Morphological Spectrum of Renal Allograft Dysfunction: An Institutional Study
Azra Bashir*, Saira Javeed, Sohaib Khalid, Faria Waqar Khan, Zubaria Rafiq and Aribah Atiq
Chughtai Institute of Pathology, Lahore, Pakistan
Received Date: 25/09/2024; Published Date: 05/11/2024
*Corresponding author: Azra Bashir, Chughtai Institute of Pathology, Lahore, Pakistan
Abstract
Objective: Renal transplantation is a critical treatment for end-stage kidney disease, significantly improving patient survival and quality of life. However, graft dysfunction remains a major challenge, with various etiologies contributing to the decline in graft function. The study aims to analyze the morphological spectrum of renal allograft dysfunction through histopathological evaluation in a single centre.
Design: It is a cross-sectional study
Place & Duration of study: We conducted a study of 50 ultrasound-guided core biopsies from live-related renal transplant patients, reported using the Banff classification (2019) from 2019 to 2023 in Chughtai institute of pathology, Lahore.
Methodology: Clinical and histopathological data, including light microscopy, immunofluorescence, and immunohistochemistry findings, were reviewed. Statistical analyses expressed as mean ± SD, frequency and pearson chi square test were applied where applicable.
Results: Among 50 patients, 38 (76%) were males and 12 (24%) females, aged 21 to 63 years. Chronic cellular rejection (IFTA Grade I) was the most common cause of graft dysfunction, observed in 24% of cases. Chronic rejection in various grades (IFTA Grade II and III) accounted for 34%, while acute T cell-mediated rejection (TCMR) and acute antibody-mediated rejection (ABMR) were each found in 6% of cases. Other etiologies included cyclosporine toxicity, diabetic nephropathy, membranous glomerulonephritis, and severe acute tubulointerstitial inflammation. Notably, 42% of the rejections occurred in more than 36 months post-transplantation.
Conclusion: Chronic rejection, particularly chronic cellular rejection, is the leading cause of renal allograft dysfunction. The high incidence of late-onset rejection emphasizes the need for prolonged monitoring and possibly more aggressive immunosuppressive strategies. Histopathological evaluation remains crucial for accurate diagnosis and management of graft dysfunction, guiding therapeutic decisions and improving patient outcomes. Further research is needed to explore molecular and genetic factors, develop non-invasive biomarkers, and evaluate novel immunosuppressive therapies to enhance graft survival and patient quality of life.
Keywords: Renal transplantation; Graft dysfunction; Chronic rejection; Histopathology; Banff classification; Immunosuppressive therapy
Introduction
Renal transplantation, also known as kidney transplantation, is a medical procedure that has significantly improved the lives of many individuals suffering from Chronic Kidney Disease (CKD). It is considered the best treatment for end-stage kidney disease. The survival rate for renal transplant patients has increased due to the availability of immunosuppressive drugs, with a one-year survival rate of nearly 90% [1,2]. However, a major challenge in transplantation is the risk of rejection, which can occur in two main forms: acute rejection and chronic rejection. Both types can be Antibody-Mediated (ABMR) or T cell-mediated (TCMR). Other causes of rejection include de novo glomerulonephritis and thrombotic microangiopathies. Histopathological evaluation is essential in diagnosing and characterizing renal transplant rejection, as it provides crucial information for guiding treatment options, monitoring patient progress, and improving graft survival [3,4].
The histopathological features of rejection are defined by the Banff classification, established by international consensus in 1991. According to the Banff classification, there are acute, chronic, and borderline cases of rejection. For TCMR, interstitial inflammation with tubulitis is critical and should be observed in non-scarred cortex. Tubulitis, along with intimal arteritis, indicates acute rejection [5,6]. Drugs such as calcineurin inhibitors can also cause renal toxicity and graft rejection. Glomerulonephritis, such as focal segmental glomerulosclerosis and membranous glomerulonephritis, can arise de novo in the renal graft. Light microscopy, immunofluorescence, and immunohistochemistry are required to diagnose graft rejection accurately.
Materials and Methods
A total of 50 ultrasound-guided core biopsies from live-related renal transplant cases, reported using the Banff classification (2019), from 2019 to 2023 were retrieved and reviewed. Approval for this study was obtained from the Institutional Review Board prior to its commencement. Cases with a history of transplant, information on the original disease, latest lab results, and complete histopathological, IHC, and IF data were included in the study. Cases unrelated to transplant or with incomplete clinical and histopathological information were excluded.
Slides of formalin-fixed paraffin-embedded renal tissue blocks (with a maximum thickness of 3–4 microns) were reviewed. For light microscopy, hematoxylin and eosin, along with Jones methenamine (JMS), periodic acid-Schiff (PAS), and trichrome special stains, were used. These special stains (JMS, PAS, and Trichrome) from Biognost were performed using the manual method. Immunohistochemical stains (IHC) such as C4d and SV40 were performed on the auto stainer Link 48 by Dako, with retrieval time of 20 minutes for C4d and 30 minutes for SV40. The immunofluorescence (IF) images were reviewed of individual cases.
Relevant clinical history for each case, including history, age, sex, time since transplant, pre-biopsy renal function tests, urine complete examination, and serological parameters, was obtained from the lab's electronic medical record and through telephonic calls. The mean duration for the development of each pathology was estimated by calculating the time between the transplant and biopsy.
Statistical analysis was performed using SPSS version 21. Data was expressed as mean ± SD, frequency and pearson chi square test was applied where applicable.
Results
In present study majority of patients were male 38 (76.0%) and there were 12 females (24.0%). The minimum age was 21 years while maximum age was 63 years with mean 37.28 ±11.65 standard deviation of all the deranged renal profiles, only serum creatinine was used as a parameter to assess transplant rejection. Its ranges from 1.40 to 8.40 with the mean of 4.36 ± 1.82 standard deviation. The maximum number of patients in which rejection was noticed belongs to 31-40 years.
Out of 50 cases in 21 cases (42.0%) the rejection time was >36 months. The frequency of transplant rejection has been calculated in months.
The data was then analyzed for pattern of diseases in which rejection was observed. The maximum number of patients in which rejection was observed was diagnosed as Chronic Cellular Rejection (IFTA Grade I) followed by Chronic Cellular Rejection (IFTA Grade II). Overall IFTA was found in 29 (58%) cases and ABMR was found in 3 (6%) cases (Table 1). Rest of the cases showed de novo glomerulonephritis, calcineurin toxicity and acute tubular injury The frequency distribution of different etiologies involved in graft rejection in our study is shown in table 1.
Table 1: The frequency of distribution of different etiologies involved in graft rejection (n=50).
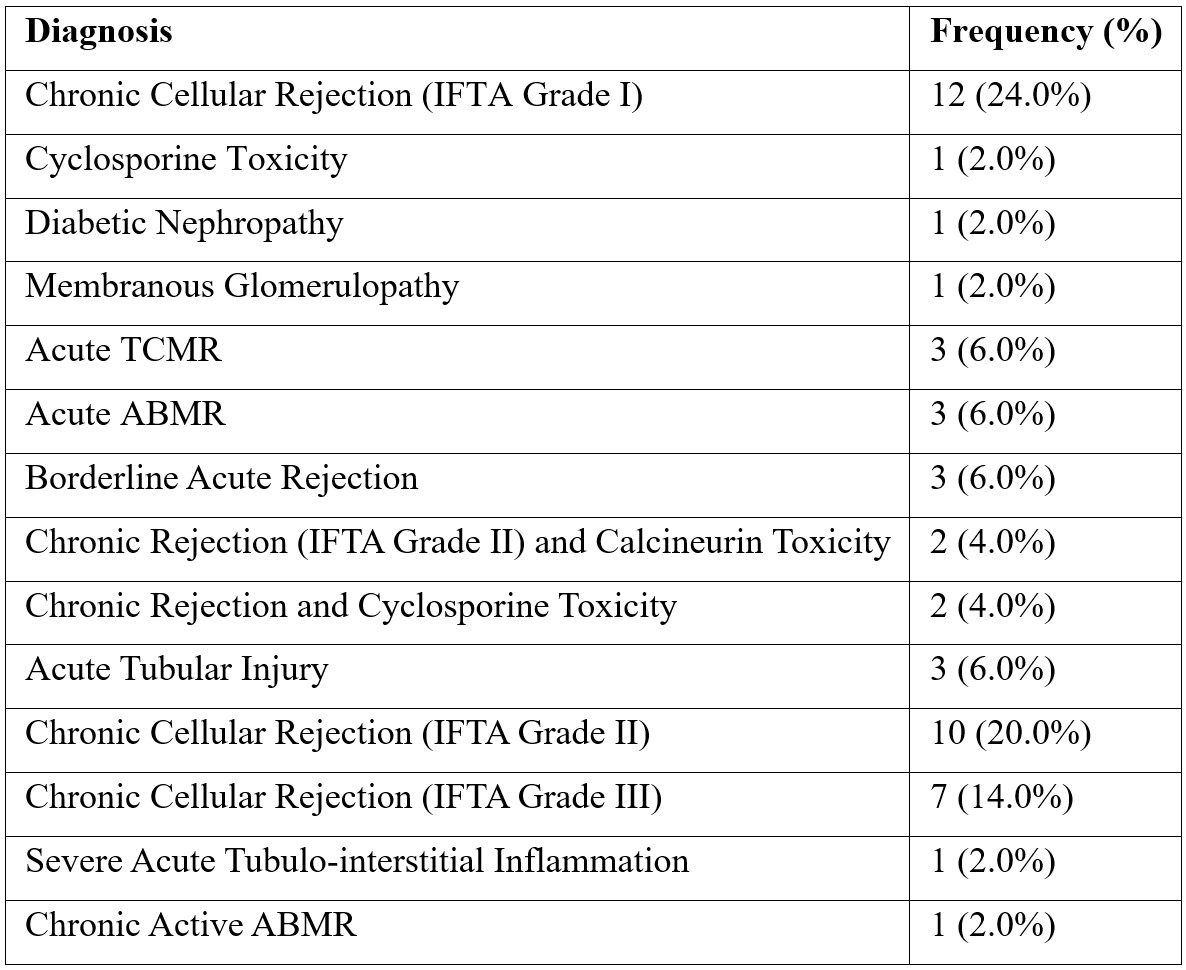
We have observed the frequency of correlation between different age groups and time of rejection (Table 2). Of 50 cases maximum rejection was seen in patient between age 31-40 years and time duration of >36 months however this was statically insignificant (pearson chi square test 0.275).
Table 2: The frequency correlation of age group distribution and time of rejection among patients (n=50).
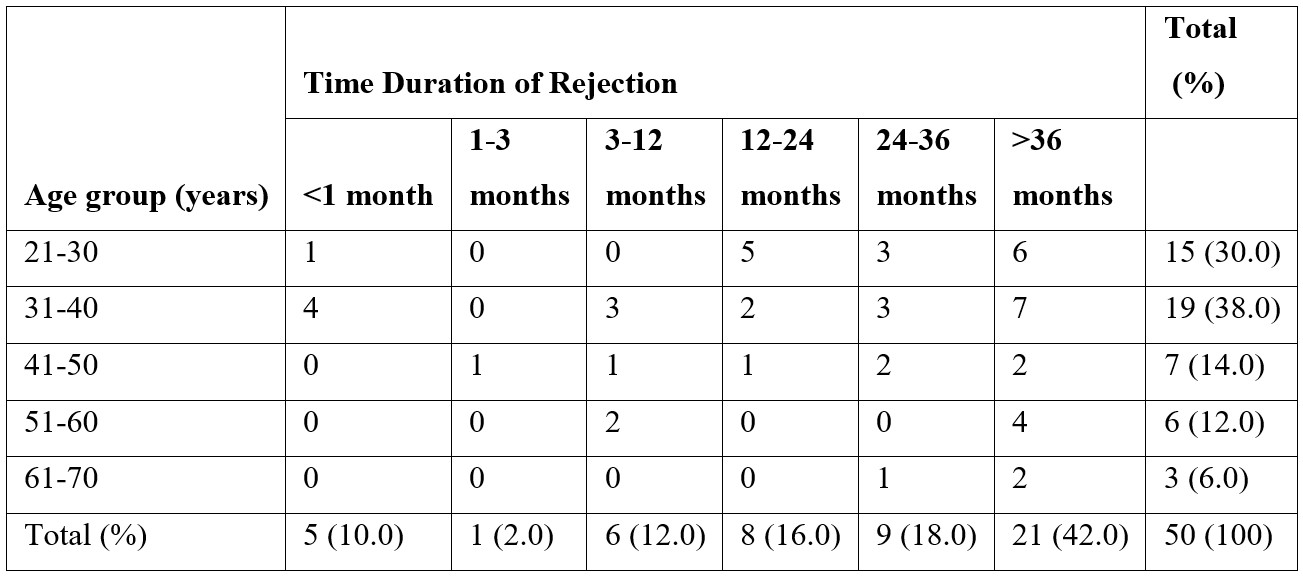
We then calculated the frequency of correlation among various diseases in which rejection was observed with the time of rejection (Table 3). Of 50 cases maximum rejection was seen in chronic cellular rejection (IFTA Grade I) and time duration of >36 months however this was also statically insignificant (pearson chi square test 0.000).
Table 3: The frequency correlation among different etiologies and time of rejection (n=50).
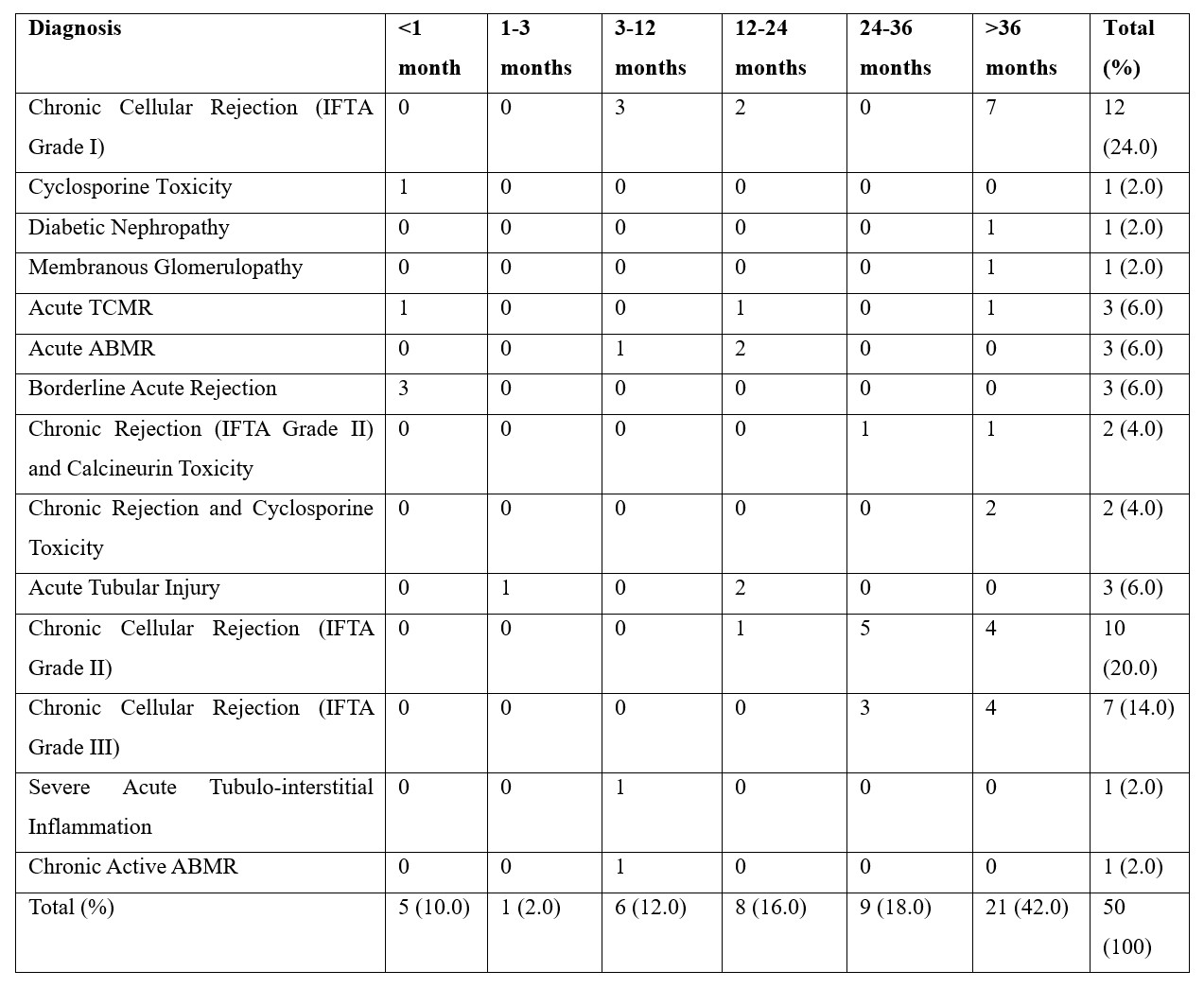
We then calculated the frequency of correlation among various diseases in which rejection was observed with the age of patients (Table 4). Of 50 cases maximum rejection was seen in chronic cellular rejection (IFTA Grade I) and age group between 31-40 years however this was also statically insignificant (pearson chi square test 0.070).
Table 4: The frequency correlation among different etiologies and age group distribution (n=50).
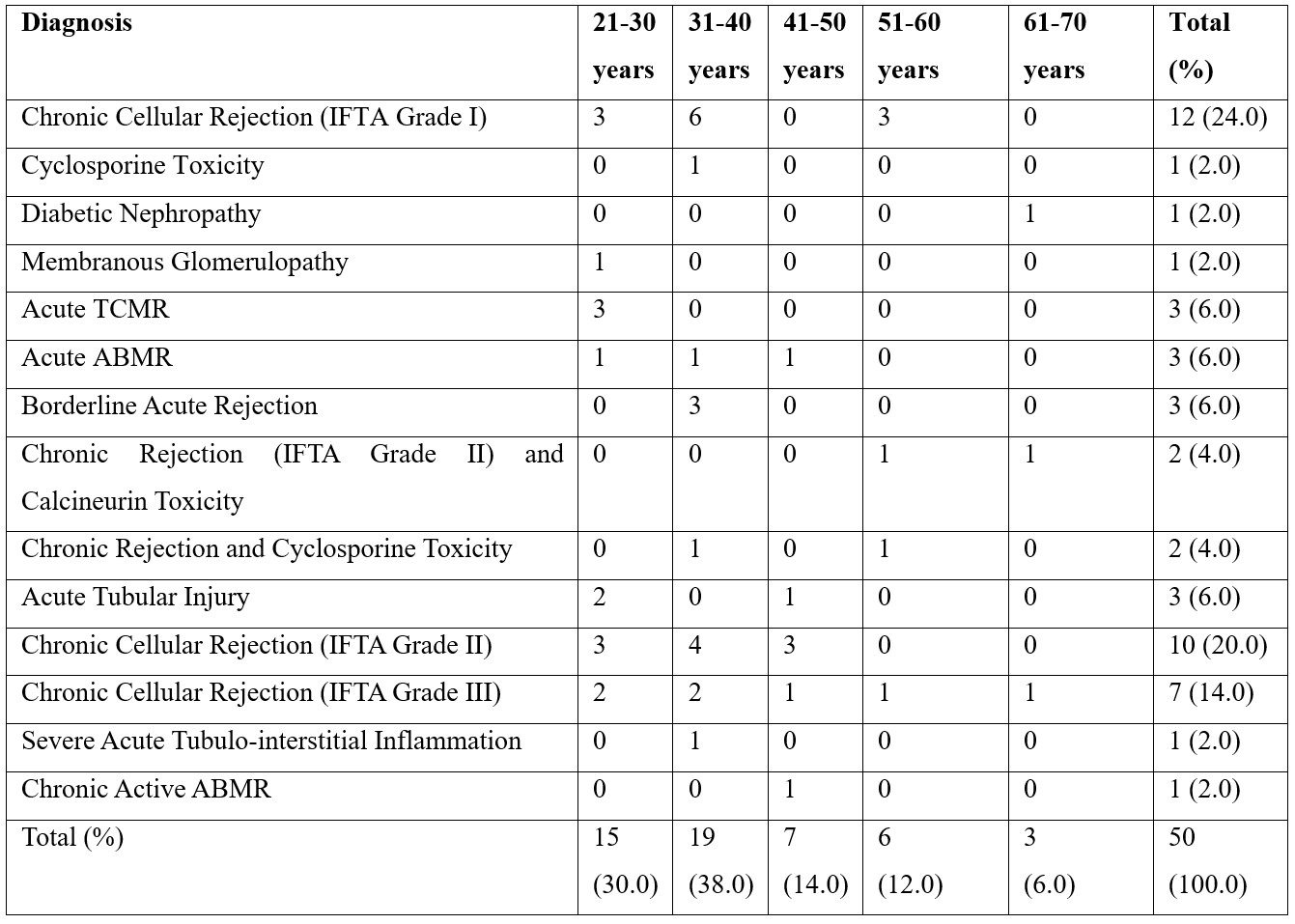
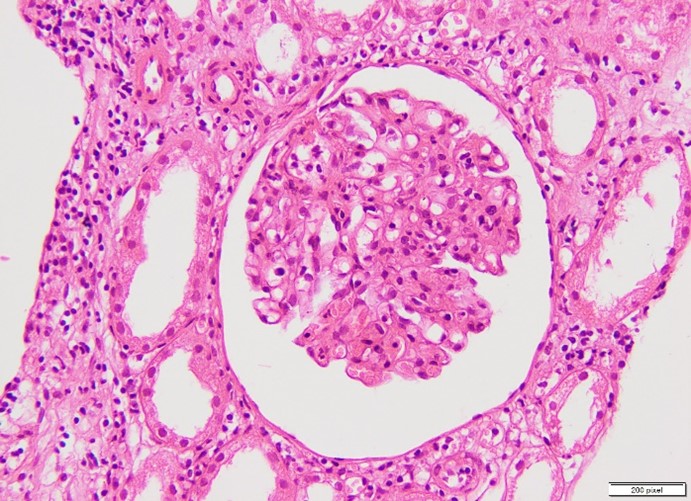
Figure 1: Glomerulitis.
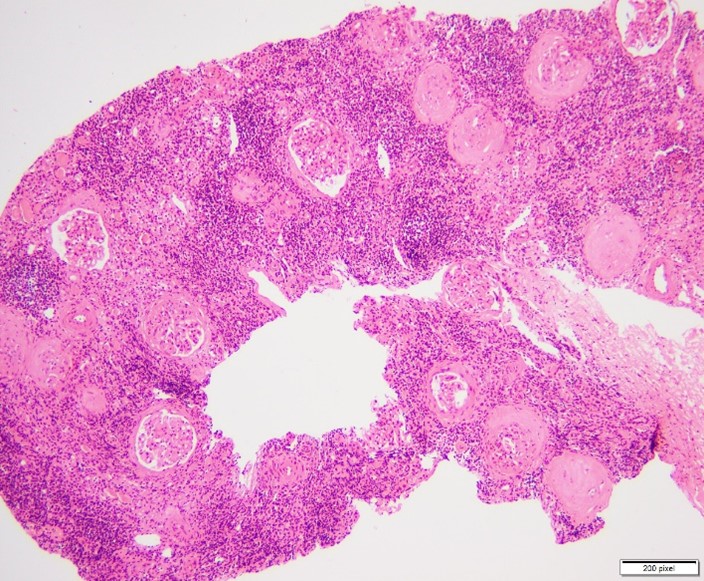
Figure 2: Chronic T-cell mediated rejection.
Discussion
In this study, we investigated the morphological spectrum of renal allograft dysfunction in 50 patients who underwent renal transplantation. Our findings revealed that chronic cellular rejection (IFTA Grade I) was the most common cause of graft dysfunction, observed in 24% of cases (Table 3). Chronic rejection in various grades (IFTA Grade II and III) accounted for a significant proportion (34%), followed by acute T cell-mediated rejection (TCMR) and acute antibody-mediated rejection (ABMR), each observed in 6% of cases (Figure 1, 2). Additionally, we identified cases of cyclosporine toxicity, diabetic nephropathy, membranous glomerulonephritis, and severe acute tubulointerstitial inflammation. Notably, 42% of the rejections occurred more than 36 months post-transplantation.
Our findings align with previous studies, which have also identified chronic rejection as a predominant cause of renal allograft dysfunction. For instance, a study by Bashir et al. (2020) reported a high prevalence of chronic rejection among renal transplant patients [1]. Similarly, the International Journal of Nephrology highlighted chronic rejection and its impact on long-term graft survival [1]. However, our study observed a lower incidence of ABMR compared to some reports, such as the one by Tawhari et al. (2022), which may be attributed to differences in patient demographics and immunosuppressive protocols [3].
Discrepancies in the prevalence of cyclosporine toxicity and diabetic nephropathy compared to other studies might be due to varying diagnostic criteria and patient management strategies. For example, Yunus et al. (2012) reported a higher incidence of post-transplant complications in regions with limited healthcare resources [7].
The clinical significance of our findings lies in the identification of chronic rejection as the primary cause of renal allograft dysfunction, emphasizing the need for early detection and management. The high prevalence of chronic cellular rejection suggests that closer monitoring and potentially more aggressive immunosuppressive strategies may be required to improve graft survival [8,9]. Additionally, the occurrence of rejection beyond 36 months post-transplantation underscores the necessity for long-term follow-up and maintenance therapy [10,11]
Our study also highlights the importance of histopathological evaluation in diagnosing and characterizing graft dysfunction, guiding therapeutic decisions, and improving patient outcomes. The observed patterns can inform clinicians about the potential risks and aid in developing tailored treatment plans for renal transplant recipients [12,13].
One of the strengths of our study is the comprehensive histopathological analysis using standardized techniques, such as light microscopy, immunofluorescence, and immunohistochemistry, following the Banff classification. This allowed for a detailed and accurate assessment of graft dysfunction causes [14-17,20] Additionally, the inclusion of a diverse patient population enhances the generalizability of our findings.
However, our study has several limitations. The retrospective design may introduce selection bias, and the relatively small sample size limits the statistical power of our conclusions. Furthermore, the lack of longitudinal data on patient outcomes post-diagnosis restricts our ability to assess the long-term impact of the identified causes of graft dysfunction [18,19]. Potential confounding factors, such as variations in immunosuppressive regimens and patient adherence, were not controlled, which could influence the results.
Future research should focus on larger, prospective studies to validate our findings and explore the long-term outcomes of patients with different types of graft dysfunction. Investigating the molecular and genetic factors underlying chronic rejection and other causes of graft dysfunction could provide insights into personalized treatment approaches.
Moreover, studies examining the efficacy of novel immunosuppressive therapies and their impact on graft survival and patient quality of life are warranted. Developing non-invasive biomarkers for early detection of rejection could revolutionize the management of renal transplant recipients, reducing the need for invasive biopsies.
Conclusion
Our study contributes to the understanding of the morphological spectrum of renal allograft dysfunction, highlighting the predominance of chronic rejection and the importance of histopathological evaluation. These findings have significant implications for clinical practice and future research, aiming to enhance the outcomes of renal transplantation.
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- Bashir S, Hussain M, Ali Khan A, Hassan U, Mushtaq KS, Hameed M, et al. Renal transplant pathology: demographic features and histopathological analysis of the causes of graft dysfunction. International Journal of Nephrology, 2020; 2020(1): 7289701.
- Jun H, Hwang JW. The most influential articles on kidney transplantation: A PRISMA-compliant bibliometric and visualized analysis.Medicine (Baltimore), 2022; 101(3): e28614. https://doi.org/10.1097/MD.0000000000028614.
- Tawhari M, Alhamadh MS, Alhabeeb A, Almutlaq M, Radwi M. Renal Transplant Experience in a Tertiary Care Center in Saudi Arabia: A Retrospective Cohort Study. Cureus, 2022; 14(3): e22974. https://doi.org/10.7759/cureus.22974.
- Rizvi SA, Naqvi SA, Zafar MN, Akhtar SF. A kidney transplantation model in a low-resource country: an experience from Pakistan.Kidney International Supplements, 2013; 3(2): 236-240. https://doi.org/10.1038/kisup.2013.11.
- Solez KI, Axelsen RA, Benediktsson H, Burdick JF, Cohen AH, Colvin RB, et al. International standardization of criteria for the histologic diagnosis of renal allograft rejection: the Banff working classification of kidney transplant pathology. Kidney international, 1993; 44(2): 411-422.
- Kim M, Martin ST, Townsend KR, Gabardi S. Antibody‐mediated rejection in kidney transplantation: a review of pathophysiology, diagnosis, and treatment options. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy, 2014; 34(7): 733-744.
- Yunus M, Aziz T, Mubarak M. Posttransplant malignancies in renal transplant recipients: 22-years experience from a single center in Pakistan. Asian Pacific Journal of Cancer Prevention, 2012; 13(2): 575-578.
- Abbas F, El Kossi M, Jin JK, Sharma A, Halawa A. De novo glomerular diseases after renal transplantation: How is it different from recurrent glomerular diseases? World Journal of Transplantation, 2017; 7(6): 285.
- Colvin RB. Antibody-mediated renal allograft rejection: diagnosis and pathogenesis. Journal of the American Society of Nephrology, 2007; 18(4): 1046-1056.
- Loupy A, Haas M, Solez K, Racusen L, Glotz D, Seron D, et al. The Banff 2015 kidney meeting report: current challenges in rejection classification and prospects for adopting molecular pathology. American journal of transplantation, 2017; 17(1): 28-41.
- Sapir-Pichhadze R, Curran SP, John R, Tricco AC, Uleryk E, Laupacis A, et al. A systematic review of the role of C4d in the diagnosis of acute antibody-mediated rejection. Kidney international, 2015; 87(1): 182-194.
- De Serres SA, Noël R, Côté I, Lapointe I, Wagner E, Riopel J, et al. 2013 Banff criteria for chronic active antibody-mediated rejection: assessment in a real-life setting. American Journal of Transplantation, 2016; 16(5): 1516-1525.
- Rao PS, Merion RM, Ashby VB, Port FK, Wolfe RA, Kayler LK. Renal transplantation in elderly patients older than 70 years of age: results from the Scientific Registry of Transplant Recipients. Transplantation, 2007; 83(8): 1069-1074.
- El-Zoghby ZM, Stegall MD, Lager DJ, Kremers WK, Amer H, Gloor JM, et al. Identifying specific causes of kidney allograft loss. American Journal of Transplantation, 2009; 9(3): 527-535.
- Cosio FG, Gloor JM, Sethi S, Stegall MD. Transplant glomerulopathy. American Journal of Transplantation, 2008; 8(3): 492-496.
- Amer H, Filder ME, Myslak M, Morales P, Kremers WK, Larson TS, et al. Proteinuria after kidney transplantation, relationship to allograft histology and survival. American Journal of Transplantation, 2007; 7(12): 2748-2756.
- Nankivell BJ, Shingde M, Keung KL, Fung CL, Borrows RJ, O’Connell PJ, et al. The causes, significance and consequences of inflammatory fibrosis in kidney transplantation: the Banff i-IFTA lesion. American journal of transplantation, 2018; 18(2): 364-376.
- Nankivell BJ, Borrows RJ, Fung CL, O'Connell PJ, Allen RD, Chapman JR. The natural history of chronic allograft nephropathy. New England Journal of Medicine, 2003; 349(24): 2326-2333.
- Chand S, Atkinson D, Collins C, Briggs D, Ball S, Sharif A, et al. The spectrum of renal allograft failure. PLoS One, 2016; 11(9): e0162278.
- Shishido S, Asanuma H, Nakai H, Mori Y, Satoh H, Kamimaki I, et al. The impact of repeated subclinical acute rejection on the progression of chronic allograft nephropathy. Journal of the American Society of Nephrology, 2003; 14(4): 1046-1052.

