Evaluating the Impact of Phytoconstituents and Nutrients on Stress Reduction and Sexual Dysfunction in Males: A Double-Blind Clinical Trial
Dr. Gayatri Ganu* and Dr. Dheeraj Nagore
Director, Mprex Healthcare Pvt. Ltd, India
Received Date: 11/09/2024; Published Date: 30/10/2024
*Corresponding author: Dr. Gayatri Ganu, Director, Mprex Healthcare Pvt. Ltd, Pune, India
Abstract
Introduction: Erectile Dysfunction (ED) is a common medical condition that affects approximately 100 million men worldwide and is currently recognized as a major public health problem. It is estimated that nearly one half of men older than 40 years have some degree of ED. The objective of this study was to evaluate the safety and efficacy of PR/HC/1718/002 (Phytoconstituents and Nutrients formulation) in patients suffering from erectile dysfunction.
Materials and Methods: Total 71 patients was randomized to test group (35 patients) and placebo group (36 patients) in 1:1 ratio. Treatment duration is of 90 days.
Results: The test demonstrated superior efficacy over the placebo after 90 days. Erectile function improved significantly in the test group (91.27% vs. 26.57%), along with increase in sexual desire (57.95% vs. 15.40%) and orgasmic function (88.14% vs. 27.21%) compared to the placebo. Intercourse satisfaction increase in the test group 73.64%, compared to the placebo (30.85%). Overall satisfaction improved by 110.85% in the test group, compared to the placebo (36.65%). Serum total testosterone levels rose by 41.90% in the test group, versus 5.23% in the placebo. There was significant improvement in quality of erection score in the test group (180.89% vs. 34.41%) compared to the placebo. No adverse events were reported during the study, and it was well tolerated.
Conclusion: The test group demonstrated significant improvement in various sexual health parameters including erectile and orgasmic function, quality of erection, testosterone level, sexual desire, and treatment satisfaction along with sexual encounter profile. Importantly, the formulation exhibited excellent safety, with no adverse events observed and consistent tolerability throughout the study.
Keywords: Erectile dysfunction; Sexual desire; Stress; Testosterone; Erectile Function
Introduction
Erectile Dysfunction (ED) is a common medical condition that affects approximately 100 million men worldwide and is currently recognized as a major public health problem. It is estimated that nearly one half of men older than 40 years have some degree of ED [1]. While in 1995, ED affected over 152 million men worldwide, it is projected that by 2025, more than 320 million patients will be afflicted with the largest projected increases in the developing world [2]. Erectile dysfunction can be classified as developing from psychological, neurological, hormonal, and vascular pathologies, or combinations of these factors [3].
Injury to the spinal cord may interrupt neural pathways to the sacral region, preventing or inhibiting the process of achieving an erection [4]. Hormones such as adrenocorticotropic hormone, oxytocin, prolactin, and androgens, especially testosterone, have been implicated in the modulation of erectile function. Hypogonadism plays a significant role in erectile dysfunction as it is believed that a threshold level of testosterone is necessary for erection to occur, and as men age there is a natural decrease in testosterone production further contributing to ED [5]. Peripheral arterial disease and endothelial dysfunction seen in diabetes mellitus, atherosclerosis, coronary disease, and hypertension also contribute to the development of ED [6]. This may develop from degeneration of the tunica albuginea, loss of myogenic venous responses, trauma, or endothelial/smooth muscle dysfunction in the corpora [7].
Nitric Oxide (NO) is the primary vasoactive neurotransmitter in erectile response, released from nonadrenergic, noncholinergic (NANC) neurons and the endothelium [8]. Nitric oxide synthase (NOS) converts L-arginine to NO and L-citrulline [9]. This process causes smooth muscle cell relaxation, dilating arteries/arterioles and increasing blood flow into corporal sinuses, leading to an erection [8-10]. Risk factors for ED include cardiovascular disease (CVD), diabetes, depression, alcohol use, smoking, surgery, neurologic disease, obesity, radiation, and Peyronie’s disease [6, 11]. Endothelial dysfunction links CVD and ED [12]. Hormone deficiency, especially hypogonadism, affects erectile function, with low testosterone prevalent in ED cases [13]. While conventional PDE5 inhibitors the treatment of erectile dysfunction, their use is associated with several limitations. As a result, there is a growing demand for alternative therapeutic options that are safer, more affordable, and better tolerated, particularly from natural sources [14].
Herbal formulations have garnered significant attention due to their potential efficacy and favorable safety profiles in the management of various medical conditions, including erectile dysfunction. These formulations offer a promising avenue for the development of new treatments that could address the limitations of conventional therapies. The present study aims to evaluate the effects of PR/HC/1718/002, a polyherbal formulation, on various aspects of erectile function and sexual performance in men with erectile dysfunction. By investigating this herbal formulation, we seek to provide a safer and more accessible therapeutic option that could improve the overall management of erectile dysfunction.
Materials and Methods
Study Design
This was a randomized, double-blind, placebo-controlled, interventional, prospective clinical study that assessed safety and efficacy of PR/HC/1718/002 (Phytoconstituents and Nutrients formulation) in patients who suffered from erectile dysfunction. The duration of the treatment was 90 days. Ethical approval was obtained from the Institutional Ethics Committee (IEC) of Lokmanya Medical Research Centre, Chinchwad. The clinical trial was registered with the Clinical Trial Registry-India (CTRI) with the registration number CTRI/2019/06/019517 on June 4, 2019.
Investigational Product Composition: Ashwagandha (400 mg), Kawach (200 mg), Tagar (100 mg), Kesar (5 mg), Vitamin C (50 mg), Vitamin D3 (7.50 mcg), Vitamin B12 (1.10 mcg), Magnesium (5 mg) Xanthum gum, Purified talcum, hydroxyl propyl methyl cellulose, isopropyl alcohol.
Dosage and Duration of the Treatment:
Group A: Two PR/HC/1718/002 capsule twice daily orally after meals with water.
Group B: Two placebo capsule twice daily orally after meals with water.
Inclusion Criteria:
Patients meeting all of the following criteria were eligible for the study: male patients aged between 21 to 50 years who suffered from erectile dysfunction (ED); patients who scored between 11 and 25 on the Erectile Function (EF) domain of the International Index of Erectile Function (IIEF) at the screening visit; patients who were in an active and stable sexual relationship for the entire duration of the study.
Exclusion Criteria:
Patients with any of the following criteria were not eligible for the study: anatomical abnormalities of the penis; history of radical prostatectomy, spinal cord injury, or urogenital surgeries; severe sexual dysfunction (IIEF-EF score < 11 or > 25); ineffective prior treatment with PDE5 inhibitors; recent spermatogenic fertility treatment; ED caused by other disorders or untreated endocrine diseases; history of pelvic surgery, penile implant, or deformity; use of ED-related drugs (e.g., nitrates, anti-androgens, chemotherapy); significant alcoholism or drug abuse in the past year; smoking or nicotine use over 10 times daily; significant lab or ECG abnormalities; current hormonal, antidepressant, antipsychotic, or psychoactive drug use; HIV/AIDS, Hepatitis C/B, cancer, major diseases; known hypersensitivity to study drug ingredients; or any condition deemed unsuitable by investigators.
Methodology
After obtaining ethics committee approval and registering the clinical study on the CTRI website. Written informed consent was obtained from eligible patients during the screening visit. Total 71 patients were randomized to test group (35 patients) and placebo group (36 patients) in 1:1 ratio (Figure 1). Treatment duration is of 90 days. Demographic details, medical history, current medications, and vital signs were recorded. Patients underwent clinical examination, including assessment for sexual dysfunction and organic causes. The IIEF questionnaire was used to assess Erectile Function (EF) domain score. Concomitant medications were recorded, and drug compliance was assessed at each follow-up visit.
Assessment of change in anthropometric parameters, Sexual health parameters assessed by the International Index of Erectile Function (IIEF) Questionnaire, quality of penile erection evaluated using the Quality of Erection Questionnaire (QEQ), male sexual health assessed by the Erectile Dysfunction Inventory of Treatment Satisfaction (EDITS) questionnaire (patient and partner versions), sexual encounter profile, including Intra-vaginal Ejaculation Latency Time (IELT), Subjective Vitality Score, Perceived Stress, and Satisfaction with Life Score were assessed at screening and end of the study.
Patients received either the test or placebo capsules for 90 days and were advised to take one capsule twice daily orally after meals with water. Safety and tolerability of the investigational treatment in terms of Adverse Events (AEs), Serious Adverse Events (SAEs), and safety in terms of abnormal laboratory parameters were assessed throughout the study.
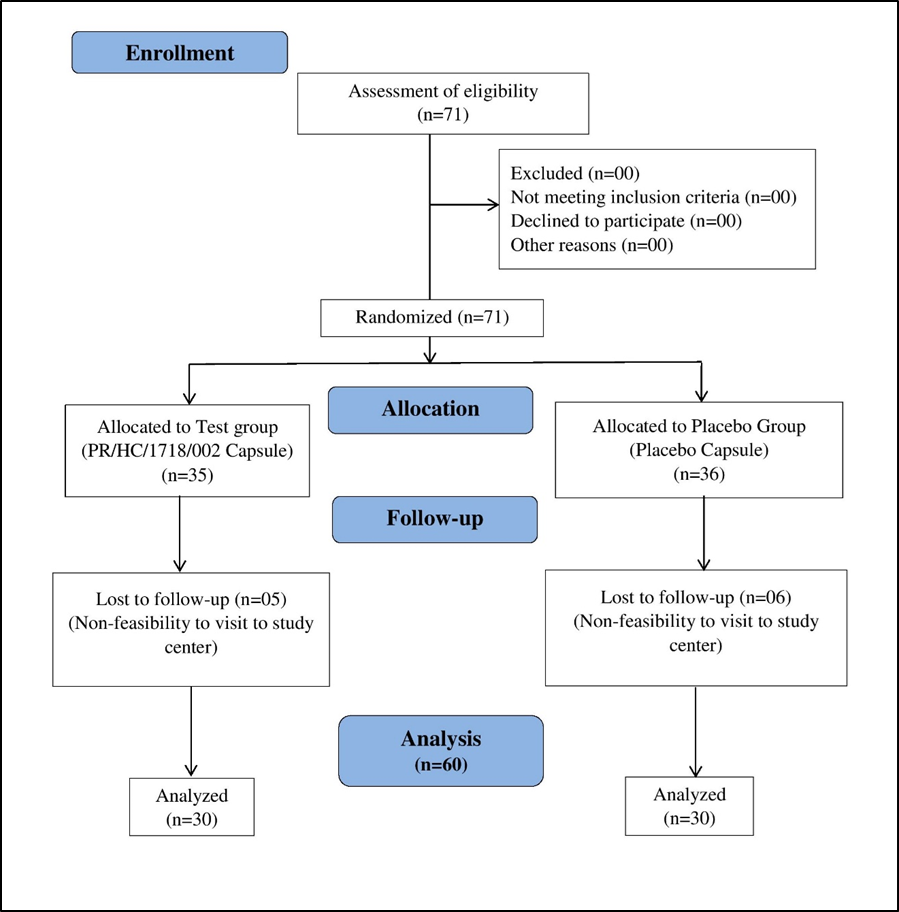
Figure 1: CONSORT diagram.
Statistical analysis
The normality of the data was assessed using the Kolmogorov-Smirnov test.
Subsequently, erectile function, sexual desire, orgasmic function, intercourse satisfaction, overall satisfaction, quality of erection, serum total testosterone, sexual encounters, IELT, EDITS, hematological and were analyzed using the Mann-Whitney U Test, Student t-Test and Wilcoxon Signed-Ranks Test. The compliance was assessed using the chi-square test.
Results
Demographic characteristics
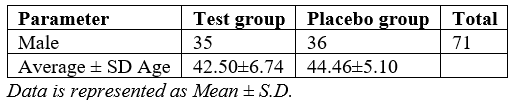
In this study, a total of 71 male patients were initially enrolled, with 60 completing the study. Of the participants, 35 males were in the test group, and 36 males were in the placebo group. The average age among test group was 42.50±6.74 years, while among the placebo group, it was 44.46±5.10 years as depicted in Table 1.
Table 1: Assessment Demographic Details.
Assessment of anthropometric details and lifestyle habits
The height measurements remained unchanged for both groups. The test group’s patients weight decreased by 1.51% from baseline (74.604 kg) to Day 90 (73.48 kg), while the placebo group’s patients weight decreased by 1.60% from baseline (75.86 kg) to Day 90 (74.63 kg). Waist circumference reduced by 0.95% in the test group and 0.68% in the placebo group. Hip circumference decreased by 1.03% in the test group and 0.53% in the placebo group. These findings suggest more pronounced changes in the test group.
Assessment of change in erectile function between the groups
At baseline, the average erectile function scores were similar between the test group (12.60) and the placebo group (12.72), with no significant difference. However, on Day 30, the test group exhibited a 74.52% increase in average erectile function (21.98) compared to a 38.68% increase in the placebo group (17.65), with a statistically significant difference (Figure 2).
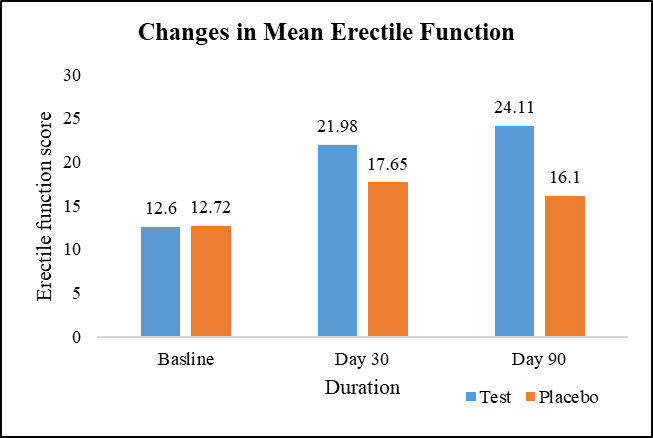
Figure 2: Changes in mean erectile function.
Assessment of change in sexual desire between the groups
At baseline, the average sexual desire scores were comparable between the test group (4.97) and the placebo group (4.74). However, on Day 30, the test group exhibited a 47.08% increase in average sexual desire (7.30) compared to a 13.92% increase in the placebo group (5.40), with a statistically significant difference. This trend persisted on Day 90, where the test group maintained a 57.95% increase in average sexual desire (7.85) compared to a 15.40% increase in the placebo group (5.47), further reinforcing the significant difference between the two groups (Figure 3).
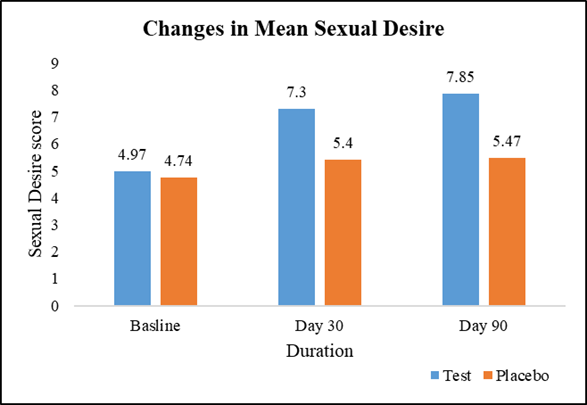
Figure 3: Changes in mean sexual desire.
Assessment of change in orgasmic function between the groups
At baseline, the average orgasmic function scores were similar between the test group (4.47) and the placebo group (4.52), with no significant difference. However, on Day 30, the test group exhibited a 76.29% increase in average orgasmic function (7.88) compared to a 27.43% increase in the placebo group (5.76), with a statistically significant difference. This trend continued on Day 90, where the test group maintained an 88.14% increase in average orgasmic function (8.41) compared to a 27.21% increase in the placebo group (5.75), further reinforcing the significant difference between the two groups (Figure 4).
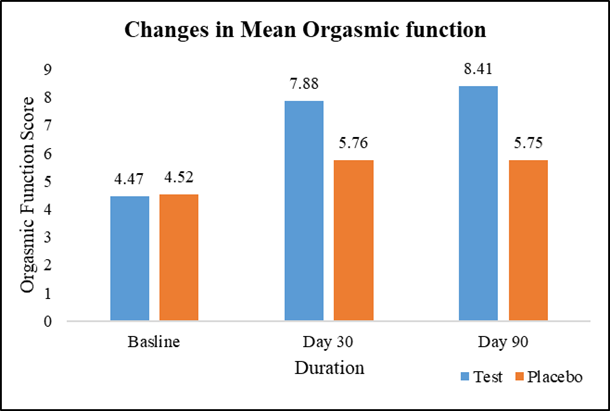
Figure 4: Changes in mean orgasmic function.
Assessment of change in intercourse satisfaction between groups
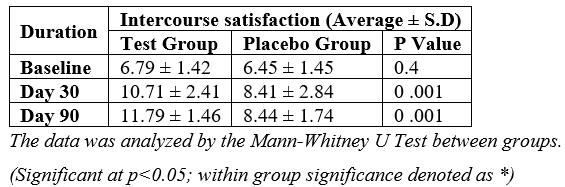
At baseline, the average intercourse satisfaction scores were comparable between the groups with no significant difference. On Day 30, the test group exhibited a 57.73% increase in average intercourse satisfaction compared to a 30.54% increase in the placebo group, with a statistically significant difference. This trend persisted on Day 90, where the test group maintained a 73.64% increase in average intercourse satisfaction compared to a 30.85% increase in the placebo group, further reinforcing the significant difference between the two groups [Table 2].
Table 2: Assessment of change in intercourse satisfaction between the groups.
Assessment of change in overall satisfaction between the groups
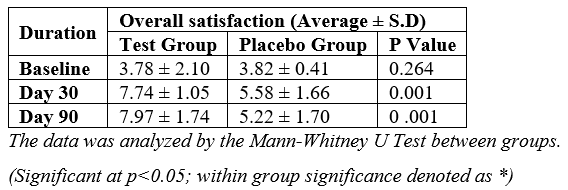
At baseline, the average overall satisfaction scores were similar between the test group and the placebo group, with no significant difference. However, on Day 30, the test group exhibited a 104.76% increase in average overall satisfaction compared to a 46.07% increase in the placebo group, with a statistically significant difference. This trend continued on Day 90, where the test group maintained a 110.85% increase in average overall satisfaction compared to a 36.65% increase in the placebo group, further reinforcing the significant difference between the two groups [Table 3].
Table 3: Assessment of change in overall satisfaction between the groups.
Assessment of change in quality of erection score between groups
At baseline, the average quality of erection scores was comparable between the test group (23.41) and the placebo group (23.77), with no significant difference. However, on Day 30, the test group exhibited a 136.90% increase in average quality of erection score (55.45) compared to a 15.48% increase in the placebo group (27.45), with a statistically significant difference. This trend persisted on Day 90, where the test group maintained a 180.89% increase in average quality of erection score (65.71) compared to a 34.41% increase in the placebo group (31.96), further reinforcing the significant difference between the two groups (Figure 5).
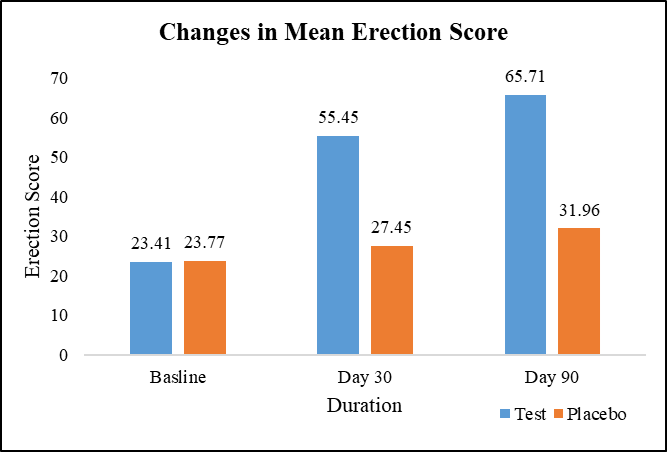
Figure 5: Changes in mean erection score.
Assessment of change in serum total testosterone level between the groups
In this study, the baseline average serum testosterone levels were comparable, with 358.63 ng/dL in the test group and 324.47 ng/dL in the placebo group. However, a significant difference emerged on Day 90, where the test group demonstrated a substantial 41.90% increase in average serum testosterone levels (508.66 ng/dL), while the placebo group experienced only a modest 5.23% increase (341.44 ng/dL). This disparity in the percent change between the two groups was statistically significant, suggesting that the intervention administered to the test group had a positive impact on enhancing serum total testosterone levels (Figure 6).
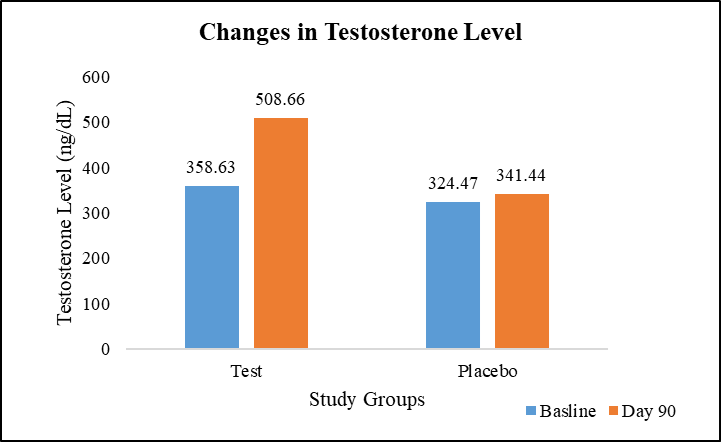
Figure 6: Changes in serum testosterone level.
Assessment of number of sexual encounters between the groups
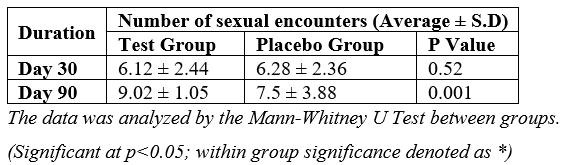
At the initial Day 30 assessment, both groups reported similar average numbers of sexual encounters. However, a significant difference emerged by Day 90, where the test group experienced a notable 47.39% increase in the average number of sexual encounters compared to a 19.43% increase in the placebo group. This substantial disparity in the percent change between the two groups was statistically significant [Table 4].
Table 4: Assessment of number of sexual encounters between the groups.
Assessment of Intra Vaginal Ejaculation Latency Time (IELT) between the groups
The assessment of IELT revealed significant differences between the test and placebo groups over the study period. At Day 30, both groups showed comparable IELT values, with the test group averaging 30.55 seconds and the placebo group at 27.79 seconds. However, by Day 90, a marked divergence was observed. The test group demonstrated a substantial increase in IELT to 63.55 seconds, more than doubling their initial value. In contrast, the placebo group showed minimal change, with an IELT of 30.50 seconds. This difference at Day 90 was statistically significant, indicating that the intervention effectively prolonged IELT in the test group compared to the placebo group.
Assessment of change in Erectile Dysfunction Inventory of Treatment Satisfaction (EDITS) (patient version) between the groups
In this study, the EDITS was assessed between the test group and the placebo group at Day 30 and Day 90. At the Day 30 evaluation, both groups exhibited comparable EDITS scores, with the test group averaging 33.62 and the placebo group averaging 36.55, indicating no significant difference in treatment satisfaction at this time point. However, a notable distinction emerged by Day 90, where the test group demonstrated a substantial improvement in EDITS scores, reaching an average of 48.58, reflecting a 44.48% increase from the Day 30 assessment. In contrast, the placebo group exhibited a more modest 16.16% increase, with an average EDITS score of 42.45.
Assessment of change in erectile dysfunction inventory of treatment satisfaction (EDITS) (partner version) between the groups
This study evaluated the EDITS from the partner's perspective between the test group and the placebo group at Day 30 and Day 90. Initially, at the Day 30 assessment, both groups reported similar levels of partner satisfaction, with the test group averaging 15.70 and the placebo group averaging 15.85, indicating no significant difference. However, a notable divergence emerged by Day 90, wherein the test group exhibited a remarkable 43.12% increase in partner satisfaction scores, reaching an average of 22.46. In contrast, the placebo group demonstrated a modest 4.23% increase, with an average score of 16.52.
Assessment of changes in laboratory investigations and vital signs
There were no statistically significant changes observed in the hematological and biochemical parameters (Renal function, Liver function, Urine analysis and Lipid profile) between the test and placebo groups. Similarly, vital parameters, also remained within normal ranges in both the groups.
Assessment of adverse events and compliance
Adverse events were recorded through self-reporting by the patients or the identification of clinical signs and symptoms during examinations. Notably, there were no adverse events observed throughout study period.
Both groups showed high compliance rate; by Day 90, the test group had a 97%, and the placebo group had a 95% throughout the study.
Assessment of Tolerability
All the patients consistently showed levels of tolerability is excellent in response to the investigational product throughout the study duration.
Discussion
The present clinical trial evaluated the efficacy and safety of an investigational product containing a combination of herbal extracts and vitamins/minerals for the management of Erectile Dysfunction (ED) in adult males. The rationale for the chosen ingredients was based on their potential mechanisms of action and previous scientific evidence supporting their roles in improving various aspects of male sexual health.
The study demonstrated notable improvements in various parameters related to male sexual function and associated factors in the test group compared to the placebo group. Significant enhancements were observed in the test group for erectile function, sexual desire, orgasmic function, intercourse satisfaction, overall satisfaction, and quality of erection scores at both Day 30 and Day 90 assessments. Furthermore, the test group exhibited a substantial 41.90% increase in serum total testosterone levels by Day 90, while the placebo group showed only a modest 5.23% increase. Notably, the test group also demonstrated a 47.39% increase in the number of sexual encounters and a prolonged intra-vaginal ejaculatory latency time by Day 90, suggesting improved sexual stamina and satisfaction. The study also revealed higher treatment satisfaction scores, as assessed by EDITS, for both patients and their partners in the test group compared to the placebo group.
The study findings demonstrate excellent drug compliance rates in both the test and placebo groups. Notably, there were no adverse events observed throughout the study duration. Comprehensive laboratory investigations, including hematological, lipid profile, liver function, renal function, and urine analysis, revealed no significant changes from baseline in either group after treatment at Day 90. Additionally, all patients consistently reported excellent tolerability levels in response to the investigational product. Furthermore, vital signs remained within normal ranges throughout the study period, with no clinically significant differences observed between the test and placebo groups. These findings collectively suggest that the intervention administered to the test group had a comprehensive and favorable impact on various aspects of male sexual health and performance.
One of the key components, Ashwagandha (Withania somnifera), is an ancient Ayurvedic herb known for its adaptogenic and rejuvenating properties. Several studies have demonstrated the potential benefits of Ashwagandha in improving sexual function, particularly in cases of ED. The proposed mechanisms involve modulating stress-related hormones, enhancing nitric oxide bioavailability, and exhibiting antioxidant and anti-inflammatory effects, all of which can contribute to improved vascular function and sexual performance. In a randomized, double-blind, placebo-controlled study, after 90 days of treatment, the Ashwagandha group showed significant improvements in sexual function, as evidenced by increased serum testosterone levels, sperm counts, and seminal motility compared to the placebo group [15].
Our study results align with previous findings by Dongre et al. investigated the effects of Ashwagandha root extract in 50 men aged 22-40 years with ED. The results showed significant improvements in the IIEF scores, indicating enhanced erectile function, sexual desire, and overall satisfaction in the Ashwagandha group compared to the placebo group [16]. The potential mechanism of action for Ashwagandha in improving erectile function is thought to be multifaceted. It may involve modulating stress-related hormones, enhancing nitric oxide bioavailability, and exhibiting antioxidant and anti-inflammatory effects, all of which can contribute to improved vascular function and sexual performance [17].
Another key ingredient, Mucuna pruriens, is renowned for its aphrodisiac properties and potential to increase testosterone levels and sperm count [18,19]. The proposed mechanisms involve stimulating the secretion of L-Dopa, which converts to dopamine, subsequently enhancing the release of FSH and LH from the pituitary gland. Increased LH levels can then facilitate testosterone production, leading to improved spermatogenesis and sexual performance. Additionally, Mucuna pruriens has been found to inhibit prolactin secretion, which can contribute to improving sexual drive and erectile function [20,21].
The stigmas of Saffron (Crocus sativus) flower have various photochemically active components such as crocin, crocetin, picrocrocin, and safranal [22]. In a randomized double-blind placebo-controlled study, 30 men with erectile dysfunction were recruited in two groups of intervention (received saffron) and control (received placebo) for 4 weeks. The findings indicated that saffron had a statistically significant effect on erectile function improvement and intercourse satisfaction among men [23]. In a clinical trial, 20 men with erectile dysfunction were studied before and after the intervention. The status of erectile dysfunction before intervention was evaluated by a questionnaire of international indicators of erectile function before the study and then after 10-day treatment with saffron tablets. The results showed that the use of saffron tablet resulted in an improvement of erectile function and all subscales including dyspepsia, sexual desire, sexual satisfaction, and general satisfaction [24].
The investigational product also contained vitamins C, D3, and B12, as well as the mineral magnesium, which have been explored for their potential roles in supporting male sexual health. Vitamin C, a potent antioxidant, has been shown to improve endothelial function and enhance nitric oxide bioavailability, contributing to improved vascular function and erectile function [25]. Vitamin B12 plays a role in cell metabolism, nerve function, and red blood cell production, which can indirectly influence erectile function [26]. Magnesium, an essential mineral, has been shown to promote vasodilation and improve endothelial function, thereby potentially enhancing erectile function [27]. Similarly, Vitamin D3 has been associated with vascular function, androgen production, and inflammatory processes, all of which can impact erectile function. A randomized, double-blind, placebo-controlled trial investigated the effects of vitamin D3 supplementation in 86 men with ED. In this study, a 3-month supplementation with 4000 IU/ day VD3 significantly increased serum 25(OH) VD3, PTH, phosphorus, and seminal and serum calcium; and total and progressive sperm motilities in infertile men [28].
The absence of adverse events related to the investigational product, alongside stable vital signs throughout the study, further underscores favorable safety profile of PR/HC/1718/002 capsules. Collectively, these findings indicate that the investigational product exhibited a high level of safety and tolerability, making it a promising therapeutic option for further exploration and potential clinical application. Further research, including long-term follow-up and comparisons with other interventions, as well as clinical trials elucidating the mechanism of action, could provide additional insights into the optimal management of erectile dysfunction using the PR/HC/1718/002 capsule.
Conclusion
In conclusion, the present clinical trial demonstrated the potential efficacy and safety of the investigational product containing a combination of herbal extracts, vitamins, and minerals for the management of erectile dysfunction in adult males. The favorable outcomes observed in the test group, including improvements in various sexual function parameters, increased testosterone levels, and enhanced treatment satisfaction, highlight the promise of this intervention. Furthermore, the excellent tolerability profile and absence of adverse events further support the potential clinical utility of the investigational product. While these findings are encouraging, further research is warranted to address the elucidate the mechanisms of action, and establish the optimal clinical applications of this intervention in the management of male sexual health and performance.
Acknowledgement: The authors would like to acknowledge the research team and the back-office team involved in the research work.
References:
- Kapoor R, Kapoor A. Erectile dysfunction: A present day coronary disease risk equivalent. Indian J Med Res, 2016; 144(3): 307-310. doi:10.4103/0971-5916.198669.
- Ayta IA, McKinlay JB, Krane RJ. The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. BJU Int, 1999; 84(1): 50-56. doi:10.1046/j.1464-410x.1999.00142. x.
- Yafi FA, Jenkins L, Albersen M, et al. Erectile dysfunction. Nat Rev Dis Primers, 2016; 2: 16003. doi:10.1038/nrdp.2016.3.
- Malhi GS, Bell E. Questions in Psychiatry (QuiP): Psychological basis for sexual dysfunction in psychiatry. Bipolar Disord, 2022; 24(8): 830-833. doi:10.1111/bdi.13273.
- Ata SI Elsayed, Azab E Azab, Rabia AM Yahya. The Role of Oxytocin, Prolactin, and Estrogen in Male Sexual Functions. J Clinical Research and Reports, 2022; 10(3). DOI:10.31579/2690-1919/228.
- Einarson TR, Acs A, Ludwig C, et al. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc Diabetol, 2018; 17: 83.
- Raheem OA, Su JJ, Wilson JR, Hsieh T-C. The Association of Erectile Dysfunction and Cardiovascular Disease: A Systematic Critical Review. American Journal of Men’s Health, 2017; 11(3): 552-563. doi:10.1177/1557988316630305.
- Burnett AL. The role of nitric oxide in erectile dysfunction: implications for medical therapy. J Clin Hypertens (Greenwich), 2006; 8(12 Suppl 4): 53-62. doi:10.1111/j.1524-6175.2006. 06026.x.
- Lasker GF, Maley JH, Kadowitz PJ. A Review of the Pathophysiology and Novel Treatments for Erectile Dysfunction. Adv Pharmacol Sci, 2010; 2010: 730861. doi:10.1155/2010/730861.
- Costa ED, Rezende BA, Cortes SF, Lemos VS. Neuronal Nitric Oxide Synthase in Vascular Physiology and Diseases. Front Physiol, 2016; 7: 206. doi:10.3389/fphys.2016.00206.
- DeLay KJ, Haney N, Hellstrom WJ. Modifying Risk Factors in the Management of Erectile Dysfunction: A Review. World J Mens Health, 2016; 34(2): 89-100. doi:10.5534/wjmh.2016.34.2.89.
- Sayadi M, Elmafshar R, Razeghian-Jahromi I, Zibaeenezhad MJ. Detection of Coronary Artery Disease by an Erectile Dysfunction Questionnaire. Cardiol Res Pract, 2021; 2021: 6647995. doi:10.1155/2021/6647995.
- Kumar P, Kumar N, Thakur DS, Patidar A. Male hypogonadism: Symptoms and treatment. J Adv Pharm Technol Res, 2010; 1(3): 297-301. doi:10.4103/0110-5558.72420
- Ausó E, Gómez-Vicente V, Esquiva G. Visual Side Effects Linked to Sildenafil Consumption: An Update. Biomedicines, 2021; 9(3): 291. doi:10.3390/biomedicines9030291
- Chandrasekhar K, Kapoor J, Anishetty S. A prospective, randomized double-blind, placebo-controlled study of safety and efficacy of a high-concentration full-spectrum extract of ashwagandha root in reducing stress and anxiety in adults. Indian J Psychol Med, 2012; 34(3): 255-262. doi:10.4103/0253-7176.106022
- Ambiye VR, Langade D, Dongre S, Aptikar P, Kulkarni M, Dongre A. Clinical Evaluation of the Spermatogenic Activity of the Root Extract of Ashwagandha (Withania somnifera) in Oligospermic Males: A Pilot Study. Evidence-based complementary and alternative medicine: eCAM, 2013; 571420.
- Mamidi P, Thakar AB. Efficacy of Ashwagandha (Withania somnifera Dunal. Linn.) in the management of psychogenic erectile dysfunction. Ayu, 2011; 32(3): 322-328. doi:10.4103/0974-8520.93907.
- Herberg LJ, Rose IC. Excitory amino acid pathway in brainstimulation reward. Behav Brain Res, 1990; 39: 230-239.
- Clark BJ, Soo SC, Caron KM, Ikeda Y, Parker KL, Stocco DM. Hormonal and developmental regulation of the steroidogenic acute regulatory protein. Mol Endocrinol, 1995; 9: 1346-1355.
- Caggiula AR, Antelman SM, Chiodo LA, Lineberry CG. Brain dopamine and sexual behavior. In: Usdin E, Kopin IJ, Barchas J, editors. Catecholamines: Basic and Clinical Frontiers. New York: Pergamon Press, 1978; p. 1765–1767.
- Ahmad MK, Mahdi AA, Shukla KK, Islam N, Jaiswar SP, Ahmad S. Mucuna pruriens improves male fertility by its action on the hypothalamus–pituitary–gonadal axis. Fertil Steril, 2009; 92: 1934-1940.
- Hausenblas HA, Heekin K, Mutchie H L, Anton S. A systematic review of randomized controlled trials examining the effectiveness of saffron (Crocus sativus L.) on psychological and behavioral outcomes. J Integr Med, 2015; 13: 231-240.
- Modabbernia A, Sohrabi H, Nasehi AA, Raisi F, Saroukhani S, Jamshidi A, et al. Effect of saffron on fluoxetineinduced sexual impairment in men: randomized double-blind placebocontrolled trial. Psychopharmacology (Berl), 2012; 223: 381-388.
- Shamsa A, Hosseinzadeh H, Molaei M, Shakeri MT, Rajabi O. Evaluation of Crocus sativus L. (saffron) on male erectile dysfunction: a pilot study. Phytomedicine, 2009; 16: 690-693.
- Lefferts EC, Hibner BA, Lefferts WK, et al. Oral vitamin C restores endothelial function during acute inflammation in young and older adults. Physiol Rep, 2021; 9(21): e15104. doi:10.14814/phy2.15104.
- Keleş M, Çırakoğlu A, Benli E, Yazıcı İ, Kadim N, Durmuş H. Correlation between erectile dysfunction and serum B12 levels: a 136-case cross-sectional analysis. Andrology, 2024; 12(3): 613-617. doi:10.1111/andr.13495.
- Toprak O, Sarı Y, Koç A, Sarı E, Kırık A. The impact of hypomagnesemia on erectile dysfunction in elderly, non-diabetic, stage 3 and 4 chronic kidney disease patients: a prospective cross-sectional study. Clin Interv Aging, 2017; 12: 437-444. doi:10.2147/CIA.S129377
- Maghsoumi-Norouzabad L, Zare Javid A, Mansoori A, Dadfar M, Serajian A. The effects of Vitamin D3 supplementation on Spermatogram and endocrine factors in asthenozoospermia infertile men: a randomized, triple blind, placebo-controlled clinical trial. Reprod Biol Endocrinol, 2021; 19(1): 102. doi:10.1186/s12958-021-00789.

