Clinical Pharmacology of Celecoxib
Gian Maria Pacifici*
Gian Maria Pacifici, Professor of Pharmacology, Via Sant’Andrea 32, 56127 Pisa, Italy
Received Date: 11/10/2024; Published Date: 06/11/2024
*Corresponding author: Gian Maria Pacifici, Professor of Pharmacology, Via Sant’Andrea 32, 56127 Pisa, Italy. E-mail: prof.pacifici.gianmaria@gmail.com
Abstract
Celecoxib is used for the management of active pain, osteoarthritis, rheumatic arthritis, ankylosing spondylitis, juvenile rheumatic arthritis, and primary dysmenorrhoea. The recommended dose of celecoxib for treating osteoarthritis is 200 mg daily and for treating rheumatic arthritis the dose of celecoxib is 100 to 200 mg twice-daily. The efficacy and safely of celecoxib, the prophylaxis with celecoxib, the treatment of patients with celecoxib, and the trials conducted with celecoxib have been reviewed and celecoxib in hydroxylated into 4-hydroxy-celecoxib by CYP2C9. The pharmacokinetics of celecoxib have been studied in healthy subjects in absence and in presence of Ojeok-san and according to CYP2C9 genotype. In absence of Ojeok-san the elimination half-life of celecoxib is about 7 hours and Ojeok-san prolongs the elimination half-life of celecoxib to about 11 hours. The peak concentration and the area under the concentration-time of celecoxib are higher in subjects who are CYP2C9 intermediate metabolisers than in subjects who are CYP2C9 normal metabolizers. Celecoxib is a safe drug and causes less gastrointestinal, renal, and myocardial toxicity than nonsteroidal anti-inflammatory drugs. The aim of this study is to review the efficacy and safely of celecoxib, the prophylaxis with celecoxib, the treatment of patients with celecoxib, and the trials conducted with celecoxib. In addition, the metabolism and the pharmacokinetics of celecoxib and the toxicity induced by celecoxib have been reviewed.
Keywords: Celecoxib; Efficacy-safely; Metabolism; Pharmacokinetics; Prophylaxis; Toxicity; Treatment; Trials
Introduction
Therapeutic uses of celecoxib
Celecoxib is used for the management of active pain and for the treatment of osteoarthritis, rheumatic arthritis, ankylosing spondylitis, juvenile rheumatoid arthritis, and primary dysmenorrhoea. The recommended dose for treating osteoarthritis is 200 mg daily as a single dose or divided in two doses. In the treatment of rheumatoid arthritis the recommended dose is 100 mg to 200 mg twice-daily. Due to cardiovascular hazard, physicians should use the lower possible dose for the shortest possible duration [1].
Absorption, distribution, metabolism, and elimination of celecoxib
The bioavailability of oral celecoxib is not known and peak plasma concentration occurs at 2 and 4 hours after administration. The elderlies (> 65 years of age) may have up to 2-fold higher peak concentrations and area under the concentration-tine curve values than younger patients (< 55 years of age). Celecoxib is bound extensively to plasma proteins. The elimination half-life is approximately 7 hours. The drug commonly is given once-daily or twice-daily during chronic treatment. Plasma concentrations are increased in patients with mild and moderate hepatic impairment requiring reduction in the dose. Celecoxib is metabolized into 4-hydroxy-celecoxib by CYP2C9 thus vigilance is necessary during co-administration of drugs that are known to inhibit CYP2C9 [1].
Adverse-effects caused by celecoxib
Celecoxib confers a risk of myocardial infarction and myocardial stroke and this appears to be related to the dose and the underlying risk of cardiovascular disease. Effects attributed to inhibition of prostaglandin production in the kidney or hypertension and oedema occur with nonselective inhibitors and also with celecoxib. Selective cyclooxygenase-2 inhibitors lose their gastrointestinal advantage over other nonsteroidal anti-inflammatory drugs alone when used in conjugation with aspirin. Chronic use of celecoxib may decrease bone mineral density particularly in the older male patients. There are some suggestions that celecoxib may slow fracture healing and tendon-to-bone healing [1].
Molecular structure of celecoxib (molecular weight = 381.37 grams/mole)
Literature search
The literature search was performed electronically using PubMed database as search engine and the following key words were used: “celecoxib efficacy, safely”, “celecoxib prophylaxis”, “celecoxib treatment”, “celecoxib trials”, “celecoxib metabolism”, “celecoxib pharmacokinetics”, and “celecoxib toxicity”. In addition the book: Goodman@Gilman’s. The Pharmacological basis of Therapeutics [1] has been consulted.
Results
Efficacy and safely of celecoxib
Eight studies have been reported on the efficacy and safely of celecoxib. Celecoxib was administered at the dose of 200 mg once-daily for six weeks to patients with osteoarthritis of the knee and this treatment effectively and safely relieved pain and improved walking [2]. Rofecoxib was administered at the daily dose of 12.5 mg and celecoxib was administered at the daily dose of 200 mg. Both drugs were administered for six weeks and effectively and safely treated osteoarthritis of the knee [3]. One-hundred-seventy-five patients had rheumatoid arthritis and 204 patients had osteoarthritis of the knee or the hip and all patients received celecoxib at the daily dose of 200 mg for four weeks. The treatment cured 263 patients (69.4%), only 108 patients (28.5%) did not show change in their symptoms, and only 8 patients (2.1%) were aggravated after treatment. Celecoxib was an efficacious treatment of rheumatoid arthritis and osteoarthritis of the knee and the hip [4]. A total of 1,061 patients with symptomatic osteoarthritis of the hip received either celecoxib at the daily doses of 100 mg, 200 mg or 400 mg or naproxen at the daily dose of 1,000 mg and treatments lasted 12 weeks. In terms of pain relief and improvement in functional capacity celecoxib administered at the daily doses of 200 mg and 400 mg was efficacious as naproxen administered at the daily dose of 1,000 mg and both drugs were well-tolerated [5]. A total of 269 patients received celecoxib at the dose of 50 mg or 100 mg 4 times-daily for treatment of acute pharyngeal pain and celecoxib effectively relieved pain and was well-tolerated [6]. Of 228 children, aged 1 to 17 years, undergoing adenotonsillectomy 75 children (33.3%) received celecoxib at the daily dose of 0.15 mg/kg to 0.20 mg/kg and 152 children (56.7%) received morphine at the daily dose of 0.20 mg/kg to 0.27 mg/kg. Celecoxib administered early after adenotonsillectomy reduced pain intensity and duration of hospital stay more effectively than morphine [7]. A total of 73,748 patients, aged ≥ 65 years, with arthritis received either celecoxib at the daily dose of 200 mg or 400 mg or traditional nonsteroidal anti-inflammatory drugs and treatments lasted 120 days. Celecoxib was associated with a lower risk of gastrointestinal bleeding than traditional nonsteroidal anti-inflammatory drugs (P-value = 0.03) but caused cardiovascular and renal risks higher than traditional nonsteroidal anti-inflammatory drugs (P-value < 0.001). Celecoxib effectively treated old patients with arthritis and was well-tolerated [8]. A total of 2,159 patients with colorectal adenomas received either celecoxib at the dose of 400 mg once-daily or 200 mg twice-daily or 400 mg twice-daily or placebo and treatments lasted 1 to 3 years. Celecoxib effectively and safely reduced the incidence of colorectal adenomas more effectively than placebo and was well-tolerated [9].
Prophylaxis with celecoxib
Only three studies have been reported on the prophylaxis with celecoxib. Heterotopic ossification is a potential complication that may have a particularly higher association with hip resurfacing. Of 198 patients, 83 patients (41.9%) received celecoxib and 115 patients (58.1%) did not. Prophylaxis with celecoxib reduced the rate of heterotopic ossification in 65.1% of patients and the reduction of the rate of heterotopic ossification was 25.0% in patients who did not receive celecoxib (P-value < 0.001). Prophylaxis with celecoxib is associated with decreased incidence and severity of heterotopic ossification after hip resurfacing [10]. Prophylaxis with celecoxib prevented the development of heterotopic ossification after total hip arthroplasty [11]. Of 480 patients with heterotopic ossification, 368 patients (76.7%) received prophylaxis with celecoxib and 112 patients (23.3%) did not receive any prophylaxis. Patients who received celecoxib had a reduction of heterotopic ossification more effectively than patients who did not receive celecoxib [12].
Treatment of patients with celecoxib
Eight studies have been reported on the treatment of patients with celecoxib. Celecoxib, administered at the daily dose of 200 mg to 400 mg, effectively treated patients with rheumatoid arthritis [13]. Celecoxib, administered at the dose of 100 mg, 200 mg, or 400 mg twice-daily, effectively reduced the signs and symptoms of rheumatoid arthritis and osteoarthritis [14]. A total of 697 patients with osteoarthritis of the knee or the hip received 200 mg of celecoxib in the morning and in the evening and this treatment relived the pain and improved the ability to walk [15]. Celecoxib, administered at the daily dose of 200 mg, effectively treated patients with musculoskeletal arthritis and this treatment was well-tolerated [16]. Two-hundred patients with pain and inflammation received celecoxib at the dose of 100 mg twice-daily. This treatment relieved pain, reduced the inflammation, was well-tolerated [17]. Thirty-six patients with low-back pain received celecoxib at the daily dose of 3 mg/kg to 6 mg/kg and pregabalin at the daily dose of 1 mg/kg in the first week and then at a daily dose of 2 mg/kg to 4 mg/kg. The combination of celecoxib with pregabalin relieved low-back pain more effectively than celecoxib or pregabalin alone [18]. Celecoxib, administered at the daily dose of 200 mg, effectively manages patients with atherosclerosis [19]. Celecoxib, administered at the daily dose of 100 mg, effectively treated patients with migraine [20].
Trials conducted with celecoxib
Six trials conducted with celecoxib have been reported. A multicentre, randomized, double-blind, placebo-controlled trial was conducted in 1,003 patients with symptomatic osteoarthritis of the knee who received either celecoxib at dose of 50 mg or 100 mg or 200 mg twice-daily or naproxen at the dose of 500 mg twice-daily or placebo and treatments lasted 12 weeks. Celecoxib led to significant improvement of osteoarthritis of the knee and relieved pain. All celecoxib doses were more efficacious than placebo although the dose of 50 mg twice-daily was minimally effective. The higher doses of celecoxib (100 mg and 200 mg twice-daily) were similarly efficacious and the magnitude of improvement observed with these doses of celecoxib was comparable to that seen with naproxen and treatments were well-tolerated [21]. A double-blind, crossover trial was conducted in 79 patients with osteoarthritis of the knee or the hip who received either celecoxib at daily dose of 200 mg or 400 mg or sustained-release paracetamol administered at the dose of 1,330 mg thrice-daily and the treatments lasted two weeks. Celecoxib treated osteoarthritis more effectively than paracetamol [22]. Two multicentre, randomized, double-blind, placebo-controlled, active-comparator trials were conducted in 179 Asian patients and in 150 non-Asian patients with osteoarthritis of the knee who received either celecoxib at the dose of 200 mg once-daily or naproxen at the dose of 500 mg twice-daily or ibuprofen at the dose of 800 mg thrice-times and treatments lasted six weeks. Celecoxib treated the osteoarthritis of the knee more effectively than naproxen and ibuprofen [23]. Two randomized, clinical trials were conducted in 342 patients who received tramadol at the dose of 50 mg or 100 mg 4 times-daily, in 181 patients who received celecoxib at the dose of 100 mg twice-daily, and in 172 patients who received placebo and all patients suffered from pain and treatments lasted six weeks. Tramadol and celecoxib relieved pain more effectively than placebo and celecoxib relieved pain more effectively than tramadol [24]. A randomized, clinical trial was conducted in 7,462 patients who received celecoxib at the daily dose of 200 mg to 800 mg, in 4,057 patients who received placebo, and in 13,990 patients who received nonsteroidal anti-inflammatory drugs such as diclofenac, ibuprofen, naproxen, ketoprofen, and loxoprofen. The rate of cardiovascular events was not significantly different in patients who received celecoxib, placebo, and other nonsteroidal anti-inflammatory drugs [25]. A clinical trial was conducted in 18,942 patients who received either celecoxib at the dose of 100 mg to 200 mg twice-daily or diclofenac at the dose of 50 mg to 75 mg twice-daily or ibuprofen at the dose of 800 mg thrice-daily or naproxen at the dose or 500 mg twice-daily or placebo. The cardiovascular thrombotic events were not different in patients who received celecoxib, diclofenac, ibuprofen, naproxen or placebo [26].
Metabolism of celecoxib
Siu et al. [27] studied the metabolism of celecoxib in human liver microsomes and stated that celecoxib is hydroxylated into 4-hydroxy-celecoxib by CYP2C9 and the hydroxylation of celecoxib by CYP2C9 was highly variable. Extensive metabolizers had the genotype CYP2C9*1*1 and slow metabolisers had the genotype CYP2C9*1*3 and CYP2C9*3*3.
Pharmacokinetics of celecoxib
Park et al. [28] studied the pharmacokinetics of celecoxib in 20 healthy subjects aged 26.0+4.1 years with a body weight of 71.9+9.0 kg and with a body-mass-index of 23.5+2.2 kg/m2 and celecoxib was administered orally at the dose of 200 mg once-daily. Ojeok-san is a frequently used herbal medication for the management osteoarthritis pain and was administered orally at the dose of 14.47 grams/pack thrice-daily. Plasma samples were collected before drug administration and on days 1 to 3 and 15 to 17 after drug administration. Genotype analysis was performed among the two most common decreased-function mutations of CYP2C9, CYP2C9*2 and CYP2C9*3. Table 1 summarizes the pharmacokinetic parameters of celecoxib in absence and in presence of Ojeok-san and according to the genotype of subjects included in the study.
Table 1: Pharmacokinetic parameters of celecoxib which have been obtained in absence and in presence of Ojeok-san and according to the genotype of subjects included in the study. Values are the mean+SD except for the Tmax which is reported as the median and (range), by Park et al. [28].
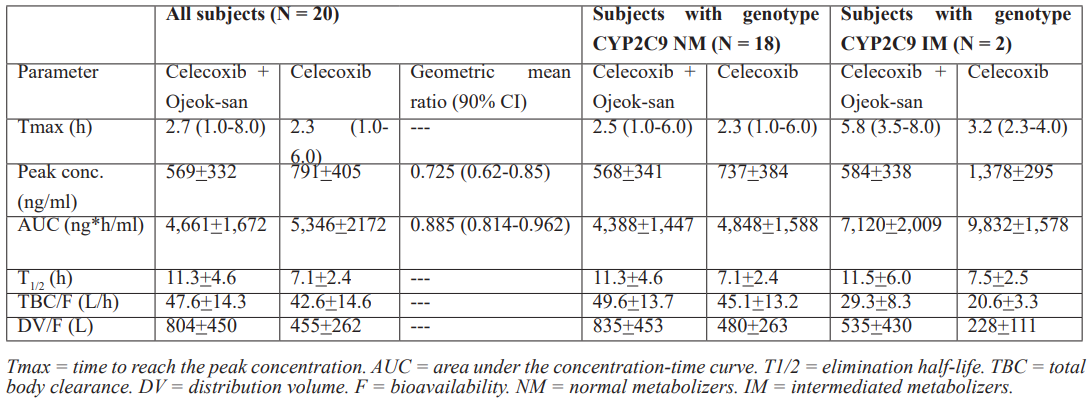
This table shows that Ojeok-san decreases the peak concentration and the area under the concentration-time of celecoxib. Ojeok-san prolongs the elimination half-life of celecoxib and celecoxib is rapidly eliminated as the elimination half-life of celecoxib is about 7 hours. Ojeok-san increases the distribution volume of celecoxib and celecoxib is distributed in a large volume as the distribution volume of celecoxib is about 800 liters. The peak concentration and the area under the concentration-time curve of celecoxib are higher in intermediate metabolizers than in normal metabolizers whereas the total body clearance and the distribution volume of celecoxib are lower in intermediate metabolizers than in normal metabolizers. In addition, there is a remarkable interindividual variability of the pharmacokinetic parameters of celecoxib and this variability is accounted by the vide variation of the vital data of the subjects included in the study.
Toxicity induced by celecoxib
Celecoxib is a safe drug and causes less toxicity than nonsteroidal anti-inflammatory drugs thus limited information is available about the toxicity induced by celecoxib. Celecoxib, administered at doses greater than those indicated clinically, was associated with a lower incidence of symptomatic ulcers and ulcer complications and caused fewer gastrointestinal adverse-effects than naproxen, ibuprofen, and aspirin administered at the standard doses [29]. Celecoxib, administered at therapeutic doses, was well-tolerated and caused less renal toxicity than nonsteroidal anti-inflammatory [30]. Celecoxib, administered at therapeutic doses, caused cardiovascular events as placebo and caused fewer cardiovascular adverse-effects than nonsteroidal anti-inflammatory drugs administered at clinically recommended doses [31]. In patients with symptomatic arthritis who had moderate-to-high risk of cardiovascular events naproxen and ibuprofen caused higher risks of cardiovascular toxicity than celecoxib [32].
Discussion
Celecoxib is used for the management of active pain and for the treatment of osteoarthritis, rheumatic arthritis, ankylosing spondylitis, juvenile rheumatoid arthritis, and primary dysmenorrhoea. The recommended dose for treating osteoarthritis is 200 mg daily and for treating the rheumatic arthritis the dose is 100 mg to 200 mg twice-daily. The peak concentration and the area under the concentration-time of celecoxib are 2-fold higher in elderlies than in younger patients [1]. The efficacy and safely of celecoxib have been reviewed. Celecoxib administered at the dose of 200 mg once-daily for six weeks to patients with osteoarthritis effectively and safely reliefs pain and improves walking [2], rofecoxib was administered at the daily dose of 12.5 mg and celecoxib was administered at the daily dose of 200 mg for six weeks and both drugs effectively and safely treat patients with osteoarthritis of the knee [3], patients with rheumatoid arthritis or with osteoarthritis of the knee or the hip received celecoxib at the daily dose of 200 mg and the treatment cures 69.3% of patients, 28.5% of patients do not respond to treatment, and only 2.1% of patients were aggravated after treatment thus celecoxib is an efficacious treatment of rheumatoid arthritis and osteoarthritis of the knee and the hip [4], patients with osteoarthritis of the hip received either celecoxib at the daily doses of 100 mg, 200 mg, or 400 mg or naproxen at the daily dose of 1,000 mg and treatments lasted 12 weeks. Celecoxib, administered at the daily doses of 200 mg and 400 mg, is efficacious as naproxen administered at the daily dose of 1,000 mg and both drugs are well-tolerated [5], patients with acute pharyngeal pain received celecoxib at the dose of 50 mg or 100 mg 4 times-daily and celecoxib effectively reliefs the pain and is well-tolerated [6], children undergoing adenotonsillectomy received either celecoxib at the daily dose of 0.15 mg/kg to 0.20 mg/kg or morphine at the daily dose of 0.20 mg/kg to 0.27 mg/kg and celecoxib reduces the pain intensity and the duration of hospital stay more effectively than morphine [7], old patients with arthritis received either celecoxib at the daily dose of 200 mg to 400 mg or traditional nonsteroidal anti-inflammatory drugs and treatments lasted 120 days. Celecoxib causes less gastrointestinal bleeding than traditional nonsteroidal anti-inflammatory drugs (P-value = 0.03) but causes higher cardiovascular and renal risks than traditional nonsteroidal anti-inflammatory drugs (P-value < 0.001). Celecoxib effectively treats old patients with arthritis and is well-tolerated [8], and patients with colorectal adenomas received either celecoxib at the dose of 400 mg once-daily or 200 mg twice-daily or 400 mg twice-daily or placebo for 1 to 3 years and celecoxib reduces the incidence of colorectal adenomas more effectively than placebo [9]. The prophylaxis with celecoxib has been reviewed. Prophylaxis with celecoxib reduces the rate of heterotic ossification in 65.1% of patients and is associated with decreased incidence and severity of heterotopic ossification after hip resurfacing [10], the prophylaxis with celecoxib prevents the development of heterotopic ossification after total hip arthroplasty [11], and the prophylaxis with celecoxib reduces heterotopic ossification in 76.7% of patients whereas the reduction of heterotopic ossification is 23.3% in patients who do not received celecoxib [12]. The treatment of patients with celecoxib has been reviewed. Celecoxib, administered at the daily dose of 200 mg to 400 mg, effectively treats patients with rheumatoid arthritis [13], celecoxib, administered at the dose of 100 mg, 200 mg, or 400 mg twice-daily, effectively treats rheumatoid arthritis and osteoarthritis [14], patients with osteoarthritis of the knee or the hip received 200 mg of celecoxib in the morning and in the evening and this treatment reliefs the pain and improves the ability to walk [15], celecoxib, administered at the daily dose of 200 mg, effectively treats patients with musculoskeletal arthritis and this treatment is well-tolerated [16], celecoxib, administered at the dose of 100 mg twice-daily, reliefs pain and inflammation and is well-tolerated [17], patients with low-back pain received celecoxib at the daily dose of 3 mg/kg to 6 mg/kg and pregabalin at the daily dose of 1 mg/kg in the first week and then at a daily dose of 2 mg/kg to 4 mg/kg. The combination of celecoxib with pregabalin reliefs pain more effectively than celecoxib or pregabalin alone [18], celecoxib, administered at the daily dose of 200 mg, effectively manages atherosclerosis [19], and celecoxib, administered at the daily dose of 100 mg, effectively treats migraine [20]. The trials conducted with celecoxib have been reviewed. A multicentre, randomized, double-blind, placebo-controlled trial was conducted in patients with symptomatic osteoarthritis of the knee who received either celecoxib at the dose of 50 mg or 100 mg or 200 mg twice-daily or naproxen at the dose of 500 mg twice-daily or placebo and treatments lasted 12 weeks. All celecoxib doses are more efficacious than placebo although the dose of 50 mg twice-daily is minimal effective. The celecoxib doses of 100 mg and 200 mg twice-daily are similarly efficacious and treats the osteoarthritis of the knee as naproxen and treatments are well-tolerated [21], a double-blind, crossover trial was conducted in patients with osteoarthritis of the knee or the hip who received either celecoxib at the daily dose of 200 mg or 400 mg or sustained-release paracetamol administered at the dose of 1,330 mg thrice-daily, treatments lasted two weeks, and celecoxib treats the osteoarthritis more effectively than paracetamol [22], two multicentre, randomized, double-blind, placebo-controlled, active-comparator trials were conducted in 179 Asian patients and in 150 non-Asian patients with osteoarthritis of the knee who received either celecoxib at the dose of 200 mg once-daily or naproxen at the dose of 500 mg twice-daily or ibuprofen at the dose of 800 mg thrice-daily, treatments lasted six weeks, and celecoxib treats the osteoarthritis of the knee more effectively than naproxen and ibuprofen [23], two randomized, clinical trials were conducted in patients suffering from pain who received either tramadol at the dose of 50 mg or 100 mg 4 times-daily or celecoxib at the dose of 100 mg twice-daily or placebo, treatments lasted six weeks, tramadol and celecoxib relief pain more effectively than placebo, and celecoxib reliefs pain more effectively than tramadol [24], a randomized, clinical trial was conducted in patients who received either celecoxib at the daily dose of 200 mg to 800 mg or placebo or nonsteroidal anti-inflammatory drugs such as diclofenac, ibuprofen, naproxen, or loxoprofen and the rate of cardiovascular events is not different in patients who received celecoxib, placebo, and nonsteroidal anti-inflammatory drugs [25], and a clinical trial was conducted in patients who received either celecoxib at the dose of 100 mg to 200 mg twice-daily or diclofenac at the dose of 50 mg to 75 mg twice-daily or ibuprofen at the dose of 800 mg thrice-daily or naproxen at the dose of 500 mg twice-daily or placebo and the cardiovascular thrombotic events are not different in patients who received celecoxib, diclofenac, ibuprofen, naproxen, or placebo [26]. The metabolism of celecoxib was studied in human liver microsomes and celecoxib is hydroxylated into 4-hydroxy-celecoxib by CYP2C9. Extensive metabolizers have the genotype CYP2C9*1*1 and slow metabolizers have the genotype CYP2C9*1*3 and CYP2C9*3*3 [27]. Park et al. [28] studied the pharmacokinetics of celecoxib in healthy subjects who received Ojeok-san and according to CYP2C9 genotype. In absence of Ojeok-san, the elimination half-life of celecoxib is about 7 hours and Ojeok-san prolongs the elimination half-life of celecoxib to about 11 hours. The peak concentration and the area under the concentration-time curve of celecoxib are higher in subjects who are CYP2C9 intermediate metabolizers than in subjects who are CYP2C9 normal metabolizers. The toxicity induced by celecoxib has been reviewed. Celecoxib is a safe drug and causes less toxicity than nonsteroidal anti-inflammatory drugs. Celecoxib, administered at doses greater than those indicated clinically, is associated with a lower incidence of ulcers and ulcer complications and causes fewer gastrointestinal adverse-effects than naproxen, ibuprofen, and aspirin administered at the standard doses [29], celecoxib administered at therapeutic doses is well-tolerated and causes less renal toxicity than nonsteroidal anti-inflammatory drugs [30], celecoxib, administered at therapeutic doses is well-tolerated and causes cardiovascular events as placebo and causes fewer cardiovascular adverse-effects than nonsteroidal anti-inflammatory drugs administered at clinically recommended doses [31], and in patients with symptomatic arthritis, who had moderate-to-high risk of cardiovascular events, naproxen and ibuprofen cause higher risks of cardiovascular toxicity than celecoxib [32].
Conclusion
Celecoxib is used for the treatment of active pain, osteoarthritis, rheumatic arthritis, ankylosing spondylitis, juvenile rheumatic arthritis, and primary dysmenorrhoea. The recommended dose of celecoxib for treating osteoarthritis is 200 mg daily and for treating rheumatoid arthritis the recommended dose of celecoxib is 100 mg to 200 mg twice-daily. Elderlies may have up to 2-fold higher peak concentrations and area under the concentration-time curve values of celecoxib than younger patients. Celecoxib confers a risk of myocardial infarction and myocardial stroke and this appears to be related to the dose and the underlying risk of cardiovascular disease. Chronic use of celecoxib may decrease bone mineral density particularly in the older male patients. The efficacy and safely of celecoxib, the prophylaxis with celecoxib, the treatment of patients with celecoxib, and the trials conducted with celecoxib have been reviewed. Celecoxib is hydroxylated into 4-hydroxy-celecoxib by CYP2C9, extensive metabolizers have the genotype CYP2C9*1*1 and slow metabolizers have the genotype CYP2C9*1*3 and CYP2C9*3*3. The pharmacokinetics of celecoxib have been studied in healthy subjects in absence and in presence of Ojeok-san and according to CYP2C9 genotype. In absence of Ojeok-san, the elimination half-life of celecoxib is about 7 hours and Ojeok-san prolongs the elimination half-life of celecoxib to about 11 hours. The peak concentration and the area under the concentration-time curve of celecoxib are longer in subjects who are CYP2C9 intermediate metabolizers than in subjects who are CYP2C9 normal metabolizers. Celecoxib is a safe drug and induces less gastrointestinal, renal, and myocardial adverse-effects than nonsteroidal anti-inflammatory drugs. The aim of this study is to review the clinical pharmacology of celecoxib.
Conflict of interests: The authors declare no conflicts of financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employments, gifts, and honoraria.
This article is a review and drugs have not been administered to men or animals.
Acknowledgments: The author thanks Dr. Patrizia Ciucci and Dr. Francesco Varricchio, of the Medical Library of the University of Pisa, for retrieving the scientific literature.
References
- Grosser T, Ricciotti E, FitzGerald GA. Pharmacotherapy of inflammation, Fever, Pain, and Gout, In Goodman@Gilman’s. The Pharmacological Basis of Therapeutics. Brunton LL, Knollmann BC editors. Mc Graw Hill. 14th Edition, 2023; pp. 829-856.
- Xu C, Gu K, Yasen Y, Hou Y. Efficacy and Safety of Celecoxib Therapy in Osteoarthritis. Medicine (Baltimore), 2016; 95(20): e3585-3595.
- Birbara C, Ruoff G, Sheldon E, Valenzuela C, Rodgers A, Petruschke RA, et al. Efficacy and safety of rofecoxib 12.5 mg and celecoxib 200 mg in two similarly designed osteoarthritis studies. Curr Med Res Opin, 2006; 22(1): 199-210.
- Seong S-S, Park Y-B, Uhm W-S, Lee J, Ji JD, Bae S-C, et al. Multicenter Study in Efficacy and Safety of Celecoxib in Patients with Rheumatoid Arthritis and Osteoarthritis. J Korean Rheum Assoc, 2006; 13(3): 209-217.
- Kivitz AJ, Moskowitz RW, Woods E, Hubbard RC, Verburg KM, Lefkowith JB, et al. Comparative efficacy and safety of celecoxib and naproxen in the treatment of osteoarthritis of the hip. Int Med Res, 2001; 29(6): 467-479.
- Schachtel BP, McCabe D, Berger M, Zhang R, Sanner KM, Savino L, et al. Efficacy of low-dose celecoxib in patients with acute pain. J Pain, 2011; 12(7): 756-763.
- Allard A, Valois-Demers J, Pellerin A, Leclerc JE, Cloutier K. Evaluation of Postoperative Efficacy and Safety of Celecoxib in Children Hospitalized After Adenotonsillectomy. J Pediatr Pharmacol Ther, 2024; 29(3): 255-265.
- Shin S. Safety of celecoxib versus traditional nonsteroidal anti-inflammatory drugs in older patients with arthritis. J Pain Res, 2018; 11(12): 3211-3219.
- Veettil SK, Nathisuwan S, Ching SM, Jinatongthai P, Lim KG, Kew ST, et al. Efficacy and safety of celecoxib on the incidence of recurrent colorectal adenomas: a systematic review and meta-analysis. Cancer Manag Res, 2019; 11(1); 561-571.
- Ng M, Brigati D, Wagner TC, Bigart K, Anton Khlopas A, Sultan AA, et al. Prophylactic Celecoxib Administration Is Associated with Decreased Incidence and Severity of Heterotopic Ossification After Hip Resurfacing by Direct Lateral Approach in Male Patients. Orthopedics, 2018; 41(6): e807-812.
- Cicirello M, Colombero D, Aprato A, Capella M. Prophylaxis of heterotopic ossification after hip surgery: Our experience with celecoxib and review of literature. Minerva Ortopedica Traumatologica, 2011; 62(4): 253-261.
- Barbato M, D'Angelo E, Di Loreto G, Menna A, Di Francesco A, Salini V, et al. Adherence to routine use of pharmacological prophylaxis of heterotopic ossification after total hip arthroplasty: results from an Italian multicenter, prospective, observational survey. J Orthop Traumatol, 2012; 13(2): 63-67.
- Sanchack K, Seales S, Seales P. Effectiveness and Safety of Celecoxib for the Treatment of Rheumatoid Arthritis. Am Fam Physician, 2018; 97(9): 573-574.
- Goldenberg MM. Celecoxib, a selective cyclooxygenase-2 inhibitor for the treatment of rheumatoid arthritis and osteoarthritis. Clin Ther, 1999; 21(9): 1497-1513.
- Stengaard-Pedersen K, Ekesbo R, Karvonen A-L, Lyster M. Celecoxib 200 mg q.d. is efficacious in the management of osteoarthritis of the knee or hip regardless of the time of dosing. Rheumatology (Oxford), 2004; 43(5): 592-595.
- Krasselt M, Baerwald C. Celecoxib for the treatment of musculoskeletal arthritis. Expert Opin Pharmacother, 2019; 20(14): 1689-1702.
- Tindall E. Celecoxib for the treatment of pain and inflammation: the preclinical and clinical results. J Am Osteopath Assoc, 1999: 99(Suppl 11): S13-17.
- Romanò CL, Romanò D, Bonora C, Mineo G. Pregabalin, celecoxib, and their combination for treatment of chronic low-back pain. J Orthop Traumatol, 2009; 10(4): 185-191.
- Papageorgiou N, Zacharia E, Briasoulis A, Charakida M, Tousoulis D. Celecoxib for the treatment of atherosclerosis. Expert Opin Investig Drugs, 2016; 25(5): 619-633.
- Ailani J, Nahas SJ, Friedman DI, Kunkel T. The Safety of Celecoxib as an Acute Treatment for Migraine: A Narrative Review. Pain Ther, 2023; 12(3): 655-669.
- Bensen WG, FiechtnerJJ, McMillen JI, Zhao WW, Yu SS, Woods EM, et al. Treatment of Osteoarthritis with Celecoxib, a Cyclooxygenase-2 Inhibitor: A Randomized Controlled Trial. Mayo Clin Prooc, 1999; 74(11): 1095-1105.
- Yelland MJ, Nikles CJ, McNairn N, Del Mar CB, Schluter PJ, Brown RM. Celecoxib compared with sustained-release paracetamol for osteoarthritis: a series of n-of-1 trials. Rheumatology (Oxford), 2007; 46(1): 135-140.
- Lubis A, Wang W, Lima G, Fayyad R, Walker C. Comparing the Safety and Efficacy of Celecoxib for the Treatment of Osteoarthritis in Asian and non-Asian Populations: An Analysis of Data from Two Randomized, Double-blind, Placebo-controlled, Active-comparator Trials. Pain Ther, 2017; 6(2): 235-242.
- Langford R, Viscusi ER, Morte A, Cebrecos J, Sust M, Giménez-Arnau JM, et al. Efficacy of Co-Crystal of Tramadol-Celecoxib (CTC) in Patients with Acute Moderate-to-Severe Pain: A Pooled Analysis of Data from Two Phase 3 Randomized Clinical Trials. Drugs, 2024; 24(2): 239-252.
- White WB, West CR, Borer JS, Gorelick PB, Lavange L, Pan SX, et al. Risk of cardiovascular events in patients receiving celecoxib: a meta-analysis of randomized clinical trials. Am J Cardiol, 2007; 99(1): 91-98.
- White WB, Faich G, Borer JS, Makuch RW. Cardiovascular thrombotic events in arthritis trials of the cyclooxygenase-2 inhibitor celecoxib. Am J Cardiol, 2003; 92(4): 411-417.
- Siu YA, Hao M-H, Dixit V, Lai WG. Celecoxib is a substrate of CYP2D6: Impact on celecoxib metabolism in individuals with CYP2C9*3 variants. Drug Metab Pharmacokinet, 2018; 33(5): 219-227.
- Park S-I, Park J-Y, Park M-J, Yim S-V, Kim B-H. Effects of Ojeok-san on the Pharmacokinetics of Celecoxib at Steady-state in Healthy Volunteers. Basic Clin Pharmacol Toxicol, 2018; 123(1): 51-57.
- Silverstein FE, Faich G, Goldstein JL, Simon LS, Pincus T, Whelton A, et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: A randomized controlled trial. Celecoxib Long-term Arthritis Safety Study. JAMA, 2000; 284(10): 1247-1255.
- Whelton A, Maurath CJ, Verburg KM, Geis GS. Renal safety and tolerability of celecoxib, a novel cyclooxygenase-2 inhibitor. Am J Ther, 2000; 7(3): 159-175.
- White WB, West CR, Borer JS, Gorelick PB, Lavange L, Pan SX, et al. Risk of cardiovascular events in patients receiving celecoxib: a meta-analysis of randomized clinical trials. Am J Cardiol, 2007; 99(1): 91-98.
- Solomon DH, Husni ME, Libby PA, Yeomans ND, Lincoff AM, Lϋscher TF, et al. The Risk of Major NSAID Toxicity with Celecoxib, Ibuprofen, or Naproxen: A Secondary Analysis of the PRECISION Trial. Am J Med, 2017; 130(12): 1415-1422.

