The Incidence of Diarrhea and Clostridium Difficile Colonization Due to Antibiotic Intake in Sample of Egyptian Pediatrics
Ebtessam Mohammed Mahmoud Silema*, Shymaa El-Sayed Ali Mohamed, Lamyaa Hussain Abdulrahman Seliem, Hebatallah Shoukry Mohamed Soliman, Marwa Foad Abdelrahman Asker, Ibrahim Mohamed Eladel Ibrahim Abouelkhir
Pediatrics department, Shebin Elkom Teaching Hospital, Shebin Elkom, Menoufia, Egypt
Received Date: 21/09/2024; Published Date: 01/11/2024
*Corresponding author: Ebtessam Mohammed Mahmoud Silema, Pediatrics department, Shebin Elkom Teaching Hospital, Shebin Elkom, Menoufia, 6132415, Egypt
Abstract
Background: Antibiotic-associated diarrhea is one of the most frequent side effects of antimicrobial therapy. Clostridium Difficile-Associated Diarrhea (CDAD) is increasingly diagnosed in children, the worldwide incidence rate of the infection is increasing.
Objective: To study antibiotic associated diarrhea and clostridium difficile colonization in pediatrics of Shebin Elkom Teaching Hospital.
Methods: A case control study conducted on two groups of patients was collected from Pediatric Department and Neonatal ICU in Shebin Elkom Teaching Hospital. Group 1(Cases): Sixty patients with antibiotic intake whether having diarrhea or not, group 2(Control): Thirty patients without antibiotic intake.
Results: There was a high percentage of Clostridium difficile detection or their toxin among studied neonates having diarrhea. Also, there was a low percentage of Clostridium difficile detection or their toxin among studied neonates not having diarrhea.
Conclusion: Antibiotic associated diarrhea is more common in infants while non-diarrheal colonization by C. difficile is more common in neonates following prolonged antibiotic uses. Also, none of the antibiotics singly or in combination could be correlated specially to C. difficile diarrhea among hospitalized neonates or infants.
Keywords: Antibiotic; Clostridium difficile; Colonization; Diarrhea
Introduction
Clostridium difficile (C. difficile) is a Gram-positive, anaerobic, toxin-producing germ first isolated from the stool of healthy newborns in 1935 [1]. It is the most common cause of antimicrobial therapy-associated diarrhea and is a common healthcare-associated pathogen [2]. In recent years, there has been an increase in the incidence of community-acquired infection in subjects without known risk factors [3], such as previous exposure to antibiotics, patients with chronic gastrointestinal pathology or inflammatory bowel disease (IBD), [4].
The clinical spectrum of C. difficile ranges from asymptomatic colonization, mild and self-limiting disease to a severe, life-threatening pseudomembranous colitis, toxic megacolon, sepsis and death [5,6]. CDI is defined when there is the presence of symptomatic diarrhea defined by three or more unformed stools per 24 h and at least one of the following criteria: a positive laboratory assay for C. difficile toxin A and/or B or toxin-producing C. difficile organism in a stool sample or pseudomembranous colitis or colonic histopathology characteristics of CDI revealed by endoscopy [7,8]. CDI is associated with an increased abundance of toxin-producing C. difficile strains, leading to high toxin concentrations within the colon resulting in inflammation and damage of the colonocytes [9,10]. Usually, the indigenous microbial communities provide a colonization resistance to C. difficile, which could also be proven in animal models. However, a disruption of this microbial system can promote the development of CDI [11,12].
Antibiotic-associated diarrhea (AAD) is defined as diarrhea that develops any time from a few hours after the onset of antibiotic therapy to eight weeks following antibiotic cessation, where diarrhea is defined as more than 2 unformed stools for ≥2 days [13]. The direct toxic effects of antibiotics on the intestines include altered digestive function secondary to reduced concentrations of gut bacteria or the overgrowth of pathogenic microorganisms [14]. The bacterial diversity of the intestinal lumen is also diminished after the administration of certain antibiotics, and these alterations in the abundance and composition of gut microbiota further lead to the dysfunction of these microbiota [15].
The incidence of CDAD in hospitalized children has been increasing since 1997 [16-18]. Accompanying this trend are studies indicating that the burden of pediatric CDAD in the community is substantial and rising [19,20]. In addition, a study from Olmstead County, Minnesota found that their rate of CA CDAD in children increased 10.5-fold from 1991 to 2007 [19]. Thus, this study aimed to study antibiotic associated diarrhea and clostridium difficile colonization in pediatrics of Shebin Elkom Teaching Hospital.
Patients and Methods
A case control study conducted on two groups of patients were collected from Pediatric Department and Neonatal ICU in Shebin Elkom Teaching Hospital.
They were divided as follows:
Group 1(Cases): Sixty patients (thirty infants or children and thirty neonates) with antibiotic intake whether having diarrhea or not.
Group 2(Control): Thirty patients (fifteen infants or children and fifteen neonates) without antibiotic intake.
Inclusion criteria: Infants or children and neonates with or without antibiotic intake whether having diarrhea or not.
Exclusion criteria: Patients with major systemic illness e.g. renal or cardiac and immune deficient patients.
The patients were subjected to:
- Full history taking as a routine Including: history of drug intake particularly antibiotics (type, dose, duration, route) more than 10 days, history of diarrhea if present.
- Full clinical examination: they were examined as regards weight, height and head circumference.
- Associated clinical manifestation as: fever, vomiting, abdominal pain or distension.
- Diarrhea: color, frequency, consistency, watery or not and presence of blood or mucus.
Materials and Preparations:
All materials were supplied by different Sources and prepared in Medical Microbiology and Immunology Department, Faculty of Medicine, Tanta University according to the manufacturer’s instructions.
- Staining: Gram stain sets.
- Bacteriological culture media:
Ready to use cefoxitin-cycloserine fructose agar (CCFA) (Biomerieux, France). It is a selective medium for the isolation of Clostridium difficile. The selective agents are usually based on cefoxitin and cyloserine, and these are usually inhibitory to most other clostridial species so the resulting growth from an active case of infection is often a pure culture of C. difficile [21].
- Absolute Alcohol: (supplied by El Nasr Pharmaceutical Chemicals Co., Egypt) used for alcohol shock method (discussed later in details)
- difficile Toxin A+B 2nd Generation ELISA Kit: (Supplied by Diagnostic Automation, Inc., USA)
Specimen collection:
From each subject of the two groups, stool samples were collected in sterile sealed disposable plastic containers. The samples were immediately transferred to the laboratory of Medical Microbiology and Immunology Department at Tanta Faculty of Medicine for further processing storage and testing.
Principle of Procedure
During the first incubation, C. difficile toxins A+B antigen present in the stool are “sandwiched” by anti-C. difficile toxins A+B antibodies attached to the wells and anti-C. difficile toxins A+B antibodies conjugated to peroxidase. After washings to remove unbound enzyme, a chromogen is added which develops a blue color in the presence of the enzyme complex and peroxide. The stop solution ends the reaction and turns the blue color to yellow. If no antigen is captured, or if there is an insufficient level of antigen, no colored reaction will take place.
Statistical analysis
All data were compiled and analyzed using SPSS version 21 (SPSS Inc., Chicago, IL, USA). Continuous variables are presented as means (± standard deviation [SD]), and categorical variables are presented using relative frequency distributions and percentages. Continuous variables were compared using Student’s t-test or the Mann-Whitney (t) test, and categorical data were analyzed using the chi-square (X2) test, Statistical significance established at p ≤ 0.05.
Results
A total of 45 patients showed that there was no statistically significant difference between studied neonates (cases) and their controls as regards, sex, and mode of delivery. But there is significant relation between antibiotic intake and diarrhea, Clostridium difficile detection by stool culture and toxin detected by ELISA. There is a highly statistically significant difference between studied neonates (cases) and their controls as regards age and type of feeding (Table 1).
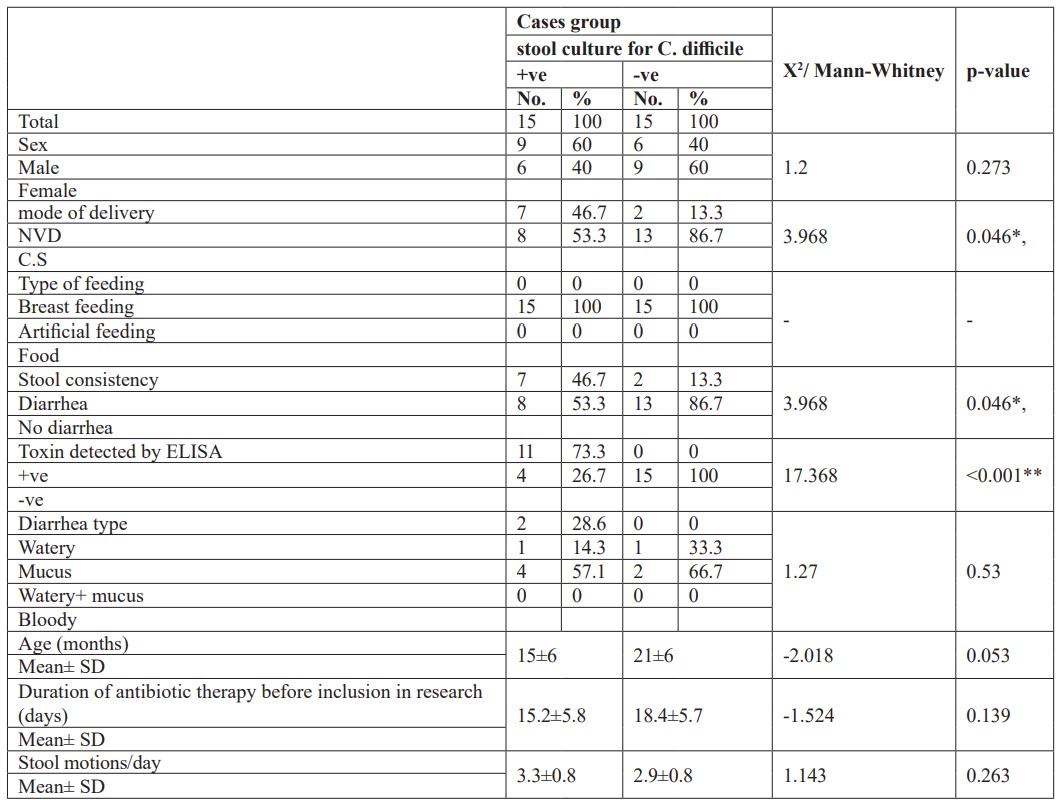
Also, that there was no statistically significance difference between Clostridium difficile positive and negative cases among studied neonates as regards, sex, type of feeding, age, duration of antibiotic intake, type of diarrhea and frequency. But there was a significant difference between clostridium difficile positive and negative cases as regards, mode of delivery and presence of diarrhea. It is to be noted that 7 out of 15 cases positive for C. difficile had diarrhea while 8 cases had colonization without diarrhea. Also 6 cases out of 7 cases with C. difficile diarrhea were positive for stool toxins by ELISA while 1 case was toxins negative. Also 2 cases had diarrhea after antibiotic intake but were negative to stool C. difficile and their toxins (Table 2).
Table 1: Clinical and laboratory data of studied neonates and their controls.
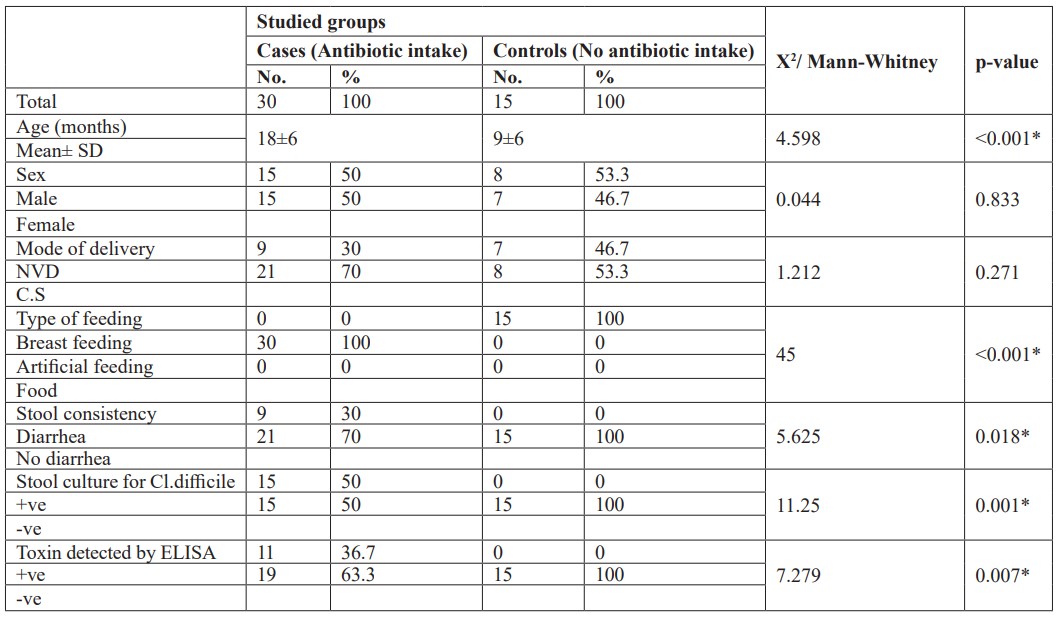
Table 2: Comparison between Clostridium difficile positive and negative cases among studied neonates.
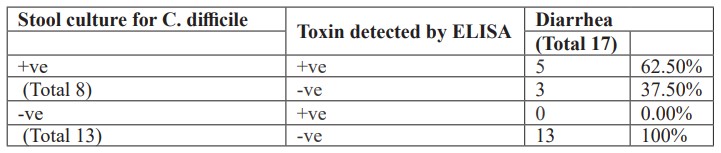
Additionally, there was a high percentage of Clostridium difficile detection or their toxin among studied neonates having diarrhea (Table 3). Also, there was a low percentage of Clostridium difficile detection or their toxin among studied neonates not having diarrhea (Table 4).
Table 3: The presence of Clostridium difficile or their toxin among studied neonates having diarrhea.
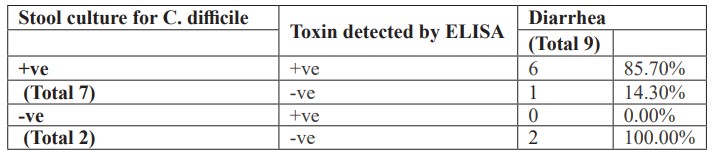
Table 4: The presence of Clostridium difficile or their toxin among studied neonates not having diarrhea.
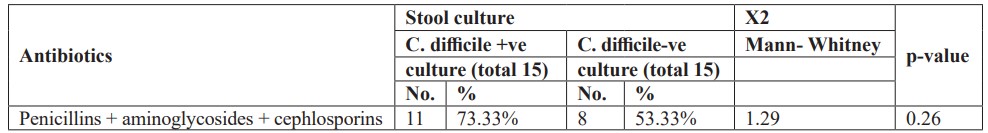
Furthermore, there was no statistically significant difference between studied neonates with Clostridium difficile positive and negative cultures and one type of antibiotic administration (Table 5). Also, there was no statistically significant difference between studied neonates with Clostridium difficile positive and negative cultures and combination of two antibiotics administration (Table 6). Moreover, there was no statistically significant difference between studied neonates with Clostridium difficile positive and negative cultures and combination of triple antibiotics administration (Table 7).
Table 5: Antibiotics administration in neonates with C. difficile positive &negative cultures.
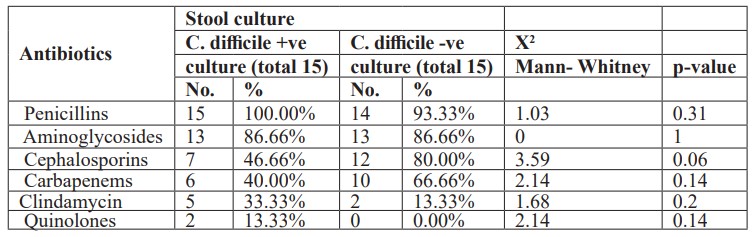
Table 6: Combination of two antibiotics administration in neonates with C.difficile positive &negative cultures.
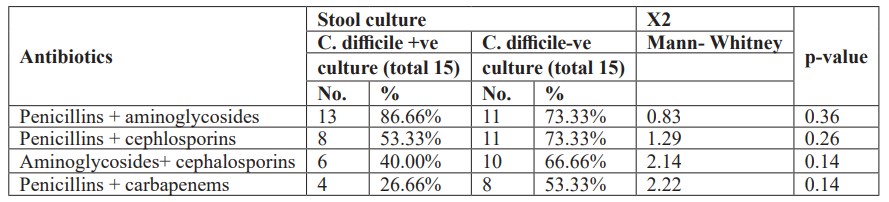
Table 7: Combination of three antibiotics administration in neonates with C.difficile positive &negative cultures.
Discussion
The emergence of antibiotics made it possible to treat many serious life-threatening infections and save the lives of many people. However, widespread and uncontrolled use of various groups of antibacterial drugs, especially in outpatient settings, leads to the development of many negative effects [22]. Clostridium difficile is an important cause of antibiotic-associated diarrhea and the most widely recognized diarrheal pathogen acquired in healthcare settings [8]. During the past 10 years, there has been a notable increase in the incidence of C. difficile-associated diarrhea (CDAD) in the United States and the emergence of disease in community settings and in individuals previously considered low risk [23].
The incidence of antibiotic-associated diarrhea in hospitals is 20-25%, but in recent years, the free supply of drugs, including antibiotics, has led to an increase in the number of antibiotic-associated diarrhea in outpatient settings [22]. Antibiotic-associated diarrhea maybe mild in patients, and in some cases, symptoms resolve when the antibiotic is discontinued. Clinical symptoms may appear even after 8 weeks after the end of the course of antibiotic therapy and cause deepening of the pathological process, which significantly complicates the diagnosis. Pseudomembranous colitis (PMK), which is one of the dangerous forms, develops [24]. So, this study aimed To study antibiotic associated diarrhea and clostridium difficile colonization in pediatrics of Shebin Elkom Teaching Hospital.
The present study showed that there is significant relation between antibiotic intake and diarrhea, clostridium difficile detection by stool culture and toxin detected by ELISA. In this concern a study by [22] found that Clostridium difficile infection was detected in 32 out of 68 patients with antibiotic-related diarrhea, which is47%. Toxins A and B were detected in children with antibiotic-related diarrhea during hospitalization, and this is 18.7%. Also, this result agreed with the study performed by [25] who reported that antibiotics lead to increased susceptibility to the acquisition of C. difficile. Antibiotics destabilize the GIT microflora and can alter the integrity of epithelial surfaces [25]. Similarly, [26] reported that diarrhea is a common complication of antibiotics which occurs in between 5% to 39% of patients. This effect is caused by disturbance of the normal balance of intestinal organisms [26,27]. Additionally, [28] reported that, of the 67 infants and children under 3 years of age with no medical disorders who were examined in a day-nursery, 25 (37%) carried C. difficile, while the carriage rate in infants < 1 year of age was much higher (65%).
Our study demonstrated that there were no statistically significant differences between studied neonates (cases) and their controls as regards, sex and mode of delivery. There was a highly statistically significant difference between studied neonates (cases) and their controls regarding age and type of feeding. The results agreed with [29], who stated that antibiotics and length of hospitalization were two significant independent risk factors in developing of C.difficile infection in pediatrics [29]. We also agreed with [30], that the microflora of formula-fed infants is more diverse, with high numbers of Enterobacteria and Clostridia compared to breastfed [30,31]. On the other hand, [32], stated that infants born by Caesarean section may be exposed to their mothers’ microflora, but the initial exposure is most likely to be from the surrounding environment such as the air, other infants and the nursing staff, which serve as vectors for transfer [32].
In the present work, there was no statistically significance difference between Clostridium difficile positive and negative cases among studied neonates as regards, sex, type of feeding, age, duration of antibiotic intake, type of diarrhea and frequency. However, there was a significance difference between Clostridium difficile positive and negative cases as regards, mode of delivery and presence of diarrhea. And high significant relation between Clostridium difficile toxin detection by ELISA and culture positive cases for C. difficile. This result agreed with [26], who stated that the occurrence of antibiotic associated diarrhea varies greatly and is influenced by a number of factors, including nosocomial outbreaks, patterns of antimicrobial use, and individual susceptibility. It is estimated that 10% to 15% of all hospitalized patients treated with antibiotics will develop antibiotic associated diarrhea. Most important, twice as many will become asymptomatic carriers. Risk factors include compromised immune status, types and prolonged use of antibiotics, and the length of hospitalization. The infection rates for C. difficile were reported to be around 10% after 2 weeks of hospitalization but may reach 50% after 4 or more weeks [26].
Also, we agreed with [33], who stated that Clostridium difficile infection causes a spectrum of symptoms including asymptomatic colonization, mild, watery diarrhea or severe pseudomembranous colitis. Infants are usually asymptomatically colonized. Most symptomatic children experience mild to moderate watery diarrhea. Among 200 Candian children with CDI, 79% present with diarrhea compared to Indian 20-30%. [33], due to environmental considerations and different population factors. Our results coincided with [34,35], who stated that in early infancy, asymptomatic carriage of C. difficile in the digestive tract is very common. Many infants are colonized by toxigenic or nontoxigenic C. difficile strains during the first two years of life. This colonization is rarely associated with C. difficile infection. Fecal microbiota is less complex in infants under 2 years of age than in adults [34,35].
This study showed that there was no statistically significant difference between studied neonates with Clostridium difficile positive and negative cultures and one type of antibiotic administration. Also, there was no statistically significant difference between studied neonates with Clostridium difficile positive and negative cultures and combination of two antibiotics administration. Moreover, there was no statistically significant difference between studied neonates with Clostridium difficile positive and negative cultures and combination of triple antibiotics administration. In a study by [22] found that a risk factor for the development of Clostridium difficile infection was combined antibiotic therapy: the frequency of combined therapy was 65.6% (22 patients). This is explained by the fact that taking several antibiotics at the same time increases their negative impact on the microecological relationship in the gastrointestinal tract, expands the range of toxic effects, and, as a result, creates additional conditions for the growth and colonization of Clostridium difficile. In our study, it was found that ampicillin (34.4%) and III generation cephalosporin (28.2%) were the most common antibiotics in the formation of C. difficile toxins and the development of the disease. [36], stated that no difference in neonatal colonization irrespective to mode of delivery whether vaginal or caesaren section [36], this disagrees with our study. We disagreed with Penders et al, that Clostridium difficile colonization is influenced by infant feeding behavior. According to their study, exclusively breastfed infants were significantly less frequently colonized (14- 16%) than breast fed infants who also receive formula (35%) and formula fed only infants (30-62%) [37]. The disagreement resulted from that all our cases were artificially fed. Furthermore, [38] noted that the majority of neonates (97.9%) in their study were breast-fed, which was reported to be associated with lower rates of C.difficile colonization [39].
In the present study, there was a high percentage of Clostridium difficile detection or their toxin among studied infants, children, and neonates having diarrhea as compared to non-diarrheal cases. These results agreed with [40], who reported that C. difficile contributed to more than 55.4% of diarrheal inpatients from five Swedish hospitals with great varience among the hospitals themselves obtained as high as 62.5% of tested patients in one of the studied centers. [40]. Other different results were obtained in the USA by [41], reporting that C. difficile-associated diarrhea was the etiology in 32% of all studied in patients with diarrhea [41].
Conclusion
Antibiotic associated diarrhea is more common in infants while non-diarrheal colonization by C. difficile is more common in neonates following prolonged antibiotic uses. Also, None of antibiotics singly or in combination could be correlated specially to C.difficile diarrhea among hospitalized neonates or infants.
References:
- Borali E, De Giacomo C. Clostridium difficile infection in children: a review. Journal of pediatric gastroenterology and nutrition, 2016; 63(6): e130-140.
- Borali E, Ortisi G, Moretti C, Stacul EF, Lipreri R, Gesu GP, et al. Community-acquired Clostridium difficile infection in children: a retrospective study. Digestive and Liver Disease, 2015; 47(10): 842-846.
- Kuntz JL, Johnson ES, Raebel MA, Petrik AF, Yang X, Thorp ML, et al. Epidemiology and healthcare costs of incident Clostridium difficile infections identified in the outpatient healthcare setting. Infection Control & Hospital Epidemiology, 2012; 33(10): 1031-1038.
- Dop D, Marcu IR, Padureanu V, Caragea DC, Padureanu R, Niculescu SA, et al. Clostridium difficile infection in pediatric patients. Biomedical Reports, 2023; 20(2): 18.
- Gerding DN, Johnson S, Peterson LR, Mulligan ME, Silva J. Clostridium difficile-associated diarrhea and colitis. Infection Control & Hospital Epidemiology, 1995; 16(8): 459-477.
- Rupnik M, Wilcox MH, Gerding DN. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nature Reviews Microbiology, 2009; 7(7): 526-536.
- Kuijper EJ, Coignard B, Tüll P, ESCMID Study Group for Clostridium difficile (ESGCD), EU Member States and the European Centre for Disease Prevention and Control (ECDC). Emergence of Clostridium difficile‐associated disease in North America and Europe. Clinical Microbiology and Infection, 2006; 12: 2-18.
- Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infection Control & Hospital Epidemiology, 2010; 31(5): 431-455.
- Meyer GK, Neetz A, Brandes G, Tsikas D, Butterfield JH, Just I, et al. Clostridium difficile toxins A and B directly stimulate human mast cells. Infection and immunity, 2007; 75(8): 3868-3876.
- Carroll Karen C. Biology of Clostridium difficile: Implications for Epidemiology and Diagnosis. Annual Review of Microbiology, 2011; 65(1): 501-21.
- Rea MC, O'Sullivan O, Shanahan F, O'Toole PW, Stanton C, Ross RP, et al. Clostridium difficile carriage in elderly subjects and associated changes in the intestinal microbiota. Journal of clinical microbiology, 2012; 50(3): 867-875.
- Buffie CG, Bucci V, Stein RR, McKenney PT, Ling L, Gobourne A, et al. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature, 2015; 517(7533): 205-208.
- Tanır Basaranoğlu S, Karaaslan A, Salı E, Çiftçi E, Gayretli Aydın ZG, Aldemir Kocabaş B, et al. Antibiotic associated diarrhea in outpatient pediatric antibiotic therapy. BMC pediatrics, 2023; 23(1): 121.
- Kuehn J, Ismael Z, Long PF, Barker CI, Sharland M. Reported rates of diarrhea following oral penicillin therapy in pediatric clinical trials. The Journal of Pediatric Pharmacology and Therapeutics, 2015; 20(2): 90-104.
- Shao H, Zhang C, Xiao N, Tan Z. Gut microbiota characteristics in mice with antibiotic-associated diarrhea. BMC microbiology, 2020; 20: 1-9.
- Zilberberg MD, Tillotson GS, McDonald LC. Clostridium difficile infections among hospitalized children, United States, 1997–2006. Emerging infectious diseases, 2010; 16(4): 604.
- Nylund CM, Goudie A, Garza JM, Fairbrother G, Cohen MB. Clostridium difficile infection in hospitalized children in the United States. Archives of pediatrics & adolescent medicine, 2011; 165(5): 451-457.
- Crews JD, Anderson LR, Waller DK, Swartz MD, DuPont HL, Starke JR. Risk factors for community-associated Clostridium difficile-associated diarrhea in children. The Pediatric infectious disease journal, 2015; 34(9): 919-923.
- Khanna S, Baddour LM, Huskins WC, Kammer PP, Faubion WA, Zinsmeister AR, et al. The epidemiology of Clostridium difficile infection in children: a population-based study. Clinical infectious diseases, 2013; 56(10): 1401-1406.
- Wendt JM, Cohen JA, Mu Y, Dumyati GK, Dunn JR, Holzbauer SM, et al. Clostridium difficile infection among children across diverse US geographic locations. Pediatrics, 2014; 133(4): 651-658.
- Stevens V, Dumyati G, Fine LS, Fisher SG, van Wijngaarden E. Cumulative antibiotic exposures over time and the risk of Clostridium difficile infection. Clinical infectious diseases, 2011; 53(1): 42-48.
- Toshtemirovna RD, Mansurovna SG. The Role of Clostridium Difficile in The Development of Antibiotic-Associated Diarrhea in Early-Aged Children. International Journal of Medical Sciences and Clinical Research, 2024; 4(06): 12-17.
- Lessa FC, Gould CV, McDonald LC. Current status of Clostridium difficile infection epidemiology. Clinical Infectious Diseases, 2012; 55(suppl_2): S65-S70.
- Larcombe S, Hutton ML, Riley TV, Abud HE, Lyras D. Diverse bacterial species contribute to antibiotic-associated diarrhoea and gastrointestinal damage. Journal of Infection, 2018; 77(5): 417-426.
- Levy J. The effects of antibiotic use on gastrointestinal function. The American journal of gastroenterology, 2000; 95(1): S8-10.
- McFarland LV. Epidemiology, risk factors and treatments for antibiotic-associated diarrhea. Digestive Diseases, 1999; 16(5): 292-307.
- Shannon-Lowe J, Matheson NJ, Cooke FJ, Aliyu SH. Prevention and medical management of Clostridium difficile infection. Bmj, 2010; 340.
- Yamamoto-Osaki T, Kamiya S, Sawamura S, Kai M, Ozawa A. Growth inhibition of Clostridium difficile by intestinal flora of infant faeces in continuous flow culture. Journal of medical microbiology, 1994; 40(3): 179-187.
- Thompson CM, Gilligan PH, Fisher MC, Long SS. Clostridium difficile cytotoxin in a pediatric population. American Journal of Diseases of Children, 1983; 137(3): 271-274.
- Harmsen HJ, Wildeboer–Veloo AC, Raangs GC, Wagendorp AA, Klijn N, Bindels JG, et al. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. Journal of pediatric gastroenterology and nutrition, 2000; 30(1): 61-67.
- Fanaro S, Chierici R, Guerrini P, Vigi V. Intestinal microflora in early infancy: composition and development. Acta paediatrica, 2003; 92: 48-55.
- Schwiertz A, Gruhl B, Löbnitz M, Michel P, Radke M, Blaut M. Development of the intestinal bacterial composition in hospitalized preterm infants in comparison with breast-fed, full-term infants. Pediatric research, 2003; 54(3): 393-399.
- Gogate A, De A, Nanivadekar R, Mathur M, Saraswathi K, Jog A, et al. Diagnostic role of stool culture & toxin detection in antibiotic associated diarrhoea due to Clostridium difficile in children. Indian Journal of Medical Research, 2005; 122(6): 518.
- Collignon A, Ticchi L, Depitre C, Gaudelus J, Delmee M, Corthier G. Heterogeneity of Clostridium difficile isolates from infants. European journal of pediatrics, 1993; 152: 319-322.
- Favier CF, Vaughan EE, De Vos WM, Akkermans AD. Molecular monitoring of succession of bacterial communities in human neonates. Applied and environmental microbiology, 2002; 68(1): 219-226.
- Matsuki S, Ozaki E, Shozu M, Inoue M, Shimizu S, Yamaguchi N, et al. Colonization by Clostridium difficile of neonates in a hospital, and infants and children in three day-care facilities of Kanazawa, Japan. International Microbiology, 2005; 8(1): 43-48.
- Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics, 2006; 118(2): 511-521.
- Furuichi M, Imajo E, Sato Y, Tanno S, Kawada M, Sato S. Characteristics of Clostridium difficile colonization in Japanese children. Journal of Infection and Chemotherapy, 2014; 20(5): 307-311.
- Jangi S, Lamont JT. Asymptomatic colonization by Clostridium difficile in infants: implications for disease in later life. Journal of pediatric gastroenterology and nutrition, 2010; 51(1): 2-7.
- Wiström J, Norrby SR, Myhre EB, Eriksson S, Granström G, Lagergren L, et al. Frequency of antibiotic-associated diarrhoea in 2462 antibiotic-treated hospitalized patients: a prospective study. Journal of antimicrobial chemotherapy, 2001; 47(1): 43-50.
- Pulvirenti JJ, Mehra T, Hafiz I, DeMarais P, Marsh D, Kocka F, et al. Epidemiology and outcome of Clostridium difficile infection and diarrhea in HIV infected inpatients. Diagnostic microbiology and infectious disease, 2002; 44(4): 325-330.

