Clinical Pharmacology of Nifedipine
Gian Maria Pacifici*
Professor of Pharmacology, Via Sant’Andrea 32, 56127 Pisa, Italy
Received Date: 12/11/2024; Published Date: 06/12/2024
*Corresponding author: Gian Maria Pacifici, Professor of Pharmacology, Via Sant’Andrea 32, 56127 Pisa, Italy. E-mail: prof.pacifici.gianmaria@gmail.com
Abstract
Nifedipine is a multiple Ca2+ channel blocker approved for clinical use, is the drug of choice to treat hypertension, and the treatment of hypertensive patients is generally performed with nifedipine at the daily dose of 20 to 30 mg but some authors used higher doses. Nifedipine is rapidly absorbed following oral dosing and is rapidly eliminated. The efficacy and safely of nifedipine, the treatment of patients with nifedipine, and the trials conducted with nifedipine and with other antihypertensive drugs have been reviewed. The metabolism of nifedipine has been reviewed in humans, nifedipine undergoes a first-pass metabolism, about one half of the dose of nifedipine is eliminated presystemically, and nifedipine is metabolized by the cytochrome CYP3A4. The pharmacokinetics of nifedipine have been studied in healthy volunteers following oral administration and nifedipine is eliminated with an elimination half-life of 1.52 hours and the distribution volume of nifedipine is lower the water volume. The interaction of nifedipine with drugs has been reviewed, nifedipine interacts with drugs, and this interaction affects the plasma concentration of nifedipine. The aim of this study is to review the efficacy and safely of nifedipine, the treatment of patients with nifedipine, and the trials conducted with nifedipine and with other antihypertensive drugs. In addition, the metabolism of nifedipine, the pharmacokinetics of nifedipine, and the interactions of nifedipine with drugs have been reviewed.
Keywords: Drug-interaction; Efficacy-safely; Metabolism; Pharmacokinetics; Treatment; Trials
Introduction
Mechanisms of action of nifedipine
Nifedipine is a multiple Ca2+ channel blocker approved for clinical use. An increased concentration of cytosolic Ca2+ causes increased concentration in both cardiac and vascular smooth muscle cells. In cardiac myocytes, the entry of extracellular Ca2+ causes a larger Ca2+ release from intracellular stores (Ca2+ induced Ca2+ release) and thereby initiates the contraction twitch. In smooth muscle cells, entry of Ca2+ plays a dominant role, but the release of Ca2+ from the intracellular storage sites also contributes to contraction of vascular smooth muscle, particularly in some vascular beds. Cytosolic Ca2+ concentrations can be increased by diverse contractile stimuli in vascular smooth cells [1].
Pharmacological actions of nifedipine
Depolarization of vascular smooth muscle cells depends primarily on the influx of Ca2+. At least three distinct mechanisms may be responsible for contraction of vascular smooth cells. First, voltage-gated Ca2+ channels open in response to depolarisation of the membrane, and extracellular Ca2+ moves down its electrochemical gradient into the cell. After closure of Ca2+ channels, a finite period of time is required before the channels open again in response to a stimulus. Second, agonist-induced contractions that occur with-out despoliation of the membrane result from stimulation of the Gq-phospholipase C (inositol 1,4,5-tripjosphate) pathway, resulting in the release of intracellular Ca2+ from the sarcoplasmic reticulum. Emptying of intracellular Ca2+ stores may trigger further influx of extracellular Ca2+ (store-operated Ca2+ entry), but its relevance in smooth muscle is unresolved. Third, receptor-operated Ca2+ channels allow the entry of extracellular Ca2+ in response to receptor occupancy. An increase in cytosolic Ca2+ results in enhanced binding of Ca2+ to calmodulin. The Ca2+-calmodulin complex in turn activates myosin light-chain kinase, with resulting phosphorylation of the myosin light chain. Such phosphorylation promotes interaction between actin and myosin and leads to sustained contraction of smooth muscle Ca2+ channel blockers inhibit the voltage dependent Ca2+ channels in vascular smooth muscle and decreases Ca2+ entry. All Ca2+ channel antagonists relax arterial smooth muscle and thereby decrease arterial resistance, blood pressure, and cardiac afterload [1].
Absorption, distribution, metabolism, and elimination of nifedipine
Immediate-release nifedipine is quickly absorbed after oral intake and produces only a briefly elevated blood level of the drug with an elimination half-life of about 1.8 hours that is associated with an abrupt decrease in blood pressure, reflex activation of the sympathetic nervous system, and tachycardia. This can cause a typical flush and can increase the risk of angina pectoris by abruptly decreasing coronary perfusion pressure concomitantly with tachycardia. Sustained-release preparations of nifedipine reduce fluctuations of plasma concentration. The bioavailability of all Ca2+ channel blockers is reduced, in some cases markedly, by first-pass metabolism by CYP3A4 enzymes in the interstitial epithelium and the liver. This has two consequences: (1) the bioavailability of these drugs may be increased by strong intake of inhibitors of CYP3A4, such as macrolide and imidazole antibiotics and antiretroviral agents, and grapefruit juice. Bioavailability is reduced by inducers of CYP3A4, such as rifampin and carbamazepine. (2) Some Ca2+ channel blockers (particularly verapamil) are strong CYP3A4 inhibitors and cause clinically relevant drug interactions with other CYP3A4 inhibitors, such as simvastatin and atorvastatin [1].
Toxicity and untoward responses of nifedipine
The profile of adverse reactions to the Ca2+ channel blockers vary among the drugs in this class. Immediate-release capsules of nifedipine often cause headache, flushing, and dizziness and can actually worse myocardial ischemia. Dizziness and flushing are much less of a problem with the sustained-release formulations and with the dihydropyridines having a long elimination half-life and providing more constant plasma drug concentrations. Peripheral oedema may occur in some patients with Ca2+ channel blockers but is not the result of generalized fluid retention; rather, it most likely results from increased hydrostatic pressure in the lower extremities owing to precapillary dilatation and reflex post-capillary constriction. Ca2+ channel blockers can cause or aggravate gastroesophageal reflux [1].
Literature search
The literature search was performed electronically using PubMed database as search engine and the following key words were used: “nifedipine efficacy, safety”, “nifedipine treatment”, “nifedipine trials”, “nifedipine metabolism”, “nifedipine pharmacokinetics”, and “nifedipine drug interactions”. In addition the book: Goodman@Gilman’s. The Pharmacological basis of Therapeutics [1] has been consulted.
Nifedipine molecular structure (molecular weight = 346.339 grams/mole)
Results
Efficacy and safely of nifedipine
Seven studies have been reported on the efficacy and safely of nifedipine. Immediate-release nifedipine effectively and safely led to a reduction of the blood pressure in hypertensive patients [2]. Sustained- and extended-release nifedipine effectively and safely treated patients with mild or moderate hypertension [3]. Sublingual nifedipine is a safe, effective, and practical agent for treatment of patients with hypertensive emergencies [4]. Nifedipine, administered at the dose of 30 mg at bedtime, effectively and safely reduced the blood pressure in hypertensive patients [5]. In Chinese and European patients with hypertension, ramipril (10 mg daily) and nifedipine (30 mg daily) were similarly efficacy and safe in lowering the blood pressure [6]. Long-term administration of nifedipine, administered at the daily dose of 80 mg, was well-tolerated and effectively and safely reduced the blood pressure in patients with essential hypertension [7]. Pregnant women received 10 mg of nifedipine tablets or 10 mg of nifedipine capsules. Nifedipine tablets were as effective as nifedipine capsules for the rapid treatment of severe hypertension in pregnant women [8].
Treatment of patients with nifedipine
Nine studies have been reported on the treatment of patients with nifedipine. Nifedipine was administered at the daily dose of 30 mg to 15 hypertensive patients and nifedipine effectively lowered the blood pressure [9]. The efficacy of nifedipine in treatment of hypertension was assessed in 15 patients who received nifedipine at the dose of 10, 20 or 30 mg thrice-daily. Nifedipine is an effective treatment of hypertensive patients but probably should be used in combination with a potassium sparing diuretic [10]. The effectiveness of nifedipine in controlling the blood pressure was studied in 20 hypertensive patients. Eleven patients (55.0%) became normotensive after the administration of nifedipine at the daily dose of 30 mg and nine patients (45.0%) became normotensive after the administration of nifedipine at the daily dose of 60 mg and both doses of nifedipine were well-tolerated [11]. Nifedipine was administered at the daily dose of 10 to 20 mg to 25 hypertensive patients and this treatment normalized the blood pressure in 98% of patients [12]. Twenty-seven hypertensive patients received a single dose of 10 mg of nifedipine which reduced the blood pressure in most of patients and nifedipine was associated with a rise in cardiac output and pulse rate [13]. Thirty-one hypertensive patients received nifedipine once-daily at the dose of 30, 60 or 90 mg and 32 hypertensive patients received propranolol once-daily at the dose of 80, 160 or 240 mg. Nifedipine was more effective than propranolol (P-value < 0.02) in reducing the blood pressure and both treatments were well-tolerated [14]. Ninety-four hypertensive patients received either nifedipine at the dose of 20 mg twice-daily or cyclopenthiazide at the daily dose of 0.25 mg and nifedipine lowered the blood pressure as cyclopenthiazide [15]. Fifty-one hypertensive patients received either nifedipine once-daily at the dose of 20 mg or clonidine which was administered at the initial dose of 0.1 mg with hourly doses of 0.1 mg. Nifedipine reduced the blood pressure in 83% of patients 45 min after dosing and clonidine reduced the blood pressure in 79% of patients four hours after dosing. Both nifedipine and clonidine were safe and effective in treatment of hypertensive patients [16]. A total of 16,069 stroke patients with a mean age of 68.3+2.1 years received short-acting nifedipine and this treatment was associated with increased risk of stroke occurrence in elderly hypertensive patients [17].
Trials conducted with nifedipine and with other antihypertensive drugs
Three studies have been reported on the trials conducted with nifedipine and other antihypertensive drugs. A prospective, randomized, double-blind trial was conducted in 6,321 hypertensive patients, aged 55 to 80 years, who received either nifedipine at the daily dose of 30 mg (group A) or hydrochlorothiazide at the daily dose of 25 mg plus amiloride at the daily dose of 2.5 mg (group B) and patients were followed for three years. The mean blood pressure decreased similarly with both treatments. There were more withdrawals in patients of group A than in patients of group B because peripheral oedema occurred in 38.1% of patients of group A and in 8.0% of patients of group B (P-value = 0.02). Deaths from any cardiovascular or cerebrovascular cause occurred in 6.3% of patients of group B and in 5.8% of patients of group A (P-value = 0.35). Total mortality (death from a vascular cause and non-fatal vascular events) occurred in 12.1% of patients of group B and in 12.7% patients of group a (P-value = 0.32). Deaths were predominantly non-vascular and occurred in 176 patients of group A and in 172 patients of group B (P-value = 0.81). Both treatments had similar efficacy in controlling the blood pressure and were well-tolerated [18]. It was compared the efficacy of nifedipine, hydralazine, and labetalol in treatment of severe hypertension during pregnancy. A total of 21 trials, including 2,183 patients, compared 7 regimens (oral nifedipine administered at the daily dose of 50, 60 and 90 mg, intravenous hydralazine administered at the daily dose of 15 and 25 mg, and intravenous labetalol administered at the daily dose of 200 and 300 mg). Nifedipine was superior to hydralazine and labetalol in treatment of severe hypertension in pregnant women [19]. A multicentre, parallel-group, open-label, randomised, controlled trial compared the efficacy of 10 mg of oral nifedipine, to that of 200 mg of oral labetalol, and to that of 1,000 mg of oral methyldopa in treatment of severe hypertension during pregnancy. All treatments reduced the blood pressure in most women and nifedipine reduced the blood pressure more effectively than labetalol and methyldopa [20].
Metabolism of nifedipine
Waller et al. [21] studied the first-pass metabolism of nifedipine in six healthy volunteers. An oral formulation of nifedipine (100 µg/ml) was provided and a single dose of 20 or 30 mg of nifedipine was administered to fasting healthy volunteers. The bioavailability of nifedipine was 0.43 suggesting that about half of the dose of nifedipine is eliminated presystemically. Holtbecker et al. [22] stated that nifedipine is almost completely metabolized in humans by CYP3A4 and this cytochrome is present in human liver, small bowel, and other extrahepatic tissues.
Pharmacokinetics of nifedipine
Walley et al. [23] studied the pharmacokinetics of nifedipine in eight healthy volunteers, aged 29 years (range, 22 to 39), and weighing 73 kg (range, 59 to 85) who received a single oral dose of 8.5 mg of nifedipine. Table 1 summarizes the pharmacokinetic parameters of nifedipine which have been obtained in eight healthy volunteers.
Table 1: Pharmacokinetic parameters of nifedipine which have been obtained in 8 healthy volunteers. A single oral dose of 8.5 mg of nifedipine was administered to healthy volunteers. Values are the mean+SEM, by Walley et al. [23].
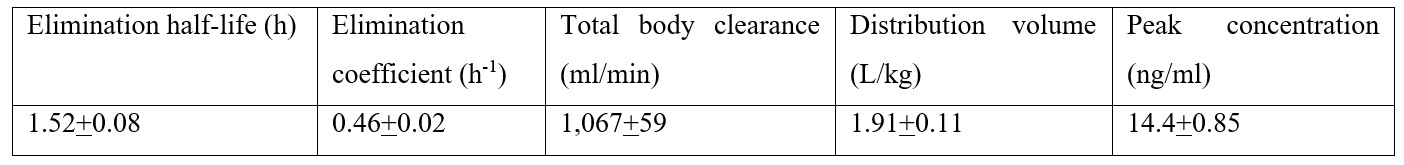
This table shows that nifedipine is rapidly eliminated as the mean elimination half-life is 1.52 hours, the distribution volume of nifedipine is lower than the water volume, and nifedipine rapidly achieves the steady-state concentration.
Interactions of nifedipine with drugs
Seven studies have been reported on the interactions of nifedipine with drugs. Eleven patients received 30 mg daily of diltiazem plus 10 mg of nifedipine 4 times-daily and both treatments lasted one week. The concomitant administration of diltiazem and nifedipine caused an increase in the plasma concentration of nifedipine [24]. Twelve healthy volunteers received cimetidine once-daily at the dose of 800 mg, ranitidine once-daily at the dose of 300 mg, and nifedipine once-daily at the dose of 20 mg. Cimetidine produced a significant increase in the area under the concentration-time curve of nifedipine whereas ranitidine did not produce any significant changes in the pharmacokinetic parameters of nifedipine [25]. Rifampicin was administered at the daily dose of 600 mg and nifedipine was co-administered at the daily dose of 20 mg and both treatments lasted 7 days. Rifampicin significantly reduced the plasma concentration of nifedipine thus reduced the antihypertensive effect of nifedipine [26]. Non-steroidal anti-inflammatory drugs were co-administered with nifedipine. Non-steroidal anti-inflammatory drugs induced the metabolism of nifedipine thus the co-administration of anti-inflammatory drugs with nifedipine reduced the plasma concentration and the antihypertensive effect of nifedipine [27]. Patients with Type II diabetes and with hypertension received metformin at the daily dose of 1,000 mg and nifedipine at the daily dose of 30 mg and nifedipine significantly increased the absorption of metformin [28]. Metformin was administered at the daily dose of 1,000 mg and nifedipine was co-administered at the daily dose of 20 mg and nifedipine reduced the concentration of glucose in human umbilical vein cells [29]. Fifteen patients received 0.3 µg/kg/min of nifedipine as an infusion and 0.5 mg/kg of enoximone as a bolus. Nifedipine plasma level dropped to values less than 15 ng/ml thus enoximone reduced the plasma concertation of nifedipine [30].
Discussion
Nifedipine is a multiple Ca2+ channel blocker approved for clinical use. An increased concentration of cytosolic Ca2+ causes increased concentration in both cardiac and vascular smooth muscle cells. In cardiac myocytes, the entry of extracellular Ca2+ causes a larger Ca2+ release from intracellular stores (Ca2+ induced Ca2+ release) and thereby initiates the contraction twitch. In smooth muscle cells, entry of Ca2+ plays a dominant role, but the release of Ca2+ from the intracellular storage sites also contributes to contraction of vascular smooth muscle, particularly in some vascular beds. At least three distinct mechanisms may be responsible for contraction of vascular smooth cells. First, voltage-gated Ca2+ channels open in response to depolarisation of the membrane, and extracellular Ca2+ moves down its electrochemical gradient into the cell. After closure of Ca2+ channels, a finite period of time is required before the channels open again in response to a stimulus. Second, agonist-induced contractions that occur with-out despoliation of the membrane result from stimulation of the Gq-phospholipase C (inositol 1,4,5-tripjosphate) pathway, resulting in the release of intracellular Ca2+ from the sarcoplasmic reticulum. Emptying of intracellular Ca2+ stores may trigger further influx of extracellular Ca2+ (store-operated Ca2+ entry), but its relevance in smooth muscle is unresolved. Third, receptor-operated Ca2+ channels allow the entry of extracellular Ca2+ in response to receptor occupancy. An increase in cytosolic Ca2+ results in enhanced binding of Ca2+ to calmodulin. Nifedipine is quickly absorbed after oral intake and the elimination half-life of nifedipine is 1.8 hours and the bioavailability of nifedipine is reduced by CYP3A4. Immediate-release capsules of nifedipine often cause headache, flushing, and dizziness and can actually worse myocardial ischemia. Dizziness and flushing are much less of a problem with the sustained-release formulations and with the dihydropyridines having a long elimination half-life and providing more constant plasma drug concentrations [1]. The efficacy and safely of nifedipine have been reviewed. Immediate-release nifedipine effectively and safely reduces the blood pressure in hypertensive patients [2], sustained- and extended-release nifedipine effectively and safely treats patients with mild or moderate hypertension [3], sublingual nifedipine is a safe, effective, and practical agent for treatment of patients with hypertensive emergencies [4], nifedipine, administered at the dose of 30 mg at bedtime, effectively and safely reduces the blood pressure in hypertensive patients [5], ramipril, administered at the daily dose of 10 mg, is effective as nifedipine, administered at the daily dose of 30 mg, in lowering the blood pressure [6], long-term administration of nifedipine, administered at the daily dose of 80 mg, is well-tolerated and effectively and safely reduces the blood pressure in patients with essential hypertension [7], and 10 mg of nifedipine tablets are effective as 10 mg of nifedipine capsules in treatment of severe hypertension in pregnant women [8]. These results indicate that nifedipine, administered at different doses, lowers the blood pressure in hypertensive patients. Long-term administration of nifedipine, administered at the daily dose of 80 mg, is well-tolerated and lowers the blood pressure in patients with essential hypertension, and ramipril, administered at the daily dose of 10 mg, lowers the blood pressure as nifedipine administered at the daily dose of 30 mg. The treatment of patients with nifedipine has been reviewed. Nifedipine, administered at the daily dose of 30 mg, lowers the blood pressure in hypertensive patients [9], nifedipine, administered at the dose of 10, 20 or 30 mg thrice-daily, treats hypertensive patients but probably should be used in combination with a potassium sparing diuretic [10], some hypertensive patients become normotensive with nifedipine administered at the daily dose of 30 mg and other hypertensive patients become normotensive with nifedipine administered at the daily dose 60 mg and both doses of nifedipine are well-tolerated [11], nifedipine, administered at the daily dose of 10 to 20 mg, normalizes the blood pressure in most of hypertensive patients [12], nifedipine, administered at the single dose of 10 mg, lowers the blood pressure in hypertensive patients and causes a rise in cardiac output and pulse-rate [13], nifedipine, administered once-daily at the dose of 30, 60 or 90 mg, reduces the blood pressure in hypertensive patients more effectively (P-value < 0.02) than propranolol administered once-daily at the dose of 80, 160 or 240 mg [14], nifedipine, administered at the dose of 20 mg twice-daily, lowers the blood pressure in hypertensive patients as cyclopenthiazide administered at the daily dose of 0.25 mg [15], nifedipine, administered once-daily at the dose of 20 mg, lowers the blood pressure in most of hypertensive patients and clonidine, administered at the initial dose of 0.1 mg with hourly doses of 0.1 mg, lowers the blood pressure in most of hypertensive patients and both treatments are safe [16], and old patients receiving short-acting nifedipine are associated with increased risk of stroke [17]. These results indicate that nifedipine, administered at different doses, lowers the blood pressure in hypertensive patients. Nifedipine, administered at the dose of 10, 20 or 30 mg thrice-daily, is an effective treatment of hypertensive patients but probably should be combined with a potassium sparing diuretic, some hypertensive patients become normotensive with nifedipine administered at the daily dose of 30 mg and other hypertensive patients become normotensive with nifedipine administered at the daily dose of 60 mg, nifedipine, administered once-daily at the dose of 30, 60 or 90 mg, lowers the blood pressure more effectively than propranolol administered once-daily at the dose of 80, 160 or 240 mg, nifedipine, administered at the daily dose of 20 mg, lowers the blood pressure as cyclopenthiazide administered at the daily dose of 0.25 mg, nifedipine, administered once-daily at the dose of 20 mg, lowers the blood pressure in most of hypertensive patients as clonidine, administered at the initial dose of 0.1 mg with hourly doses of 0.1 mg, and old patients receiving short-acting nifedipine are associated with increased risk of stroke. The trials conducted with nifedipine and with other hypertensive drugs have been reviewed. A prospective, randomized, double-blind trial was conducted in old hypertensive patients who received either nifedipine at the daily dose of 30 mg or hydrochlorothiazide at the daily dose of 25 mg plus amiloride at the daily dose of 2.5 mg. The blood pressure decreases similarly with both treatments and both treatments are well-tolerated but there was more withdrawals (P-value = 0.02) because peripheral oedema in patients treated with nifedipine [18], a total of 21 trials, including 2,183 hypertensive pregnant women, tested the efficacy of oral nifedipine, administered at the daily dose of 50, 60 and 90 mg, versus that of intravenous hydralazine, administered at the daily dose of 15 and 25 mg, and versus that of intravenous labetalol, administered at the daily dose of 200 and 300 mg, in treatment of severe hypertension in pregnant women. Nifedipine is superior to hydralazine and labetalol in treatment of severe hypertension during pregnancy [19], a multicentre, parallel-group, open-label, randomized, controlled trial compared the efficacy of 10 mg of oral nifedipine, to that of 200 mg of oral labetalol and to that of 1,000 mg of oral methyldopa in treatment of severe hypertension during pregnancy. All treatments reduce the blood pressure in most women and nifedipine reduces the blood pressure more effectively than labetalol and methyldopa [20]. The metabolism of nifedipine has been reviewed. The first-pass metabolism of nifedipine has been studied in six healthy volunteers who received nifedipine orally at a single dose of 20 or 30 mg and about half of the dose of nifedipine is eliminated presystemically [21] and nifedipine is almost completely metabolized by CYP3A4 and this cytochrome is present in human liver, small bowel, and other extrahepatic tissues [22]. Walley et al. [23] studied the pharmacokinetics of nifedipine in eight healthy volunteers who received a single oral dose of 8.5 mg of nifedipine. Nifedipine is rapidly eliminated as the elimination half-life is 1.52+0.08 hours and the distribution volume of nifedipine is lower than the water volume. The interactions of nifedipine with drugs have been reviewed. Diltiazem was administered at the daily dose of 30 mg and nifedipine was co-administered at a dose of 10 mg 4 times-daily, both treatments lasted one week, and diltiazem increases the plasma concentration of nifedipine [24], cimetidine was administered once-daily at the dose of 800 mg, ranitidine was administered once-daily at the dose of 300 mg, and nifedipine was administered once-daily at the dose of 20 mg. Cimetidine increases the area under the concentration-time curve of nifedipine whereas ranitidine does not produce any significant changes in the pharmacokinetic parameters of nifedipine [25], rifampicin was administered at the daily dose of 600 mg and nifedipine was co-administered at the daily dose of 20 mg and both treatments lasted 7 days. Rifampicin reduces the plasma concentration of nifedipine thus reduces the antihypertensive effect of nifedipine [26], non-steroidal anti-inflammatory drugs were co-administered with nifedipine. The co-administration of nonsteroidal anti-inflammatory drugs with nifedipine induced the metabolism of nifedipine and decreased the plasma concentration of nifedipine thus reduces the antihypertensive effect of nifedipine [27], patients with Type II diabetes and with hypertension received metformin at the daily dose of 1,000 mg and nifedipine at the daily dose of 30 mg and nifedipine significantly increases the absorption of metformin [28], metformin was administered at the daily dose of 1,000 mg and nifedipine was co-administered at the daily dose of 20 mg and nifedipine reduces the concentration of glucose in human umbilical vein cells [29], and nifedipine was administered at the dose of 0.3 µg/kg/min as an infusion and enoximone was administered at the dose of 0.5 mg/kg as a bolus and enoximone reduces the plasma concentration of nifedipine [30]. These results indicate that nifedipine interacts with drugs and the interaction of nifedipine with drugs affects the plasma concentration of nifedipine.
Conclusion
Nifedipine is a multiple Ca2+ channel blocker approved for clinical use. The efficacy and safely of nifedipine, the treatment of patients with nifedipine, and the trials conducted with nifedipine and with other antihypertensive drugs have been reviewed. Nifedipine undergoes a first-pass metabolism in humans, about half of the dose of nifedipine is eliminated presystemically, and nifedipine is metabolized in humans by the cytochrome CYP3A4. The pharmacokinetics of nifedipine have been studied in healthy volunteers after oral administration of nifedipine and nifedipine is eliminated with a mean elimination half-life of 1.52 hours. The interactions of nifedipine with drugs have been reviewed and nifedipine interacts with drugs and this interaction affects the plasma concentration of nifedipine. The aim of this study is to review the clinical pharmacology of nifedipine.
Conflict of interests: The authors declare no conflicts of financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employments, gifts, and honoraria.
This article is a review and drugs have not been administered to men or animals.
Acknowledgments: The author thanks Dr. Patrizia Ciucci and Dr. Francesco Varricchio, of the Medical Library of the University of Pisa, for retrieving the scientific literature.
References
- Escenhagen T. Treatment of Ischemic Heart Disease. In Goodman@Gilman’s. The Pharmacological Basis of Therapeutics. Brunton LL, Knollmann BC editors. Mc Graw Hill. 14th Edition, 2023; pp. 604-624.
- Seto SL, Barra ME, Hamidi A, Sin JH, Devine LT. Efficacy and Safety of Immediate-Release Nifedipine in Critically Ill Patients. J Pharm Pract, 2023; 36(3): 614-619.
- Stason WB, Schmid CH, Niedzwiecki D, Whiting GW, Caubet JF, Luo D, et al. Safety of nifedipine in patients with hypertension: a meta-analysis. Hypertension, 1977; 30(1 Pt 1): 7-14.
- Ellrodt AG, Ault MJ, Riedinger MS, Murata GH. Efficacy and safety of sublingual nifedipine in hypertensive emergencies. Am J Med, 1985; 79(4A): 19-25.
- Hermida RC, Ayala DE, Mojón A, Fernández JR. Chronotherapy With Nifedipine GITS in Hypertensive Patients: Improved Efficacy and Safety with Bedtime Dosing. Am J Hypert, 2008; 21(8): 948-954.
- Zhang W, Chang-Yuan L, Grzegorz B, Soranna, D, Zambon A, Kyriakoulis, K, et al. Efficacy and safety of nifedipine gastrointestinal therapeutic system versus ramipril in Chinese and European patients with hypertension. J Hypert, 2022; 40(Suppl 1): e228-234.
- Shimamoto K, Kimoto M, Matsuda Y, Asano K, Kajikawa M. Long-term safety and efficacy of high-dose controlled-release nifedipine (80 mg per day) in Japanese patients with essential hypertension. Hypert Res, 2015; 38(4): 695-700.
- Brown MA, Buddle ML, Farrell T, Davis GK. Efficacy and safety of nifedipine tablets for the acute treatment of severe hypertension in pregnancy. Am J Obstet Gynecol, 2002; 187(4): 1046-1050.
- William HF, Charlap S. Nifedipine in the Treatment of Systemic Hypertension. Arch Intern Med, 1984; 144(12): 2335-2336.
- Murphy MB, Scriven AJ, Dollery CT. Role of nifedipine in treatment of hypertension. Br Med J (Clin Res Ed), 1983; 287(6387): 257-259.
- Dean S, Kendall MJ. Nifedipine in the treatment of difficult hypertensives. Eur J Clin Pharmacol, 1983; 24(1): 1-5.
- Houston MC. Treatment of hypertensive urgencies and emergencies with nifedipine. Am Heart J, 1986; 111(5): 963-969.
- Olivari MT, Bartorelli C, Polese A, Fiorentini C, Moruzzi P, Guazzi MD. Treatment of hypertension with nifedipine, a calcium antagonistic agent. Circulation, 1979; 59(5): 1056-1062.
- Frishman WH, Garofalo JL, Rothschild A, Rothschild M, Greenberg SM, Greenberg J. The nifedipine gastrointestinal therapeutic system in the treatment of hypertension. Am J Cardiol, 1989; 64(11): F65-69.
- Sullivan FM, Murray TS, Gaw N, Langan JJ, Adams-Strump BJ. Is nifedipine a suitable first-line treatment for essential hypertension in general practice. Eur Heart J, 1987; 8(Suppl 1): 21-25.
- Jaker M, Atkin S, Soto M, Schmid G, Brosch F. Oral nifedipine vs oral clonidine in the treatment of urgent hypertension. Arch Intern Med, 1989; 149(2): 260-265.
- Jung S-Y, Choi N-K, Kim J-Y, Chang Y, Song HJ, Lee J, et al. Short-acting nifedipine and risk of stroke in elderly hypertensive patients. Neurology, 2011; 77(13): 1229-1234.
- Brown MJ, Palmer CR, Castaigne A, de Leeuw PW, Mancia G, Rosenthal T, et al. Principal results from the International Nifedipine GITS Study: Intervention as a Goal in Hypertension Treatment (INSIGHT). Eur Heart J, 2001; (Suppl B): B20-26.
- Wu H-Z, Cheng Y, Yu D, Li J-B, JiangY-F, Zhu Z-N. Different dosage regimens of nifedipine, labetalol, and hydralazine for the treatment of severe hypertension during pregnancy: a network meta-analysis of randomized controlled trials. Hypertens Pregnancy, 2022; 41(2): 126-138.
- Easterling T, Mundle S, Bracken H, Parvekar S, Mool S, Magee LA, et al. Oral antihypertensive regimens (nifedipine retard, labetalol, and methyldopa) for management of severe hypertension in pregnancy: an open-label, randomised controlled trial. Lancet, 2019; 394(10203): 1011-1021.
- Waller DG, Renwick AG, Gruchy BS, George CF. The first pass metabolism of nifedipine in man. Br J Clin Pharmacol, 1984; 18(6): 951-954.
- Holtbecker N, Fromm MF, Kroemer HK, Ohnhaus EE, Heidemann H. The nifedipine-rifampin interaction. Evidence for induction of gut wall metabolism. Drug Metab Dispos, 1996; 24(10): 1121-1123.
- Walley TJ, Heagerty AM, Woods KL, Bing RF, Pohl JE, Barnett FB. Pharmacokinetics and pharmacodynamics of nifedipine infusion in normal volunteers. Br J Clin Pharmacol, 1987; 23(6): 693-701.
- Toyosaki N, Toyo-oka T, Natsume T, Katsuki T, Tateishi T, Yaginuma T, et al. Combination therapy with diltiazem and nifedipine in patients with effort angina pectoris. Circulation, 1988; 77(6): 1370-1375.
- Smith SR, Kendall MJ, Lobo J, Beerahee A, Jack DB, Wilkins MR. Ranitidine and cimetidine; drug interactions with single dose and steady-state nifedipine administration. Br. J. Clin. Pharmac, 1987; 23(5): 311-315.
- Wanjie N, Size L, Shasha J, Xiying L, Mengwan Z, Weimin C, et al. Investigating the interaction between nifedipine- and ritonavir-containing antiviral regimens: A physiologically based pharmacokinetic/pharmacodynamic analysis. Br J Clin Pharmacol, 2021; 87(7): 2790-2806.
- Salvetti A, Magagna A, Abdel-Haq B, Lenzi M, Giovannetti R. Nifedipine interactions in hypertensive patients. Cardiovasc Drugs Ther, 1990; (Suppl 5): 963-968.
- Bello SS, Sani FB, Odunola MT, Garba M, Garba MA. Interaction of Metformin and Nifedipine in Type II Diabetic Patients with Hypertension. J Chem Pharmacol Res, 2017; 9(4): 103-107.
- Wang L-P, Jiang Y, Yang H, Peng C, Zhang C, Tao X, et al. Combination Therapy of Nifedipine and Sulphonylureas Exhibits a Mutual Antagonistic Effect on the Endothelial Cell Dysfunction Induced by Hyperglycemia Linked to Vascular Disease. Cell Physiol Biochem, 2016; 38(9): 2337-2347.
- Boldt J, Kling D, Dietevich HA, Hempelmann G. Drug interactions: the new phosphodiesterase inhibitor enoximone and the calcium channel blocker nifedipine in coronary surgery patients--influence on hemodynamics and plasma concentrations. J Cardiovasc Pharmacol, 1990; 15(1): 37-43.

