, Lestini BJ, Kottke-Marchant KK, Eppell SJ, Wilson DL, Marchant Shear-dependent changes in the three-dimensional structure of human von Willebrand factor. Blood, 1996; 88: 2939–2950.
- Sonnenberg A, Modderman PW, Hogervorst Laminin receptor on platelets is the integrin VLA-6. Nature, 1988; 336: 487–489. doi: 10.1038/336487a0.
- Sugidachi A, Asai F, Yoneda K, Iwamura R, Ogawa T, Otsuguro K, Koike Antiplatelet action of R-99224, an active metabolite of a novel thienopyridine-type G(i)-linked P2T antagonist, CS-747. Br J Pharmacol, 2001; 132: 47–54. doi: 10.1038/sj.bjp.0703761.
- Suzuki K, Nishioka J, Hashimoto S. Protein C inhibitor: Purification from human plasma and J Biol Chem, 1983; 258: 163–168.
- Tangelder GJ, Slaaf DW, Arts T, Reneman Wall shear rate in arterioles in vivo: least estimates from platelet velocity profiles. Am J Physiol, 1988; 254: H1059–H1064. doi: 10.1152/ajpheart.1988.254.6.H1059.
- Terraube V, O’Donnell JS, Jenkins Factor VIII and von Willebrand factor interaction: biological, clinical and therapeutic importance. Haemophilia, 2010; 16: 3–13. doi: 10.1111/j.1365-2516.2009.02005.x.
- Tollefsen DM, Majerus DW, Blank Heparin cofactor II. Purification and properties of a heparin-dependent inhibitor of thrombin in human plasma. J Biol Chem, 1982; 257: 2162–2169.
- Varga-Szabo D, Pleines I, Nieswandt Cell adhesion mechanisms in platelets. Arterioscler Thromb Vasc Biol, 2008; 28: 403–412. doi: 10.1161/ATVBAHA.107.150474.
- Vu TK, Hung DT, Wheaton VI, Coughlin Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell, 1991; 64: 1057–1068. doi: 10.1016/0092-8674(91)90261-v.
- Walker FJ. Regulation of activated protein C by a new protein. A possible function for bovine protein J Biol Chem, 1980; 255: 5521–5524.
- Weber R, Weimar C, Diener HC. Medical prevention of stroke and stroke recurrence in patients with TIA and minor Expert Opin Pharmacother, 2009; 10: 1883–1894. doi: 10.1517/14656560903048934.
- Weitz Low-molecular-weight heparins. N Engl J Med, 1997; 337: 688–698. doi: 10.1056/NEJM199709043371007.
- Wuillemin WA, Minnema M, Meijers JC, Roem D, Eerenberg AJ, Nuijens JH, et al. Inactivation of factor XIa in human plasma assessed by measuring factor XIa-protease inhibitor complexes: major role for C1-inhibitor. Blood, 1995; 85: 1517–1526.
- Xiao T, Takagi J, Coller BS, Wang JH, Springer TA. Structural basis for allostery in integrins and binding to fibrinogen-mimetic therapeutics. Nature, 2004; 432: 59–67. doi: 1038/nature02976.
- Yago T, Lou J, Wu T, Yang J, Miner JJ, Coburn L, et al. Platelet glycoprotein Ibalpha forms catch bonds with human WT vWF but not with type 2B von Willebrand disease J Clin Invest, 2008; 118: 3195–3207. doi: 10.1172/JCI35754.
- Zucker-Franklin Megakaryocyte and platelet structure. In: Hoffman R, Benz EJ, Shattil SJ, Furie B, Silberstein LE, McGlave P, editors. Hematology Basic Principles and Practice. 3. Churchill Livingstone New York, 2000; pp.1730–1740.

