Hydralazine vs Labetalol for the Treatment of Severe Hypertension During Pregnancy: An RCT
Muhammad Zafar Siddiq1, Aqna Malik1,2,*, Ashfaq ul Hassan2, Sadaf Fayyaz1, Arooj Fatima1, Syed Noman Aslam1, Nayyab Ghazal1 and Dr. Ahmad Hassan3
1Department of Pharmacy, University of Lahore, Lahore
2Department of Pharmacy, University of Chenab, Gujrat
3Avicenna Tajik State Medical University, Tajikstan
Received Date: 14/11/2024; Published Date: 27/11/2024
*Corresponding author: Dr. Aqna Malik, Department of Pharmacy, University of Chenab, Gujrat;
Muhammad Zafar, Department of Pharmacy, University of Lahore, Lahore
Abstract
Background: Severe increase in BP during pregnancy may lead to Pre-eclampsia accompanied by many maternal side effects. In cases of severe hypertension during pregnancy, there is broad consensus regarding which drug hydralazine or labetalol is better at treating hypertension. Every drug has pros and cons of its own.
Method: To treat severe HTN in pregnant women, A prospective, randomized, and controlled trial has been established under the supervision of Dr Shazia. This study evaluated the safety and efficacy of oral and IV labetalol and hydralazine over a range of HTN values. An envelope-based method was used in which 86 pregnant women with mild, chronic, or severe HTN were divided into 2 groups of 43 subjects each. Group A received labetalol and Group B was administered hydralazine, Upon admission, mean arterial blood pressure, diastolic blood pressure, and systolic blood pressure were noted. 3-way ANOVA was used to access interaction BP range and mode of treatment among patients.
Results: Labetalol was able to achieve the desired BP in Normal (≥120 & 80) and Elevated (120-129≥80) ranges in 13&20 patients respectively which is much better than hydralazine with only 2 patients in normal and 29 in elevated HTN range. Labetalol may help to reduce severely High BP more quickly than Hydralazine. The incidence of adverse effects in mothers or fetus at lower risk than hydralazine
Conclusion: Labetalol indicated better antihypertensive action with fewer maternal and fetal side effects than the hydralazine-treated group and proved to be the drug of choice in severe HTN.
Keywords: Severe HTN in pregnancy; Pre-eclampsia; Labetalol; Hydaralzine
Introduction
Hypertensive disorders are a primary contributor to peripartum morbidity and mortality during pregnancy. Pregnant women of low socioeconomic status can be 10-30 times more hypertensive than normal women. Such Unfavorable consequences are more prevalent in women exhibiting target organ damage, uncontrolled severe hypertension, and noncompliance with prenatal visits [1]. Pregnancy-related hypertension, especially when diastolic pressure in the first trimester of pregnancy is ≥110 mm Hg , is linked with a higher risk of both short- and long-term adverse outcomes for the mother like abruptio placentae or superimposed preeclampsia, [2] and the fetus (higher chance of perinatal morbidity and mortality) [3]. Failure of HTN treatment can cause placental abruption, eclampsia, HELLP syndrome, pulmonary edema, and even death [4,5]. For non-pregnant patients with a BP= ≥140/90 mm Hg require antihypertensive is the standard of care; however, treatment during pregnancy is debatable (Angras et al., 2022; Braunthal & Brateanu, 2019). HTN in men and non-pregnant women is frequently categorized as mild, moderate, severe, or very severe based on the SBP or diastolic BP [4,6]. While chronic HTN in pregnancy is classified as mild or severe diastolic BP = ≥ 110 mm Hg (Korotkoff phase V) [7,8]. Type of pre-eclampsia known as Superimposed preeclampsia includes exacerbation of HTN, edema, proteinuria, hyperuricemia, or a combination of these symptoms [9].
Antihypertensive treatment of pregnant women during mild chronic <160/110 mm Hg, and chronic HTN to target <140/90 mm Hg would reduce the chance of unfavorable pregnancy outcomes rather than a control strategy of no antihypertensive treatment unless the SBP = 160 mm Hg or higher or the diastolic pressure was 105 mm Hg or higher. Women who received no anti-hypertensive treatment suffered a risk of being underweight for gestational age, medically indicated preterm birth at less than 35 weeks gestation, placental abruption, or fetal or neonatal death. The significance of antihypertensive therapy in women may be further clarified by research on the long-term effects on women and their fetus like on cardiovascular and other body organs. Hydralazine [10] has long been the primary prescribed antihypertensive for severe HTN in pregnancy with associated deteriorating pre-eclampsia symptoms like headache, nausea, and vomiting [11].
Parenteral hydralazine proved more effective inducing maternal hypotension [1]. However, Labetalol demonstrates a comparative efficacy due to 7times more selectivity for β-blockade than α blockade [3]. However, women with mild chronic or chronic HTN can better select the antihypertensive medication for them [12] however, during the first trimester, only 1.9% of women received antihypertensive therapy [13]. Labetalol is rarely used by women to treat hypertension outside of pregnancy, it is significant for those who are actively trying to conceive. because embryogenesis begins during the first trimester of pregnancy, which can last up to 13 weeks of gestation, it is imperative to assess every possible risk [14,15] resulting from the theoretical implications of drug-induced teratogenicity during this critical time. There exists a critical demand for randomized RCT. Because findings of RCT will facilitate the identification of a pharmaceutical intervention anticipated to yield superior outcomes in safeguarding women from potentially life-threatening complications [1,16].
The current study aims to compare the effectiveness of two anti-hypertensive medications, labetalol and hydralazine in the treatment of severe hypertension during pregnancy, by readily achieving desired BP [17,18].
Methodology
A comparative observational study was designed in district Gujrat from October 2023 to January 2024. Convenient and simple sampling technique was used to collect data via self-constructed questionnaire according to the American Journal of Questionnaire for Collection of Data During Pregnancy. A Randomized clinical trial was conducted under the guidance of Dr. Shazia Ijaz. among pregnant women who were visiting her. Before beginning the therapy procedure, patients gave their written consent. The inclusion criteria for RCT were pregnant women with severe HTN. Assessment of hypertensive pregnant women was done for elevated BP (severe hypertension)> 110 mmHg. The formula was applied to calculate the sample size of 86 pregnant women and divided them into 2 groups each with 43 patients. Women in their 2nd and 3rd trimesters who received oral and intravenous labetalol and hydralazine medication were compared to two groups, each consisting of 43 members. They were administered antihypertensive medications, namely ‘labetalol’ and ‘hydralazine’. Group A received labetalol, while group B was administered hydralazine. All participants provided written consent. Most patients enrolled during the 4th week, while a subset joined in the 8th week of pregnancy. Group A was administered Labetalol. Group B was administered hydralazine. Three-way ANOVA with Scheffe correction and the student t test were used to compare the utilization of different drugs based on patient characteristics and to evaluate the collected data to comprehend the intricate relationships between the variables that were affecting the outcomes. Tables, charts, and graphs were used to illustrate the findings. Simple descriptive statistics were used. Statistical significance was defined as P values <0.0001.
Table 1: Demographic Profile of Participants in RCT.

Table 2: Health Profile of Participants in RCT.
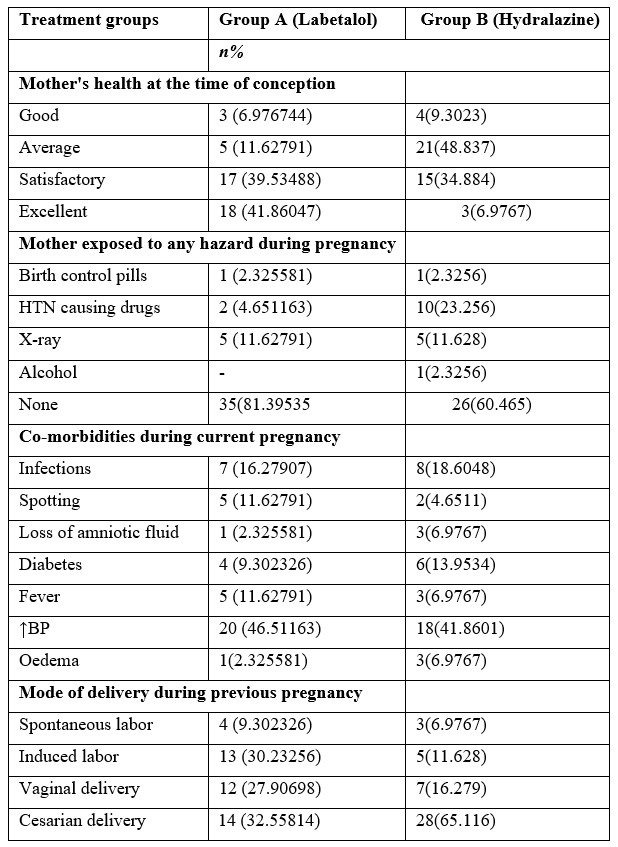
Highest number of pregnant women was observed in 19-29 years than any other age group in both treatment groups. The financial circumstances of most couples throughout the current pregnancy were satisfactory, especially in group B. The average income of participant patients was 50k-1lakh in both groups. Most patients were happily living together in both treatment groups (Table 1). Pregnant women in the Labetalol treatment group had relatively better health at the time of conception than hydralazine. Exposure to hazards in both groups was minimal except intake of drugs causing HTN in group B. As far as comorbid diseases or pathological conditions are concerned (46.5%) pregnant women in group A and 18(41.8%) in group B were observed suffering HTN. Mode of birth in prior pregnancies was mostly C-section with a greater number of abortions in hydralazine group 28(65.2%) as compared to Labetalol 14(32.5%).
The medical history of the mother and her immediate family plays a major role in the health state of the woman. Patients in Group A had a family history of heart failure and other diseases. While in Group B had a family history of heart failure and other diseases. Figure 1 represents frequency of increase in BP during Pregnancy among patients.
Table 3: Medical History among Pregnant Women in both groups.
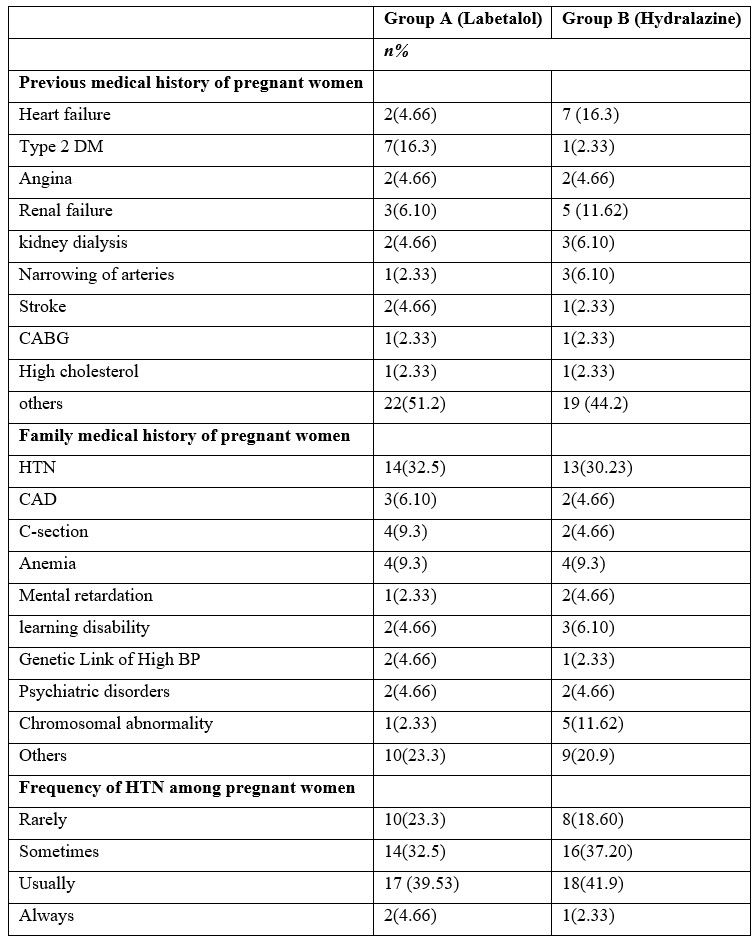
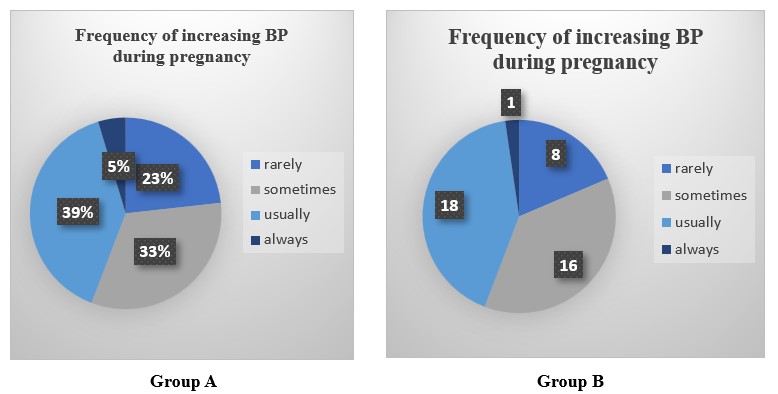
Figure 1: Pathological Condition of Pregnant Women Before Treatment.
In Group A 25 participants exhibited baseline HTN levels with elevated BP during the 1st trimester, while a few were classified under stage I (6) & stage II (7). In addition, 5 out of 43 experienced a hypertensive crisis. In Group B, 19 women had elevated BP, 7 were in stage I & 6 were in stage II while 10 experienced a hypertensive crisis (Table 4).
Mode of Treatment received by pregnant women during RCT
Labetalol Group
This group received a 20 mg intravenous slow bolus dosage of labetalol with an escalation of 20mg every 20 minutes after every 20 minutes. Oral labetalol was administered during the 2nd trimester, which normalized BP in 2 patients. However, pregnant women in stages I&II and hypertensive crisis remained approximately the same till the administration of IV Labetalol, led to immense improvement in BP. In Group A. No patient experienced a hypertensive crisis following IV Labetalol infusion, and the number of pregnant women with normal BP increased.
Hydralazine Group
A gradual IV bolus of 5 mg of hydralazine was administered every 20 minutes in RCT until the desired outcome was achieved, with a maximum of 3 doses possible. BP of all patients monitored continuously during the procedure, every 15 minutes, while also taking their overall health into account. Administration of oral hydralazine during the 2nd trimester only normalizes pregnant women with elevated BP. However, patients in stages I&II and hypertensive crisis remained approximately the same. IV Hydralazine was administered which led to significant improvement. 2 patients experienced a hypertensive crisis following IV infusion, till the last trimester.
Impact on Overall BP range during pregnancy
Overall Variation of BP among Group A patients was 77% in 120-129 systolic and > 80 diastolic and 16% in>120 systolic and 80 diastolic while 86% of Group B were in 120-129 systolic and > 80 diastolic and 7% in >120 systolic and 80 diastolic.
Statistical Analysis
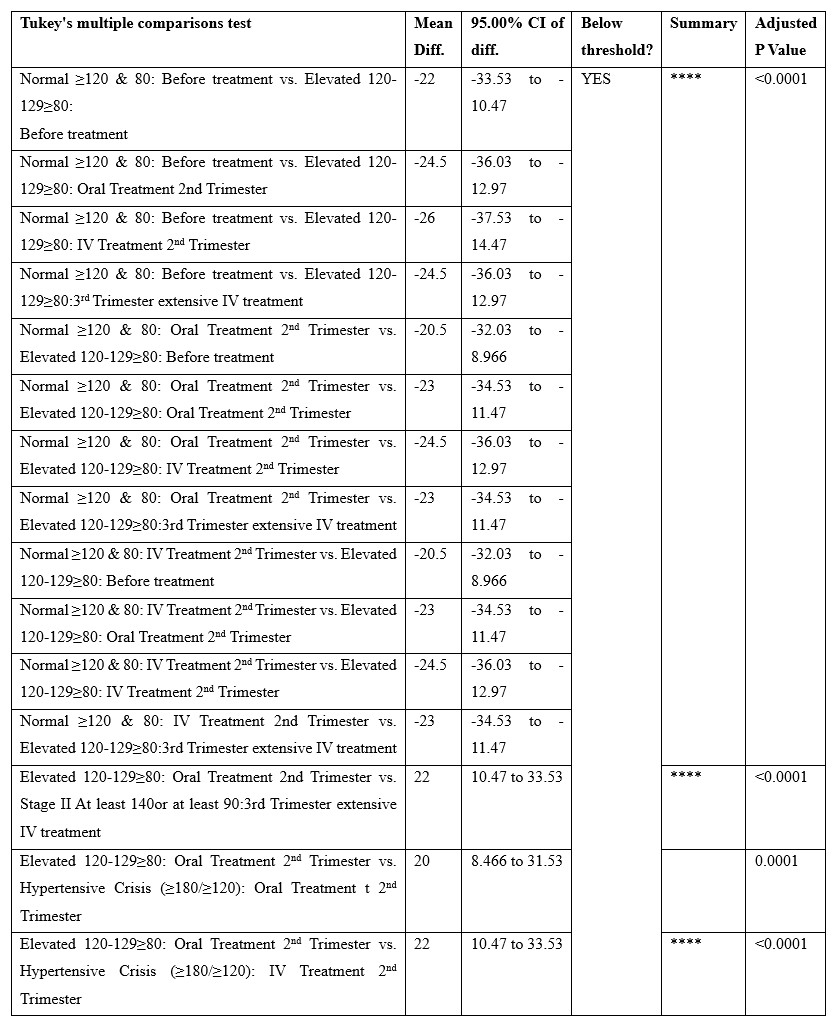
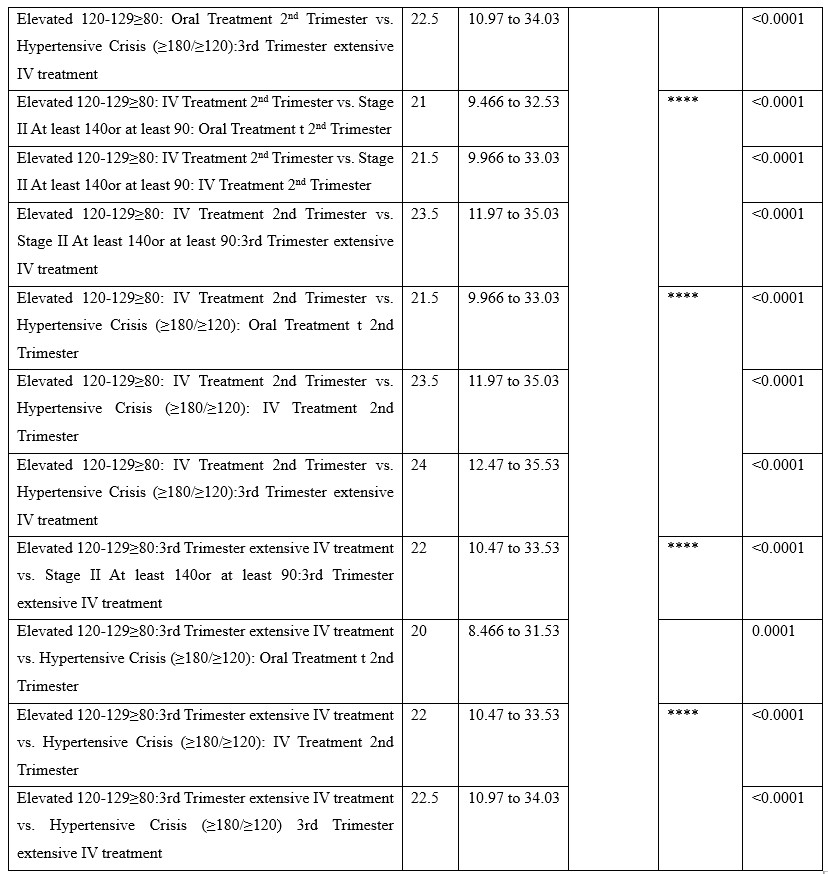
Tukey's multiple comparisons test was applied under 3way ANOVA (multiple comparisons) to determine the effects of 3 independent categorical variables (or factors) on a continuous dependent variable. HTN levels in different stages were compared with before and after treatment in 3 trimesters. A comparison was made for both oral and IV treatment. results showed a significant <0.0001 between variables.
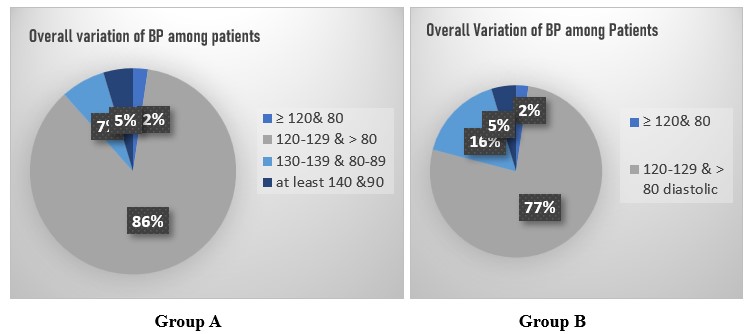
Table 4: Baseline values of HTN patients before treatment women in both groups (1st Trimester) in both Treatment groups.
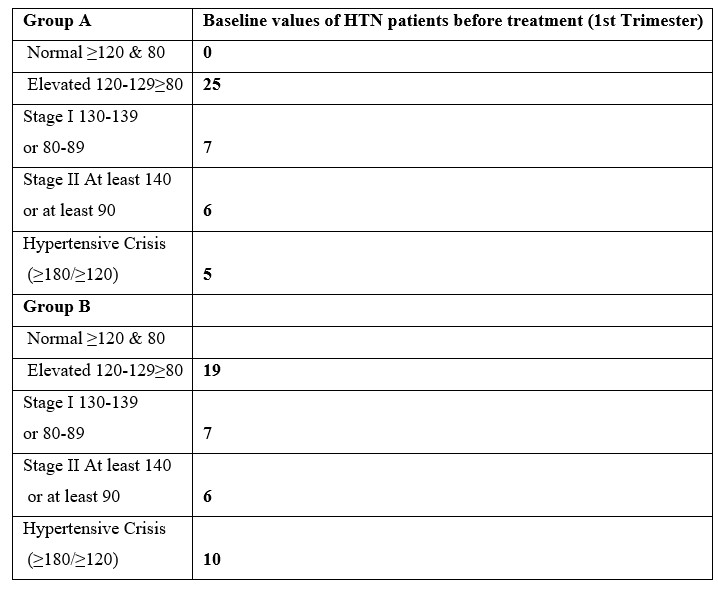
Table 5: Impact of treatment on HTN levels of pregnant women.
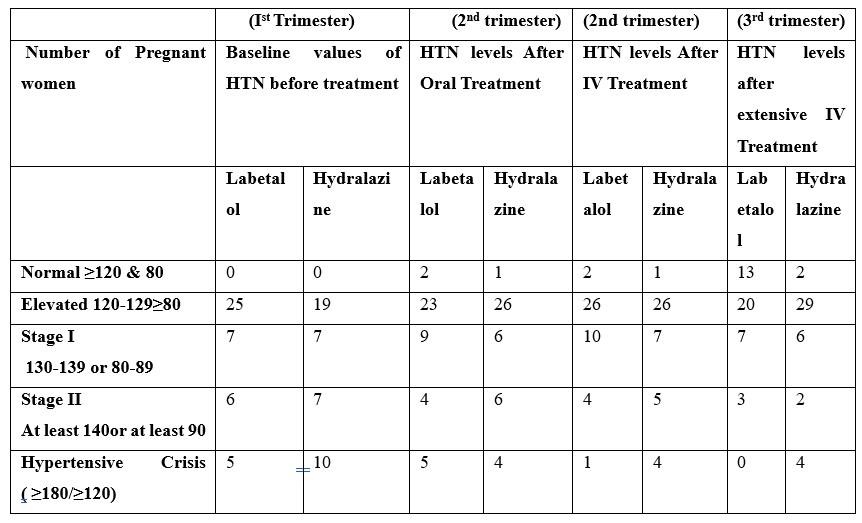
Table 6: Tukey's multiple comparisons test.
Discussion
Both Labetalol and Hydralazine can be recommended as antihypertensives for the treatment of severe HTN during pregnancy. Although hydralazine has been used safely in pregnant women for many years, there is a greater chance of adverse consequences. Incidence of maternal issues were observed during hydralazine treatment though statistically insignificant.
Labetalol is a commonly used α-&β-adrenergic blocker because of its rapid and sustained effects and low risk of side effects in both mother and infant. (27%) pregnant women experienced nausea & vomiting, headaches mild chest pain with Labetalol Which was less with hydralazine (25%). The study identified a significant association between HTN during pregnancy and a family history of increased BP. Individuals with a familial predisposition to HTN exhibited elevated blood pressure levels during pregnancy, regardless of their group allocation. This indicates a genetic predisposition to hypertension, highlighting the importance of considering family medical history in prenatal care. Elevated blood pressure poses significant risks to maternal and fetal health, potentially resulting in pre-eclampsia, eclampsia, or intrauterine growth restriction.
Conclusion
Current study concluded that labetalol improves BP regulation. Pregnancy, particularly critical circumstances, demands meticulous evaluation and prudent decision-making in the administration of medications. The safety profile of a medication must be evaluated in conjunction with an appropriate dosage. The selection of medication should be guided by the practitioner's experience and judgment until conclusive data on the superiority of labetalol or hydralazine are available.
Limitations of the study: After 2 blood pressure readings, the study proforma's blood pressure records were abandoned. Despite the American College of Obstetricians and Gynecologists' recommendation of a 10 mg parenteral Hydralazine dose, a 5 mg dose was chosen because the women in the study population were not overweight.
Recommendations: In cases of severe HTN diagnosed during pregnancy before 20 weeks of gestation, labetalol should be recommended for treatment, targeting a systolic BP =160 mm Hg or a diastolic BP= 110 mm Hg, or a systolic range of 120 to 159 mm Hg and a diastolic range of 80 to 109 mm Hg. The decision to treat chronic hypertension at lower BP levels should be guided by discussions with the patient and the presence of comorbid conditions that may necessitate reduced BP. Induction of delivery in pregnant women with chronic hypertension is not recommended before 37 weeks of gestation, except in the presence of additional indications.
References
- Khedagi AM, Bello NA. Hypertensive Disorders of Pregnancy. Cardiol Clin, 2021; 39(1): 77-90. https://doi.org/10.1016/j.ccl.2020.09.005
- Firoz T, Magee L, MacDonell K, Payne B, Gordon R, Vidler M, et al. Oral antihypertensive therapy for severe hypertension in pregnancy and postpartum: a systematic review. BJOG: An International Journal of Obstetrics & Gynaecology, 2014; 121(10): 1210-1218.
- Miller JJ, Higgins V, Ren A, Logan S, Yip PM, Fu L. Chapter Three - Advances in preeclampsia testing. In G. S. Makowski (Ed.), Advances in Clinical Chemistry. Elsevier, 2023; 117: pp. 103-161 https://doi.org/https://doi.org/10.1016/bs.acc.2023.08.004
- Angras K, Sullivan M, Young AJ, Paglia MJ, Mackeen AD. A retrospective review of pregnancy outcomes in women with uncomplicated mild to moderate chronic hypertension. The Journal of Maternal-Fetal & Neonatal Medicine, 2022; 35(25): 9071-9077.
- Saxena N, Bava AK, Nandanwar Y. Maternal and perinatal outcome in severe preeclampsia and eclampsia. International Journal of Reproduction, Contraception, Obstetrics and Gynecology, 2016; 5(7): 2171-2177.
- Braunthal S, Brateanu A. Hypertension in pregnancy: Pathophysiology and treatment. SAGE open medicine, 2019; 7: 2050312119843700.
- Scott G, Gillon TE, Pels A, von Dadelszen P, Magee LA. Guidelines—similarities and dissimilarities: a systematic review of international clinical practice guidelines for pregnancy hypertension. American journal of obstetrics and gynecology, 2022; 226(2): S1222-S1236.
- Tykarski A, Filipiak KJ, Januszewicz A, Litwin M, Narkiewic K, Prejbisz A, et al. 2019 Guidelines for the management of hypertension—part 1–7. Arterial Hypertension, 2019; 23(2): 41-87.
- Koulouraki S, Paschos V, Pervanidou P, Christopoulos P, Gerede A, Eleftheriades M. Short- and Long-Term Outcomes of Preeclampsia in Offspring: Review of the Literature. Children (Basel), 2023; 10(5). https://doi.org/10.3390/children10050826
- Magee LA, Cham C, Waterman EJ, Ohlsson A, von Dadelszen P. Hydralazine for treatment of severe hypertension in pregnancy: meta-analysis. Bmj, 2023; 327(7421): 955-960. https://doi.org/10.1136/bmj.327.7421.955
- Program NHBPE. Report of the national high blood pressure education program working group on high blood pressure in pregnancy. American journal of obstetrics and gynecology, 2000; 183(1) s1-s22.
- Garovic VD, Dechend R, Easterling T, Karumanchi SA, McMurtry Baird S, Magee LA, et al. Hypertension in pregnancy: diagnosis, blood pressure goals, and pharmacotherapy: a scientific statement from the American Heart Association. Hypertension, 2022; 79(2): e21-e41.
- Bateman BT, Hernandez-Diaz S, Huybrechts KF, Palmsten K, Mogun H, Ecker JL, et al. Patterns of outpatient antihypertensive medication use during pregnancy in a Medicaid population. Hypertension, 2012; 60(4): 913-920. https://doi.org/10.1161/hypertensionaha.112.197095
- Martinez A, Lakkimsetti M, Maharjan S, Aslam MA, Basnyat A, Kafley S, et al. Beta-Blockers and Their Current Role in Maternal and Neonatal Health: A Narrative Review of the Literature. Cureus, 2023; 15(8): e44043. https://doi.org/10.7759/cureus.44043
- Moturu S, Adkins EL, Delgado-Lebron JM. Empowering Women’s Health after Spinal Cord Injuries: A Timely and Practical Update. Physical Medicine and Rehabilitation Clinics, 2024.
- Sharma C, Soni A, Gupta A, Verma A, Verma S. Hydralazine vs nifedipine for acute hypertensive emergency in pregnancy: a randomized controlled trial. American journal of obstetrics and gynecology, 2017; 217(6): e681-687.
- Chahine KM, Sibai BM. Chronic hypertension in pregnancy: new concepts for classification and management. American journal of perinatology, 2019; 36(02): 161-168.
- Hurrell A, Webster L, Chappell LC, Shennan AH. The assessment of blood pressure in pregnant women: pitfalls and novel approaches. American journal of obstetrics and gynecology, 2022; 226(2): S804-S818.

