Linkage Imbalance of C108T and L55M Haplotypes is a High-Risk Predictor of Coronary Disease in Young Patients from Northeast Brazil
Rochelle Pinheiro Ribeiro1,2,3, Felipe Pantoja Mesquita1, Luina Benevides Lima1, Lais Lacerda Brasil de Oliveira1, Carlos Eduardo Lopes Soares1, Emerson Lucena da Silva1, Pedro Filho Noronha Souza1,*, Maria Elisabete Amaral de Moraes1, Manoel Odorico de Moraes Filho1 and Raquel Carvalho Montenegro1,4,*
1Pharmacogenetics Laboratory, Drug Research and Development Center (NPDM), Federal University of Ceará, Brazil
2Cardiology Service at University Hospital Walter Cantídeo, Federal University of Ceará, Fortaleza, Brazil
3Cardiology Service at Messejana Hospital Dr. Carlos Alberto Studart Gomes, Fortaleza, Brazil
4Red Latinoamericana de Implementación y Validación de guias clinicas Farmacogenomicas (RELIVAF), Brazil
Received Date: 11/10/2024; Published Date: 13/11/2024
*Corresponding author: Pharmacogenetics Laboratory, Drug Research and Development Center (NPDM), Federal University of Ceará, Fortaleza, CE, 60430-275, Brazil; Red Latinoamericana de Implementación y Validación de guias clinicas Farmacogenomicas (RELIVAF), Brazil
Abstract
Atherosclerotic and Coronary Artery Disease (CAD) is the leading cause of death worldwide. In the last decade, has been growing in groups of younger age. Many of these individuals do not have the risk factors classically known from the Framingham Study for the development of the disease. As for it, there are a few known environmental factors that may act on an individual with a genetic predisposition to an early onset of the disease. Paraoxonase 1 (PON1) is believed to be involved in preventing atherosclerosis through lipid-modifying features, antioxidant activity, anti-inflammatory, and many other ways. This enzyme is associated with HDL and has been shown to prevent LDL oxidation. In order to evaluate the association of PON1 polymorphism C108T and L55M, a cross- sectional, case-control study, was carried out from January 2016 to January 2019 with individuals (Men<45 years old; women <55 years old) with premature CAD. A hundred and thirteen individuals were evaluated where 81 were diagnosed with CAD and 32 were healthy. Total cholesterol, HDL, and LDL-cholesterol values were significantly lower in the CAD group (p < 0.001), whereas the blood glucose and glycated hemoglobin values were significantly higher (p < 0.05). Regarding the clinical features, high levels of AC and BMI were significantly observed. In the genotypic evaluation, those with the T allele of the C108T had a significant association with dyslipidemia (p <0.042) and those with the M allele of the L55M polymorphism had significantly higher BMI values (p <0.011). The linkage imbalance of C108T and L55M haplotypes was significantly associated with the risk of coronary disease (p <0.013). The MM genotype was significantly associated with the involvement of the coronary territory of the circumflex artery (Cx) (p <0.038). The TT genotype was associated with higher levels of TSH in CAD cases (p <0.021). The presence of PON1 polymorphisms C108T and L55M, alone, does not produce a significant increase in the occurrence of atherosclerotic coronary disease in young subjects however the rise of total cholesterol levels and low HDL levels produce a risk greater than 3 (p <0.033) and 9 times (p <0.021), respectively, in the chance of premature CAD, with the prevalence that these risk factors are under possible genetic modulation.
Keywords: Atherosclerosis and Coronary disease; Young subjects; Human paraoxonase 1; C108T; L55M
Introduction
Coronary Artery Disease (CAD) also known as coronary heart disease or ischemic heart disease, comprises a spectrum of symptomatic and asymptomatic clinical conditions typically related to plaque buildup in the wall of the coronary arteries leading to inadequate blood and oxygen supply to the heart [1]. CAD is a chronic disease of variable condition, which progresses from a long asymptomatic phase to Stable Angina (SA), Myocardial Infarction (MI), and/or unstable angina (IA) [2]. CAD is a common cause of heart failure, with reduced or preserved left ventricular ejection, ventricular arrhythmias, and sudden cardiac arrest. CAD has been also the leading cause of death and a major contributor to disability in the world in the last decade. In 2024, it was estimated that the incidence and mortality of CAD will continue to be high. Among several modifiable risk factors, high systolic blood pressure, alone, has been responsible for more than 10 million premature deaths globally in 2021 [3]. According to the World Health Organization (WHO), CAD was responsible for over 17 million deaths, which is 31% of all global deaths. It is also projected that by 2023, the number of deaths due to CAD will increase to over 23 million. Furthermore, it is estimated that by 2023, CAD will be the leading cause of death in low- and middle-income countries [4,5].
Asymptomatic individuals with atherosclerosis and thus at risk of acute cardiovascular incidents, including premature Myocardial Infarction (MI) and death, have been increasing by 2% every year among young adults [6,7]. Together with worsening risk factor control in elderly adults, young adults have been contributing massively to the cardiovascular burden worldwide. Moreover, even though the number of young adults (<40 years) with high cholesterol decreased from 40.5% to 36.1% in the past years, it was observed an increase in diabetes and obesity [6]. Moreover, the classic risk factors for CAD seem to be lower in the younger population and thus, do not completely explain the rising burden of CAD in these populations [8,9].
In general, one-third of patients with CAD have a family history of CAD, resulting in 1.5 times more likely to suffer from CAD than those without a family history. Moreover, 40%–60% of predisposition for CAD are inherited, usually a result of both genetic predisposition and traditional risk factors [10,11]. The risk from the family history of CAD depends on the number and age of first-degree relatives affected, with a 60 to 75% risk increase in the offspring of parents with premature cardiovascular disorder [12]. Therefore, genetic interference is well accepted as an important risk factor for these young adults and may be a better estimate factor of the true attributable risk to CAD development.
Among all genetic factors, paraoxonase-1 (PON1) is one of the most studied risk factors playing a crucial role as an anti-atherosclerotic factor [13,14]. PON1 is a calcium-dependent hydrolytic enzyme that plays a crucial role in protecting the body against oxidative stress and inflammation and is present in high concentrations in the blood primarily after its production by the liver [15,16]. Mackness et al. described the role of high-density lipoprotein (HDL)- associated PON1 in decreasing lipid peroxide accumulation on low-density lipoprotein (LDL) [17–19]. Recent studies have shown that genetic variability in the PON1 gene is associated with an increased risk of coronary artery disease (CAD) [20–25]. Additionally, there is an association of PON1 gene polymorphism with lipid profile, such as high-density lipoprotein cholesterol (HDL-C), which is known as "good cholesterol" and has a protective role against CAD [26,27]. Among all polymorphisms studied in the PON1 coding region, L55M and Q192R are the most studied and relevant, where both show independent associations with the PON1 enzyme activity and lead to a wide inter-/intra-individual variability [15,28,29]. Specifically, variants of the PON1 gene, such as the Q192R and L55M variants, are associated with a higher risk of CAD in older subjects [26,30–32]. The Q192R missense variant (c.575A>G) has been associated with a decrease in PON1 activity, which leads to an increase in oxidative stress and inflammation in the body. On the other hand, the L55M variant is associated with an increase in PON1 activity, which leads to a decrease in oxidative stress and inflammation [33–35]. Moreover, PON1 - L55M polymorphism was not associated with CAD risk in the Chinese population [28] showing variability within the population for this polymorphism. Despite all these observations, further studies with a strict selection of patients in different ethnic populations are required, especially in young subjects. Therefore, this present study aims to evaluate Human Paraoxonase 1 (PON1) genetic polymorphisms and metabolic abnormalities in young individuals with atherosclerotic coronary artery disease.
Methodology
Study design
This is a cross-sectional and case-control study that evaluates the clinical and metabolic profile considering the presence of genetic polymorphisms of PON-1 C108T and L55M in 113 young individuals (men under 45 years and women under 55 years) diagnosed with atherosclerotic coronary artery disease. The individuals enrolled in this research were evaluated from January 2016 to January 2019 at the Heart Hospital Dr. Carlos Alberto Studart Gomes, located in Fortaleza City, Ceará (Brazil). The control group is represented by 32 individuals, men under the age of 45 years old, and women under the age of 55 years old, matched by sex and age, with no family history of CAD.
These criteria were considered as exclusion factors: individuals under 18 years of age, with thyroid diseases, collagen diseases, advanced congestive heart failure (functional class III and IV), pregnancy, neoplasms, kidney diseases, calcium disorders, uncontrolled hypertension, allergy to iodinated contrast users of illicit drugs, patients with suspected familial hypercholesterolemia l, individuals with coronary anomalies or patients with non- atherosclerotic coronary disease on coronary angiography.
Clinical evaluation
The following parameters were evaluated in the case and control group: family history of early CAD, smoking, weight, height, body mass index (BMI), measurement of abdominal and hip circumference, blood pressure, and angiographic evaluation. A first-degree relative of a male with coronary artery disease under 55 years of age or female under 65 years of age was considered a family history of early CAD. We considered smokers individuals who have smoked one or more cigarettes in the last 180 days, and ex-smokers for individuals who have smoked for more than 180 days. Weight and height were measured on an anthropometric scale while BMI was calculated using the formula: weight divided by height squared. The abdominal circumference was measured at the midpoint of the distance between the anterior superior iliac crest and the lower edge of the costal arch (referred to as the waist) and the hip circumference at the anterosuperior iliac crest (referred to as hip) was measured using an inelastic tape measured with the individual in an upright position with the relaxed abdomen. Systolic blood pressure and diastolic blood pressure were measured with an aneroid sphygmomanometer, after 15 minutes of rest in the sitting position. Also, the following diseases were considered during the analysis of the CAD case group.
- Systemic Arterial Hypertension: patients using antihypertensive drugs and those with SBP above 140 mmHg and/or DBP above 90 mmHg were considered hypertensive.
- Diabetes mellitus: those who previously used oral antidiabetic drugs and/or insulin, or with two fasting glucose levels ≥ 126 mg/dL, were considered diabetic
- c) Dyslipidemia: The diagnosis of dyslipidemia was established according to the criteria of the V Brazilian Dyslipidemia Directive.
Angiographic evaluation
The cineangiocoronariography classification was performed according to the percentage of coronary artery lumen obstruction: obstructive lesions were classified as non-critical if they present obstruction < 50% of the arterial lumen; critical, if they present obstruction of 50% or more of the arterial lumen; and occlusive, with total obstruction, no flow through the artery defined as TIMI (Thrombolysis in Myocardial Infarction) zero. Individuals with only one lesion > 50% were classified as single-vessel and those with more than one lesion > 50% as multi- vessel. Analysis was performed by two independent physicians.
Genotyping
DNA extraction
Peripheral blood samples were collected in EDTA tubes and then extracted using the PureLink™ Genomic DNA kit (Thermo Fisher Scientific) according to the manufacturer's instructions. After genomic material isolation, the samples were stored in a -20ºC freezer until the stage of polymorphism analysis by real-time qPCR.
Primers and probes
The polymorphisms of the PON1 gene analyzed in this study were C108T (dbSNP: rs705379) and L55M (dbSNP: rs854563). Moreover, the β-actin gene was used as an endogenous control. All primers and probes were obtained from Thermo Fisher Scientific (São Paulo, Brazil).
Allelic Discrimination by Real-time qPCR
Single Nucleotide polymorphisms (SNPs) detection of the PON1 gene mentioned above was performed by real-time qPCR using the QuantStudio® 5 device (Applied Biosystems) and the commercial TaqMan® Genotyping Master Mix kit (ThermoFisher Scientific).
Genotyping of SNPs by real-time qPCR is an extremely sensitive technique that allows allelic discrimination even when small amounts of DNA are available. This technique uses Taqman® probes which differ in the nucleotide to be searched with emission of distinct fluorescence, according to the cleavage of the probe from the amplified allele.
Different genotype identification is done by classifying the samples as homozygous for the normal allele, homozygous for the mutated allele, or heterozygotes. The reaction protocol uses 9.25 µL of deionized water, 12.5 µL Buffer, 1.25 µL of TaqMan® genotyping assay, and 2 µL of DNA. The amplification protocol consisted of the following: 50ºC/2 min, 95ºC/10 min, and 50 cycles of 95ºC/15 seconds and 60ºC/1 min. All requirements proposed in Minimum Information for Publication of Quantitative Real-Time PCR Experiments - MIQE Guidelines were followed [36].
Laboratory methods
Blood determinations of total cholesterol and HDL-cholesterol were performed by the enzymatic colorimetric method (Labtest Diagnóstica, Brazil). Triglycerides were measured using the glycerine phosphate oxidase peroxidase (GPO-PAP) method (Labtest Diagnóstica, Brazil). Glucose measurement was performed using the colorimetric PAP method (Labtest Diagnóstica, Brazil). LDL-cholesterol was calculated using the Friedewald formula for values < 400 mg/dl of triglycerides. All measurements were made using the HITACHI 917 (Roche® device). TSH was determined by chemiluminescence microparticle immunoassay. This third-generation assay was performed on the Analytics E170-Roche® modular instrument.
Ethical aspects
The project was approved by the Federal University of Ceará Research Ethics Committee – CEP/UFC/PROPESQ under the number 2.206.472, and by Carlos Alberto Studart Gomes Hospital under the number 2.217.472, as a co-participating institution. The researchers are aware of compliance with ethical research precepts, based on Resolution 466/12 of the National Health Council. The individuals in the case-control group were informed about the objectives and importance of the research developed in this work. All those involved signed the Informed Consent Term.
Statistical analysis
Quantitative data were submitted to the Kolmogorov-Smirnov normality test. Data was represented as mean and standard deviation, and compared using Student's t-test and ANOVA/Bonferroni. Categorical data were expressed as absolute and percentage frequencies and compared using Pearson's chi-square test (clinical data) or McNemar's test (analysis between probes). All analyses were performed using the Statistical Package for the Social Sciences (SPSS) software, version 20.0, adopting a confidence level of 95%.
Results
Clinico-pathological characterization
In Table 1, the epidemiological and clinical features of the CAD group are shown. The study population consisted of 55 men (48.7%) and 24 women (30.4%). The mean age at diagnosis of coronary heart disease was 39.7 ± 5.0 in men and 43.4 ± 5.2 in women. About 43% of the CAD group were smokers and 46.0% were sedentary. The presence of a positive family history for the CAD group was found in 46.8% of the CAD group. In 59.5% of the individuals in the CAD group, atherosclerotic involvement was observed in only one coronary territory. As for the presence of comorbidities, 68.4% were diabetic, 54.4% were hypertensive and 46.2% had alterations in the lipid profile. Most of these patients had a low level of education (56.4%) characterized by not concluding elementary and/or high school. Upon physical examination, we observed that in both sexes, all of them had both a high BMI (mean of 27.8± 2.8) and abdominal obesity, confirmed by the mean waist circumference of 97.0 ± 9.3 cm in the men and 93.7 ± 7.1 cm in women (Table 1).
Table 1: General characteristics of the coronary arterial disease patients.

Biochemical evaluation
The laboratory tests and comparative biochemical analysis results are described in Figure 1. There were no significant differences between CAD and control groups in the mean values of triglycerides, creatinine, and TSH. Total cholesterol, HDL, and LDL- cholesterol values were significantly lower in the CAD group (p < 0.001), whereas the blood glucose and glycated hemoglobin values were significantly higher (p < 0.05).
Genotypic characterization and allelic frequency in CAD patients and controls
The genotypes and the relative frequency of C108T and L55M polymorphism alleles in individuals are presented in Table 2. It is possible to observe that, concerning PON1 L55M polymorphism, the prevalence of the LL genotype was observed in 61.6% CAD group, which is statistically significant when compared to healthy controls (p < 0.032, 95% CI = 0.17 – 0.93). The MM genotype was found in only 6.7% of the CAD group. Regarding the L and M alleles, there was a significant difference in M allele prevalence in healthy controls compared to the CAD group (p < 0.032, 95% CI = 0.17-0.93). Regarding PON1 C108T polymorphism, there was no significant difference between groups, with the TT genotype being observed in 27.7% of CAD patients. Regarding the frequency of T and C alleles, no significant difference was observed between cases and controls.
Table 2: Analysis of genotypic distribution and allelic frequency between CAD cases and controls.
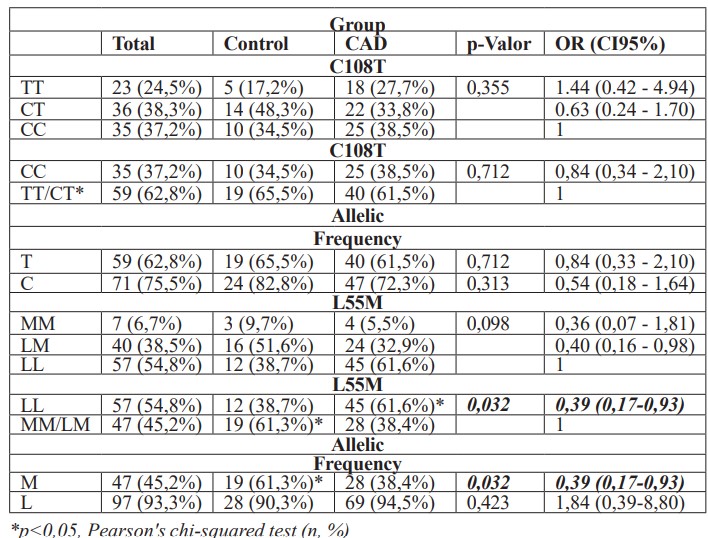

Figure 1: Biochemical measurements observed in young patients with CAD group and healthy control group. Data are represented as mean ± standard deviation and compared using the student t-test. p<0.05. HDL: High-density lipoprotein; LDL: Low-density lipoprotein; HBA1C: Glycosylated hemoglobin; TSH: thyroid stimulating hormone.
Association of risk factors with the genotypic characteristics in CAD patients
According to Table 3, there is an imbalance in the haplotypes in the CAD cases that seems to be significantly associated with disease risk (p < 0.013). In other words, homozygous individuals for C108T polymorphism will not necessarily have a homozygous L55M. For the healthy control group, however, there is an association between the haplotypes, that is, a heterozygous individual for C108T polymorphism is also heterozygous for L55M polymorphism.
Table 3: Evaluation of PON1 C108T and L55M haplotypes in young patients with and without atherosclerotic coronary artery disease.

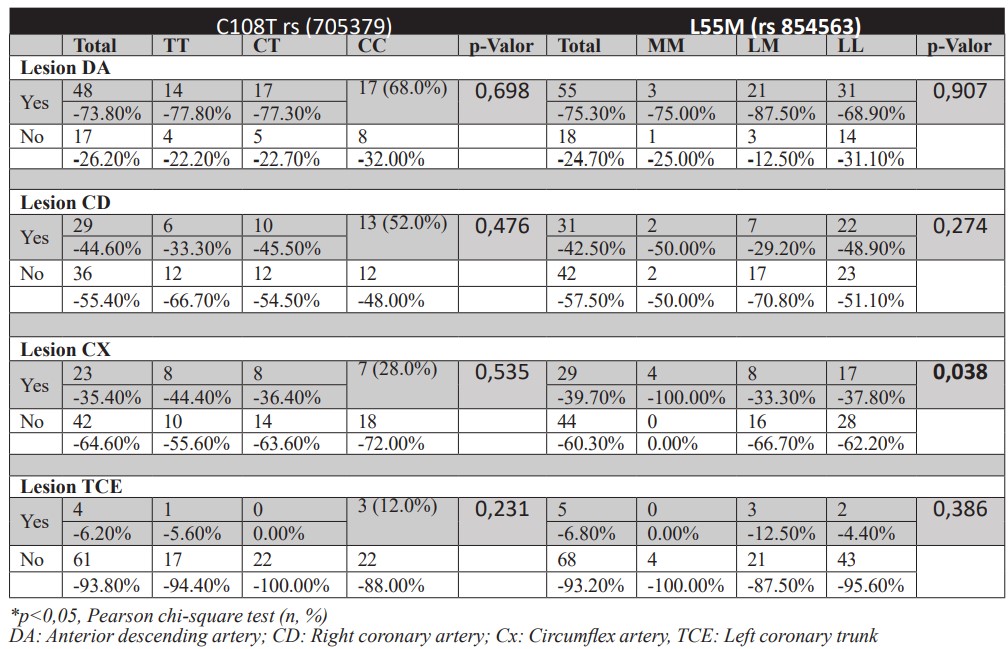
Table 4 shows that T allele carriers have a major diagnosis of dyslipidemia (p < 0.042), while individuals with M allele have significantly higher BMI values (p < 0.011). No significant difference was observed for other risk factors and CAD cases or healthy control genotypes (Table 4). Another variable analyzed was the involvement of genotypes with Circumflex artery (Cx) territory. However, there was no significant difference between genotypic patterns and the involvement of other coronary territories (Supplementary Table 1).
Table 4: Association of risk factors with the genotypic characteristics of CAD cases and healthy controls.

Supplementary table 1: Genotypic distribution according to arterial involvement pattern observed on coronary angiography in individuals with atherosclerotic coronary artery disease.
Biochemical profile and its relationship with genotypic distribution and allelic frequency
It was observed a significant difference in TSH values (p < 0.021) with lower TSH levels in CAD patients with TC genotype for C108T polymorphism of PON1 (Figure 2). No significant difference was observed in values of all the other laboratory parameters in comparison to genotypic characteristics evaluated in this study (Supplementary Table 2).
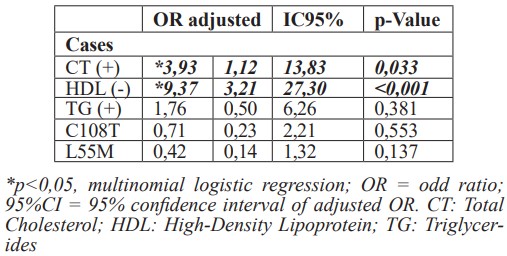
Finally, in Table 5, through multinomial logistic regression analysis, it was observed that the presence of high levels of cholesterol and low levels of HDL cholesterol in the young CAD group, represents a risk of more than three times (p < 0.033) and nine times (p < 0.001) of coronary atherosclerotic disease development, respectively.
Table 5: Risk assessment of premature CAD.
Supplementary Table 2: Analysis of the genotypic distribution of PON1 Polymorphisms with laboratory parameters in young individuals with and without atherosclerotic coronary artery disease.


Figure 2: Analysis of thyroid-stimulating hormone (TSH) according to genotypic distribution of PON1 polymorphisms (C108T) in young individuals with atherosclerotic coronary artery disease. Data are represented as mean ± standard deviation and compared using ANOVA and Tukey's multiple comparisons test. Significant differences: p<0.05 *.
Discussion
Premature coronary artery disease (CAD) has been proven to have a multifactorial etiology and is most likely a mixture of genetic and environmental factors, being the myocardial infarction occurrence, the most prevalent factor affecting offspring before the age of 55 in men and 65 in women [37,38]. In the Framingham study, after 8 years of follow-up of male and female offspring with parental history of premature cardiovascular disease (CVD) (father < 55 years, mother < 65 years), CVD increased 75% with paternal and about 60% with maternal history of premature CVD. After 16 years of follow-up, the study also found that a family history of early CAD (age <50 years) conferred a 44% increased risk of CVD mortality [11,12,39,40].
In the present study, 48.7% of atherosclerotic coronary artery disease was found in young men, with a mean age of 39.7 years at clinical diagnostic. It was observed that the majority were sedentary individuals, with overweight and visceral obesity, with no family history of premature CAD and high levels of fasting glucose, glycated hemoglobin, and very low levels of HDL-cholesterol. In the coronary angiography performed, no differences were observed in the coronary disease involvement, with the number of single and multivessel arteries being similar. Likewise, there was no predominant involvement of any coronary territory.
The prevalence of male cases agrees with several studies that confirm that men are more affected by early CAD [41,42]. This fact can be related to hormonal protection that women have while they do not enter the climacteric phase, with estrogen being a potent atheroprotective as exerts many beneficial effects against arterial diseases, including vasodilatation, acceleration of healing in response to arterial injury, arterial collateral growth and protection [43,44]. It is well established that smoking causes thrombosis, promoting the development of an inflammatory and thrombogenic profile, associated with reduced levels of prostacyclin action and increased levels of nitric thrombosis, favoring endothelial vasospasm occurrence [45]. However, no association with smoking was found, since 57% of the CAD group were not smokers.
Several studies have shown that a family history of early coronary artery disease increases CAD risk in first-degree family members increasing susceptibility to atherosclerosis growth [12,38,39,46]. However, in our study, 53% of CAD patients had no family history of CAD before in first-degree family members which disagrees with [47], who evaluated 165 North American patients with acute myocardial infarction under the age of 45 years, where it was observed that 70% were smokers and 70% had a family history of early CAD. Moreover, a large international study (INTERHEART) found an increased risk of myocardial infarction (MI) of 2.36 (Odds ratio) if at least one parent had MI and 6.56 (OR) if both parents had MI before age 50 [48]. Furthermore, when a section is made for Latin America, the INTERHEART study shows that interventions aimed at lowering blood pressure, and modifying lipids could have a large impact on the risk of acute myocardial infarction among this population [49]. Probably, the mixed population in Brazil, its genetic background, ethnicity, and other environmental exposure may influence these differences between populations around the world.
Regarding the lipid profile, patients showed similarity to what is observed in most studies that describe low levels of HDL-cholesterol (HDL-C), where all our patients had HDL-C lower than 40 mg/dl, being the lipid alteration, most found in individuals with early CAD [50– 52]. These studies emphasize that for HDL levels below 35 mg/dl, there is an increased risk of CAD occurrence by at least 4 times, and conversely, an increase of 2 mg/dl of HDL- C confers a reduction in the risk of new events and lower incidence of percutaneous coronary interventions [53]. Moreover, the Framingham study and others that followed could show that HDL-C is an independent cardiovascular risk factor and that the increase of HDL-C of only 10 mg·L−1 leads to a risk reduction of 2–3% [54–57]. Despite the existence of consistent epidemiological evidence that attributes the decrease in HDL-C to an important atherosclerotic risk factor, its exact role in CAD pathogenesis is unclear. [58–60] did not observe further reductions in coronary heart disease (CHD) risk with HDL-C values higher than 90 mg/dL in men and 75 mg/dL in women. However, several studies have shown that not only the severity of angiographic lesions but also the risk of cardiovascular events related to low concentrations of HDL-cholesterol are due to an ineffective mobilization of cholesterol from the arteries and peripheral tissues, characterizing an ineffective reverse cholesterol transport [61,62]. It is believed that, in this context, the presence of genetic alterations, characterized by single nucleotide polymorphisms (SNP), may influence the presence or absence of these risk factors [63,64]. Moreover, a significant difference was also observed between groups in the levels of glycated hemoglobin. The literature shows that an altered glycemic profile and thus insulin resistance, before the diagnosis of diabetes, generates an imbalance in glucose metabolism that generates chronic hyperglycemia, which in turn triggers oxidative stress and causes an inflammatory response that leads to cardiovascular damage [65,66]. It is important to emphasize that most of our CAD cases were unaware of any change in the glycemic profile prior to their diagnosis.
As for the extent of coronary involvement, several authors argue that multivessel coronary disease is uncommon in individuals with early CAD [67–69]. Although previous studies have reported that coronary involvement in young patients is mainly single-vessel and to a lesser extent, this study did not demonstrate a significant predominance of the pattern of single-vessel or multivessel coronary involvement. To date, we have not identified studies that correlate the presence of genotypic alterations with the predominance of involvement of specific coronary territories. Some studies [70–73] point to the possibility that patients with specific genotypic characteristics could present a greater severity in the extension of the coronary territory demonstrating that combined polymorphisms could be associated with the extension of coronary disease and more severity of the disease [74].
One of the hypotheses raised for the presence of genetic influence is on Paraoxonase 1 (PON1), the main constituent enzyme of HDL-C particles which is responsible for its antioxidant, anti-inflammatory, and reverse cholesterol transport properties, that could be enrolled in promoting changes in HDL-C concentration [75–77]. The genetic alterations would make the individual predisposed to develop atherosclerosis early even in the absence of high levels of LDL cholesterol and could intensify the role of other risk factors such as low levels of HDL cholesterol, high blood sugar, overweight, and abdominal obesity.
Few studies have been performed to analyze the association of the PON1 L55M polymorphism with coronary heart disease, and many of these have shown inconsistent results [13,28,78]. Blatter et al., [79] and Mackness and Mackness [80] found that the PON1 L55M polymorphism has a significant effect on its activity independently. Schmidt et al.,
[81] also observed an independent association of CAD with this polymorphism in Austrian subjects.
Garin et al., [82] investigated C108T PON1 polymorphism (promoter region) in patients with ischemic heart disease and their results showed that individuals with CC genotype had significantly higher levels of HDL cholesterol than heterozygotes. In contrast, no significant difference was observed in LDL and HDL levels for the PON1 L55M polymorphisms. In the present study, an LL genotype prevalence for L55M PON1 polymorphism was found to be 2.54 times higher in CAD cases than in healthy controls. This finding disagrees with Bounafaa et al., [83] who showed in a sample of 305 North African individuals that those with the PON1 55 MM genotype had a higher risk of coronary heart disease than those with the 55 LL genotype, showing once more that etinicity may play a role in CAD. Moreover, several contradictions are noticed in available studies. In a review by Litvinov et al. [84], it was reported that the antioxidant activity of PON1 decreases sequentially in MM > LM > LL genotypes, with wild-type homozygotes (LL) showing about half the activity of the paraoxonase enzyme in comparison to homozygotes mutated (MM). Allelic variation frequency among populations is enormous. The frequency of polymorphic M allele is higher in whites than in blacks. A lower frequency of the M allele has been reported in the Chinese population [85].
As for C108T polymorphism, there was no significant difference in the presence between CAD cases and healthy controls, observing a balance in allele frequency. CC genotype is responsible for the highest activity of PON1, while TT genotype is related to low gene expression and reduced serum activity of the enzyme [86]. Litvinov et al. [87], demonstrated an association between C108T polymorphism and CAD. Levieve and James [88], observed that CC genotype carriers were protected from CAD involvement at age younger than 60 years, but not in older individuals [32]. Also was no association of C108T polymorphism with CAD after adjustment with traditional risk factors in this study.
When CAD cases and healthy control genotypes were analyzed together as a haplotype, there was an imbalance in haplotypes or linkage imbalance that seemed to be significantly associated with disease risk. This observation is supported by Gupta et al.[32], who emphasize that haplotype determination is gaining more and more attention due to the finding that multiple associated SNPs have a greater potential to promote disease than when they are analyzed alone. Much information is lacking regarding PON1 haplotypes and the risk of CAD. Levieve and James [88], also showed a strong linkage disequilibrium between C108T and L55M polymorphisms among CAD carriers.
An interesting observation in the present study is that a significant difference in TSH values was found in cases with CT or TT genotype for C108T polymorphism. It should be noted that, to date, we are not aware of any studies that evaluated the relationship between activity or concentration of human PON 1 with thyroid function and its consequences in early CAD development.
Several studies recognize the relationship between higher TSH values, even within a normal reference value, and cardiovascular risk, but in general they correlate this finding with the fact that these TSH values are associated with a diagnosis of metabolic syndrome [89–91]. This observation may agree with the actions of thyroid hormones on the cardiovascular system. It is known that myocardium and vascular endothelial tissue have receptors for such hormones and are sensitive to changes in their serum concentrations, even in minimal variations [92]. These observations lead to the discussion of whether TSH serum values added to PON1 polymorphisms contribute to an environment of higher cardiovascular risk.
Finally, in this study, we observed through multinomial analysis by logistic regression that, regardless of the presence of PON1 polymorphisms evaluated here, the risk intensity of symptomatic coronary disease at an early age is 3 times greater in those individuals with high total cholesterol and about 9 times if they also have low levels of HDL cholesterol. The presence of the allelic mutation will indirectly interfere with disease developing risk by promoting a favorable environment such as overweight and visceral obesity that contribute to metabolic alterations occurring mainly in glycemic and lipid profile and of an atherogenic character in young patients. To the best of our knowledge, this is the first time that these genetic modifiers are described in young Brazilian subjects, being an important risk factor that can be used in practice clinics to manage patients with CAD.
Conclusion
The main finding of this study is the presence of PON1 polymorphisms associated with an increased risk of coronary artery disease (CAD) in young patients. PON1 C108T polymorphism should be a relevant monitor for dyslipidemia and PON1 L55M polymorphism for BMI. Moreover, the rise of total cholesterol levels and low HDL levels produce a risk greater than 3 and 9 times (p <0.021), respectively, in the chance of premature CAD.
Acknowledge: This study was funded by the Brazilian agencies: National Council for Scientific and Technological Development (CNPq) and Coordination for the Improvement of Higher Education Personnel (CAPES). Also, the authors acknowledge the Red Latinoamericana de Implementación y Validación de guias clinicas Farmacogenomicas (RELIVAF)
Data Availability Statement: The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflict of Interest Statement: The authors declare no conflicts of interest related to this manuscript.
References
- CDC, Coronary Artery Disease. cdc.gov, Cent. Dis. Control Prev, 2021.
- Bottardi A, Prado GFA, Lunardi M, Fezzi S, Pesarini G, Tavella D, et al. Clinical Updates in Coronary Artery Disease: A Comprehensive Review, Clin. Med, 2024; 13: 4600. https://doi.org/10.3390/jcm13164600.
- Stark B, Johnson C, Roth GA. Global prevalence of coronary artery disease: an update from the global burden of disease study, J. Am. Coll. Cardiol, 2024; 83: 2320–2320. https://doi.org/10.1016/S0735-1097(24)04310-9.
- Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, Abyu G, et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015, J. Am. Coll. Cardiol, 2017; 70: 1–25. https://doi.org/10.1016/j.jacc.2017.04.052.
- Vaduganathan M, Mensah GA, Turco JV, Fuster V, Roth GA. The Global Burden of Cardiovascular Diseases and Risk: A Compass for Future Health, Am. Coll. Cardiol, 2022; 80: 2361–2371. https://doi.org/10.1016/j.jacc.2022.11.005.
- Aggarwal R, Yeh RW, Joynt Maddox KE, Wadhera RK. Cardiovascular Risk Factor Prevalence, Treatment, and Control in US Adults Aged 20 to 44 Years, 2009 to March JAMA, 2023; 329: 899–909. https://doi.org/10.1001/jama.2023.2307.
- Allen N, Wilkins JT. The Urgent Need to Refocus Cardiovascular Disease Prevention Efforts on Young Adults, JAMA, 2023; 329: 886–887. https://doi.org/10.1001/jama.2023.2308.
- Emoto T, Yamashita T, Sasaki N, Hirota Y, Hayashi T, So A, et al. Analysis of Gut Microbiota in Coronary Artery Disease Patients: a Possible Link between Gut Microbiota and Coronary Artery Disease, Atheroscler. Thromb, 2016; 23: 908– 921. https://doi.org/10.5551/jat.32672.
- Kang Y, Park H-J, Kang M-I, Lee H-S, Lee S-W, Lee S-K, et al. Adipokines, inflammation, insulin resistance, and carotid atherosclerosis in patients with rheumatoid arthritis, Arthritis Res. Ther, 2013; 15: R194. https://doi.org/10.1186/ar4384.
- Wang H, Liu Z, Shao J, Jiang M, Lu X, Lin L, et al. Pathogenesis of premature coronary artery disease: Focus on risk factors and genetic variants, Genes Dis, 2022; 9: 370–380. https://doi.org/10.1016/j.gendis.2020.11.003.
- Hajar R. Genetics in Cardiovascular Disease, Heart Views J. Gulf Heart Assoc, 2020; 21: 55–56. https://doi.org/10.4103/HEARTVIEWS.HEARTVIEWS_140_19.
- Kolber MR, Scrimshaw C. Family history of cardiovascular disease, Fam. Physician, 2014; 60: 1016.
- Hernández-Díaz Y, Tovilla-Zárate CA, Juárez-Rojop IE, González-Castro TB, Rodríguez-Pérez C, López-Narváez ML, et al. Effects of paraoxonase 1 gene polymorphisms on heart diseases: Systematic review and meta-analysis of 64 case-control studies, Medicine (Baltimore), 2016; 95: https://doi.org/10.1097/MD.0000000000005298.
- Ponce-Ruiz N, Murillo-González FE, Rojas-García AE, Hernández YYB, Mackness M, Ponce-Gallegos J, et al. Phenotypes and concentration of PON1 in cardiovascular disease: The role of nutrient intake, Nutr. Metab. Cardiovasc. Dis, 2020; 30: 40–48. https://doi.org/10.1016/j.numecd.2019.08.013.
- Chistiakov DA, Melnichenko AA, Orekhov AN, Bobryshev YV. Paraoxonase and atherosclerosis-related cardiovascular diseases, Biochimie, 2017; 132: 19–27. https://doi.org/10.1016/j.biochi.2016.10.010.
- Kumar S, Maniya N, Wang C, Senapati S, Chang H-C. Quantifying PON1 on HDL with nanoparticle-gated electrokinetic membrane sensor for accurate cardiovascular risk assessment, Nat. Commun, 2023; 14: 557. https://doi.org/10.1038/s41467-023-36258-w.
- Mackness MI, Abbott C, Arrol S, Durrington PN. The role of high-density lipoprotein and lipid-soluble antioxidant vitamins in inhibiting low-density lipoprotein oxidation, Biochem. J, 1993; 294(Pt 3): 829–834. https://doi.org/10.1042/bj2940829.
- Mackness MI, Arrol S, Abbott C, Durrington PN. Protection of low-density lipoprotein against oxidative modification by high-density lipoprotein associated paraoxonase, Atherosclerosis, 1993; 104: 129–135. https://doi.org/10.1016/0021- 9150(93)90183-u.
- Mackness MI, Arrol S, Durrington PN. Paraoxonase prevents accumulation of lipoperoxides in low-density lipoprotein, FEBS Lett, 1991; 286: 152–154. https://doi.org/10.1016/0014-5793(91)80962-3.
- Aviram M, Rosenblat M, Bisgaier CL, Newton RS, Primo-Parmo SL, La Du BN. Paraoxonase inhibits high-density lipoprotein oxidation and preserves its functions. A possible peroxidative role for paraoxonase, Clin. Invest, 1998; 101: 1581–1590. https://doi.org/10.1172/JCI1649.
- Navab M, Reddy ST, Van Lenten BJ, Fogelman AM. HDL and cardiovascular disease: atherogenic and atheroprotective mechanisms, Rev. Cardiol, 2011; 8: 222–232. https://doi.org/10.1038/nrcardio.2010.222.
- Soflaei SS, Baktashian M, Moghaddam KH, Saberi-Karimian M, Kosari N, Hashemi SM, et al. Associação do Genótipo e Fenótipo da Paraoxonase-1 com Angiografia Positiva para Doença Arterial Coronariana, Arq Bras Cardiol, 2022; 119: 593–601. https://doi.org/10.36660/abc.20210422.
- Tang WHW, Hartiala J, Fan Y, Wu Y, Stewart AFR, Erdmann J, et al. Clinical and genetic association of serum paraoxonase and arylesterase activities with cardiovascular risk, Arterioscler. Thromb. Vasc. Biol, 2012; 32: 2803–2812. https://doi.org/10.1161/ATVBAHA.112.253930.
- Wysocka A, Cybulski M, Wysokiński AP, Berbeć H, Stążka J, Zapolski T. Paraoxonase 1 Activity, Polymorphism and Atherosclerosis Risk Factors in Patients Undergoing Coronary Artery Surgery, J. Clin. Med, 2019; 8: 441. https://doi.org/10.3390/jcm8040441.
- Yildiz A, Gur M, Yilmaz R, Demirbag R, Polat M, Selek S, et al. Association of paraoxonase activity and coronary blood flow, Atherosclerosis, 2008; 197: 257–263. https://doi.org/10.1016/j.atherosclerosis.2007.04.004.
- Bayrak A, Bayrak T, Bodur E, Kılınç K, Demirpençe E. The effect of HDL-bound and free PON1 on copper-induced LDL oxidation, Chem. Biol. Interact, 2016; 257: 141–146. https://doi.org/10.1016/j.cbi.2016.08.007.
- Vavlukis M, Vavlukis A, Krsteva K, Topuzovska S. Paraoxonase 1 gene polymorphisms in lipid oxidation and atherosclerosis development, Genet, 2022; 13.
- Zhang K, Zhuo H, Guo J, Li D, Dai R. Paraoxonase 1 -L55M polymorphism and coronary heart disease risk in the Chinese population: evidence from a meta- analysis, Food Sci. Technol, 2021; 42: e56721. https://doi.org/10.1590/fst.56721.
- Moreno-Godínez ME, Galarce-Sosa C, Cahua-Pablo JA, Rojas-García AE, Huerta-Beristain G, Alarcón-Romero LDC, et al. Genotypes of Common Polymorphisms in the PON1 Gene Associated with Paraoxonase Activity as Cardiovascular Risk Factor, Med. Res, 2018; 49: 486–496. https://doi.org/10.1016/j.arcmed.2019.02.002.
- Corredor-Orlandelli D, Sambracos-Parrado S, Mantilla-García S, Tovar-Tirado J, Vega-Ramírez V, Mendoza-Ayús SD, et al. Association between Paraoxonase-1 p.Q192R Polymorphism and Coronary Artery Disease susceptibility in the Colombian Population, Vasc. Health Risk Manag, 2021; 17: 689–699. https://doi.org/10.2147/VHRM.S330766.
- Godbole C, Thaker S, Kerkar P, Nadkar M, Gogtay N, Thatte U. Association of PON1 gene polymorphisms and enzymatic activity with risk of coronary artery disease, Future Cardiol, 2021; 17: 119–126. https://doi.org/10.2217/fca-2020-0028.
- Gupta N, Singh S, Maturu VN, Sharma YP, Gill KD. Paraoxonase 1 (PON1) Polymorphisms, Haplotypes and Activity in Predicting CAD Risk in North-West Indian Punjabis, PLOS ONE, 2011; 6: e17805. https://doi.org/10.1371/journal.pone.0017805.
- Chen H, Ding S, Zhou M, Wu X, Liu X, Liu J, et al. PON1 L55M and Q192R gene polymorphisms and CAD risks in patients with hyperlipidemia, Herz, 2018; 43: 642–648. https://doi.org/10.1007/s00059-017-4611-0.
- Peng W, Shi X, Xu X, Lin Y. Both CYP2C19 and PON1 Q192R Genotypes Influence Platelet Response to Clopidogrel by Thrombelastography in Patients with Acute Coronary Syndrome, Cardiovasc. Ther, 2019; 2019: 3470145. https://doi.org/10.1155/2019/3470145.
- Yigittürk O, Turgay F, Kızıldağ S, Özsoylu D, Balcı GA. Do PON1-Q192R and PON1-L55M polymorphisms modify the effects of hypoxic training on paraoxonase and arylesterase activity? J. Sport Health Sci, 2023; 12: 266–274. https://doi.org/10.1016/j.jshs.2020.11.004.
- Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments, Clin. Chem, 2009; 55: 611–622. https://doi.org/10.1373/clinchem.2008.112797.
- Mayer B, Erdmann J, Schunkert H. Genetics and heritability of coronary artery disease and myocardial infarction, Clin. Res. Cardiol. Off. J. Ger. Card. Soc, 2007; 96: 1–7. https://doi.org/10.1007/s00392-006-0447-y.
- Sivapalaratnam S, Boekholdt SM, Trip MD, Sandhu MS, Luben R, Kastelein JJP, et al. Family history of premature coronary heart disease and risk prediction in the EPIC-Norfolk prospective population study, Heart, 2010; 96: 1985–1989. https://doi.org/10.1136/hrt.2010.210740.
- Lloyd-Jones DM, Nam B-H, D’Agostino RB, Levy D, Murabito JM, Wang TJ, Wilson PWF, et al. Parental cardiovascular disease as a risk factor for cardiovascular disease in middle-aged adults: a prospective study of parents and offspring, JAMA, 2004; 291: 2204–2211. https://doi.org/10.1001/jama.291.18.2204.
- Murabito JM, Pencina MJ, Nam B-H, D’Agostino RB, Wang TJ, Lloyd- Jones D, et al. Sibling cardiovascular disease as a risk factor for cardiovascular disease in middle-aged adults, JAMA, 2005; 294: 3117–3123. https://doi.org/10.1001/jama.294.24.3117.
- Bots SH, Peters SAE, Woodward M. Sex differences in coronary heart disease and stroke mortality: a global assessment of the effect of ageing between 1980 and 2010, BMJ Glob. Health, 2017; 2: e000298. https://doi.org/10.1136/bmjgh-2017-
- Tan YY, Gast G-CM, van der Schouw YT. Gender differences in risk factors for coronary heart disease, Maturitas, 2010; 65: 149–160. https://doi.org/10.1016/j.maturitas.2009.09.023.
- Barrett-Connor E. Postmenopausal estrogen and prevention bias, Intern. Med, 199; 115: 455–456. https://doi.org/10.7326/0003-4819-115-6-455.
- Fontaine C, Morfoisse F, Tatin F, Zamora A, Zahreddine R, Henrion D, et al. The Impact of Estrogen Receptor in Arterial and Lymphatic Vascular Diseases, Int. J. Mol. Sci, 2020; 21: 3244. https://doi.org/10.3390/ijms21093244.
- Stallones RA. The association between tobacco smoking and coronary heart disease, J. Epidemiol, 2015; 44: 735–743. https://doi.org/10.1093/ije/dyv124.
- Mulders TA, Sivapalaratnam S, Stroes ESG, Kastelein JJP, Guerci AD, Pinto-Sietsma S-J. Asymptomatic individuals with a positive family history for premature coronary artery disease and elevated coronary calcium scores benefit from statin treatment: a post hoc analysis from the St. Francis Heart Study, JACC Cardiovasc. Imaging, 2012; 5: 252–260. https://doi.org/10.1016/j.jcmg.2011.11.014.
- Zarich S, Luciano C, Hulford J, Abdullah A. Prevalence of metabolic syndrome in young patients with acute MI: does the Framingham Risk Score underestimate cardiovascular risk in this population? Diab. Vasc. Dis. Res, 2006; 3: 103–107. https://doi.org/10.3132/dvdr.2006.012.
- Chow CK, Islam S, Bautista L, Rumboldt Z, Yusufali A, Xie C, et al. Parental History and Myocardial Infarction Risk Across the World, J. Am. Coll. Cardiol, 2011; 57: 619–627. https://doi.org/10.1016/j.jacc.2010.07.054.
- Lanas F, Avezum A, Bautista LE, Diaz R, Luna M, Islam S, et al. INTERHEART Investigators in Latin America, Risk factors for acute myocardial infarction in Latin America: the INTERHEART Latin American study, Circulation, 2007; 115: 1067–1074. https://doi.org/10.1161/CIRCULATIONAHA.106.633552.
- Berry JD, Dyer A, Cai X, Garside DB, Ning H, Thomas A, et al. Lifetime Risks of Cardiovascular Disease, N. Engl. J. Med, 2012; 366: 321–329. https://doi.org/10.1056/NEJMoa1012848.
- Besler C, Lüscher TF, Landmesser U. Molecular mechanisms of vascular effects of High-density lipoprotein: alterations in cardiovascular disease, EMBO Med, 2012; 4: 251–268. https://doi.org/10.1002/emmm.201200224.
- Saleheen D, Scott R, Javad S, Zhao W, Rodrigues A, Picataggi A, et al. Association of HDL cholesterol efflux capacity with incident coronary heart disease events: a prospective case- control study, Lancet Diabetes Endocrinol, 2015; 3: 507–513. https://doi.org/10.1016/S2213-8587(15)00126-6.
- Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines, 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, J. Coll. Cardiol, 2014; 63: 2889–2934. https://doi.org/10.1016/j.jacc.2013.11.002.
- Ali KM, Wonnerth A, Huber K, Wojta J. Cardiovascular disease risk reduction by raising HDL cholesterol – current therapies and future opportunities, Br. J. Pharmacol, 2012; 167: 1177–1194. https://doi.org/10.1111/j.1476- 2012.02081.x.
- Emerging Risk Factors Collaboration, Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, et al. Major lipids, apolipoproteins, and risk of vascular disease, JAMA, 2009; 302: 1993–2000. https://doi.org/10.1001/jama.2009.1619.
- Gordon DJ, Probstfield JL, Garrison RJ, Neaton JD, Castelli WP, Knoke JD, et al. High-density lipoprotein cholesterol and cardiovascular Four prospective American studies, Circulation, 1989; 79: 8–15. https://doi.org/10.1161/01.cir.79.1.8.
- Wilson PW, Garrison RJ, Castelli WP, Feinleib M, McNamara PM, Kannel WB. Prevalence of coronary heart disease in the Framingham Offspring Study: role of lipoprotein cholesterols, Am. J. Cardiol, 1980; 46: 649–654. https://doi.org/10.1016/0002-9149(80)90516-0.
- Cho YK, Jung CH, HDL-C. Cardiovascular Risk: You Don’t Need to Worry about Extremely High HDL-C Levels, J. Lipid Atheroscler, 2021; 10: 57–61. https://doi.org/10.12997/jla.2021.10.1.57.
- Kjeldsen EW, Thomassen JQ, Frikke-Schmidt R. HDL cholesterol concentrations and risk of atherosclerotic cardiovascular disease - Insights from randomized clinical trials and human genetics, Biophys. Acta Mol. Cell Biol. Lipids, 2022; 1867: 159063. https://doi.org/10.1016/j.bbalip.2021.159063.
- Liu C, Dhindsa D, Almuwaqqat Z, Ko Y-A, Mehta A, Alkhoder AA, et al. Association Between High-Density Lipoprotein Cholesterol Levels and Adverse Cardiovascular Outcomes in High-risk Populations, JAMA Cardiol, 2022; 7: 672– 680. https://doi.org/10.1001/jamacardio.2022.0912.
- Ference BA, Kastelein JJP, Ginsberg HN, Chapman MJ, Nicholls SJ, Ray KK, et al. Association of Genetic Variants Related to CETP Inhibitors and Statins with Lipoprotein Levels and Cardiovascular Risk, JAMA, 2017; 318: 947–956. https://doi.org/10.1001/jama.2017.11467.
- Ouimet M, Barrett TJ, Fisher EA. HDL and Reverse Cholesterol Transport, Res, 2019; 124: 1505–1518. https://doi.org/10.1161/CIRCRESAHA.119.312617.
- Rankinen T, Sarzynski MA, Ghosh S, Bouchard C. Are There Genetic Paths Common to Obesity, Cardiovascular Disease Outcomes, and Cardiovascular Risk Factors? Circ. Res, 2015; 116: 909–922. https://doi.org/10.1161/CIRCRESAHA.116.302888.
- Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic M, Jensen MK, et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study, Lancet Lond. Engl, 2012; 380: 572–580. https://doi.org/10.1016/S0140-6736(12)60312-
- Clemmons N. Diabetes and Functional Medicine, Diabetes Care, 2015.
- Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuñiga FA. Association between insulin resistance and the development of cardiovascular disease, Cardiovasc. Diabetol, 2018; 17: 122. https://doi.org/10.1186/s12933-018-0762-4.
- Sharma SK, Makkar JS, Bana A, Sharma K, Kasliwal A, Sidana SK, et al. Premature coronary artery disease, risk factors, clinical presentation, angiography and interventions: Hospital based registry, Indian Heart J, 2022; 74: 391–397. https://doi.org/10.1016/j.ihj.2022.08.003.
- Nazli SA, Rosman A, Mohd Kasim NA, Al-Khateeb A, Ul-Saufie AZ, Md Radzi AB, et al. Coronary risk factor profiles according to different age categories in premature coronary artery disease patients who have undergone percutaneous coronary intervention, Sci. Rep, 2024; 14: 15326. https://doi.org/10.1038/s41598-024-53539-6.
- Zafrir B, Jubran A, Lavie G, Halon DA, Flugelman MY, Shapira C. Clinical Features and Gaps in the Management of Probable Familial Hypercholesterolemia and Cardiovascular Disease, Circ. J. Off. J. Jpn. Circ. Soc, 2017; 82: 218–223. https://doi.org/10.1253/circj.CJ-17-0392.
- Ellis KL, Zhou Y, Beshansky JR, Ainehsazan E, Selker HP, Cupples LA, et al. Genetic modifiers of response to glucose–insulin–potassium (GIK) infusion in acute coronary syndromes and associations with clinical outcomes in the IMMEDIATE trial, Pharmacogenomics J, 2015; 15: 488–495. https://doi.org/10.1038/tpj.2015.10.
- Izar MC, Helfenstein T, Ihara SS, Relvas WG, Santos AO, Fischer SC, et al. Association of lipoprotein lipase D9N polymorphism with myocardial infarction in type 2 diabetes: The genetics, outcomes, and lipids in type 2 diabetes (GOLD) study, Atherosclerosis, 2009; 204: 165–170. https://doi.org/10.1016/j.atherosclerosis.2008.08.006.
- Palmer BR, Slow S, Ellis KL, Pilbrow AP, Skelton L, Frampton CM, et al. Genetic Polymorphism rs6922269 in the MTHFD1L Gene Is Associated with Survival and Baseline Active Vitamin B12 Levels in Post-Acute Coronary Syndromes Patients, PLOS ONE, 2014; 9: e89029. https://doi.org/10.1371/journal.pone.0089029.
- Song Z, Cao H, Qin L, Jiang Y. A Case-Control Study between Gene Polymorphisms of Polyunsaturated Fatty Acid Metabolic Rate-Limiting Enzymes and Acute Coronary Syndrome in Chinese Han Population, BioMed Int, 2013; 2013: e928178. https://doi.org/10.1155/2013/928178.
- Fischer SCPM, Pinto SP, do S Lins LC, Bianco HT, de C Monteiro CM, Pinheiro LFM, et al. Associação de Múltiplas Variantes Genéticas com a Extensão e Gravidade da Doença Coronária, Arq. Bras. Cardiol, 2018; 110: 16–23. https://doi.org/10.5935/abc.20170177.
- Liu T, Zhang X, Zhang J, Liang Z, Cai W, Huang M, et al. Association between PON1 rs662 polymorphism and coronary artery disease, J. Clin. Nutr, 2014; 68: 1029–1035. https://doi.org/10.1038/ejcn.2014.105.
- Munshi R, Panchal F, Chaurasia A, Rajadhyaksha G. Association between Paraoxonase 1(PON1) Gene Polymorphisms and PON1 Enzyme Activity in Indian Patients with Coronary Artery Disease (CAD), Pharmacogenomics Pers. Med, 2018; 16: 219–229.
- da S Pinheiro D, Jesuíno A. O Gene da Paraoxonase 1 (PON1) no Contexto Doença Arterial Coronariana, Arq. Bras. Cardiol, 2022; 119: 602–603. https://doi.org/10.36660/abc.20220645.
- Kocakap DBS, Doğru MT, Şimşek V, Çabuk F, Yıldırım N, Çelik Y, et al. The association of paraoxonase 1 gene L55M polymorphism with the extent and severity of coronary artery disease in the Turkish population and its dependence on gender, J. Cardiol, 2016; 16: 175–182. https://doi.org/10.5152/akd.2015.6010.
- Blatter MC, James RW, Messmer S, Barja F, Pometta D. Identification of a distinct human high-density lipoprotein subspecies defined by a lipoprotein- associated protein, K-45. Identity of K-45 with paraoxonase, J. Biochem, 1993; 211: 871–879. https://doi.org/10.1111/j.1432-1033.1993.tb17620.x.
- Mackness M, Mackness B. Paraoxonase 1 and atherosclerosis: is the gene or the protein more important? Free Radic. Biol. Med, 2004; 37: 1317–1323. https://doi.org/10.1016/j.freeradbiomed.2004.07.034.
- Schmidt H, Schmidt R, Niederkorn K, Gradert A, Schumacher M, Watzinger N. Paraoxonase PON1 polymorphism leu-Met54 is associated with carotid atherosclerosis: results of the Austrian Stroke Prevention Study, Stroke, 1998; 29: 2043–2048. https://doi.org/10.1161/01.str.29.10.2043.
- Garin M-CB, Moren X, James RW. Paraoxonase-1 and serum concentrations of HDL-cholesterol and apoA-I, J. Lipid Res. 47 (2006) 515–520. https://doi.org/10.1194/jlr.M500281-JLR200.
- Bounafaa, H. Berrougui, N. Ghalim, B. Nasser, A. Bagri, A. Moujahid, S. Ikhlef, P. Camponova, N. Yamoul, O.K. Simo, A. Essamadi, A. Khalil, Association between Paraoxonase 1 (PON1) Polymorphisms and the Risk of Acute Coronary Syndrome in a North African Population, PLoS ONE, 2015; 10: e0133719. https://doi.org/10.1371/journal.pone.0133719.
- Litvinov D, Mahini H, Garelnabi M. Antioxidant and Anti-Inflammatory Role of Paraoxonase 1: Implication in Arteriosclerosis Diseases, North J. Med. Sci, 2012; 4: 523–532. https://doi.org/10.4103/1947-2714.103310.
- Imai Y, Morita H, Kurihara H, Sugiyama T, Kato N, Ebihara A, et al. Evidence for association between paraoxonase gene polymorphisms and atherosclerotic diseases, Atherosclerosis, 2000; 149: 435–442. https://doi.org/10.1016/S0021-9150(99)00340-8.
- Kim DS, Marsillach J, Furlong CE, Jarvik GP. Pharmacogenetics of paraoxonase activity: elucidating the role of high-density lipoprotein in disease, Pharmacogenomics, 2013; 14: 1495–1515. https://doi.org/10.2217/pgs.13.147.
- Najafi M, Gohari LH, Firoozrai M. Paraoxonase 1 gene promoter polymorphisms are associated with the extent of stenosis in coronary arteries, Thromb. Res, 2009; 123: 503–510. https://doi.org/10.1016/j.thromres.2008.03.004.
- Leviev I, James RW. Promoter Polymorphisms of Human Paraoxonase PON1 Gene and Serum Paraoxonase Activities and Concentrations, Thromb. Vasc. Biol, 2000; 20: 516–521. https://doi.org/10.1161/01.ATV.20.2.516.
- Cappola AR, Desai AS, Medici M, Cooper LS, Egan D, Sopko G, et al. Thyroid and Cardiovascular Disease Research Agenda for Enhancing Knowledge, Prevention, and Treatment, Circulation, 2019. https://doi.org/10.1161/CIRCULATIONAHA.118.036859.
- Kannan L, Shaw PA, Morley MP, Brandimarto J, Fang JC, Sweitzer NK, et al. Thyroid Dysfunction in Heart Failure and Cardiovascular Outcomes, Circ. Heart Fail. 11 (2018) e005266. https://doi.org/10.1161/CIRCHEARTFAILURE.118.005266.
- Lee YK, Kim JE, Oh HJ, Park KS, Kim SK, Park SW, et al. Serum TSH Level in Healthy Koreans and the Association of TSH with Serum Lipid Concentration and Metabolic Syndrome, Korean Intern. Med, 2011; 26: 432–439. https://doi.org/10.3904/kjim.2011.26.4.432.
- Mourouzis I, Forini F, Pantos C, Iervasi G. Thyroid hormone and cardiac disease: from basic concepts to clinical application, J. Thyroid Res, 2011; 2011: 958626. https://doi.org/10.4061/2011/958626.

