Clinical Pharmacology of Mesalazine
Gian Maria Pacifici*
Professor of Pharmacology: via Sant’Andrea 32, Pisa, Italy
Received Date: 05/09/2024; Published Date: 28/10/2024
*Corresponding author: Gian Maria Pacifici, Professor of Pharmacology, Via Sant’Andrea 32, 56127 Pisa, Italy. Email: prof.pacifici.gianmaria@gmail.com
Abstract
Mesalazine (5-aminosalicylic acid) is a salicylate, is used to treat ulcerative colitis, and the oral daily dose of mesalazine is 2.4 to 4.0 grams. The efficacy and safely of mesalazine, the treatment with mesalazine, and the trials conducted with mesalazine have been reviewed. Mesalazine is N-acetylated mainly by N-acetyltransferase type 1 whereas N-acetyltransferase type 2 has no major effect on the N-acetylation of mesalazine. The pharmacokinetic parameters of mesalazine and N-acetyl mesalazine have been measured in the serum of healthy volunteers following the oral administration of mesalazine and N-acetyl mesalazine is cleared from the serum more slowly than mesalazine. Mesalazine is an inducer of human CYP1A2, CYP2B6, CYP2C9, and CYP3A4. The pharmacokinetics of mesalazine have been studied in healthy volunteers following the oral administration of 1 gram of mesalazine after the 1st, 5th, and 7th dose and mesalazine is slowly absorbed and is rapidly eliminated. The serum peak concentration and the area under the concentration-time curve of mesalazine are similar after the 1st, 5th, and 7th dose suggesting that mesalazine does not accumulate in serum. The toxicity induced by mesalazine has been reviewed and mesalazine may induce hepatic, lung, and cardiac toxicity. The aim of this study is to review the efficacy and safely of mesalazine, the treatment with mesalazine, and the trials conducted with mesalazine. In addition, the metabolism and the pharmacokinetics of mesalazine and the toxicity induced by mesalazine have been reviewed.
Keywords: Efficacy-safely; Mesalazine; Metabolism; Pharmacokinetics; Toxicity; Treatment; Trials
Introduction
Mesalazine (5-aminosalicylic acid) is a salicylate and is used to treat ulcerative colitis. In adults, the oral daily dose of mesalazine is 2.4 to 4.0 grams and mesalazine is slowly absorbed following oral administration. The mesalazine is partly absorbed from the stomach but mostly from the upper small intestine. The rate of absorption is determined by disintegration and dissolution-rates of the tablet administered, by the pH of the mucosa surface, and by gastric emptying time, and food delays the absorption of mesalazine. After absorption, mesalazine is distributed throughout most body tissues and transcellular fluids. Mesalazine is N-acetylated in human liver and mesalazine induces the following cytochromes P-450: CYP1A2, CYP2B6, CYP2C9, and CYP3A4 [1].
Figure 1: Mesalazine molecular structure (molecular weight = 153.137 grams/mole).
Literature Search
The literature search was performed electronically using PubMed database as search engine and the following key words were used: “mesalazine efficacy, safely”, “mesalazine treatment”, “mesalazine trials”, “mesalazine metabolism”, “mesalazine pharmacokinetics”, and “mesalazine toxicity”. In addition, the book: Goodman@Gilman’s. The Pharmacological basis of Therapeutics [1] has been consulted.
Results
Efficacy and safely of mesalazine
Four studies on the efficacy and safely of mesalazine have been reported. Mesalazine, administered at the daily dose of 2 to 4 grams for 8 weeks, effectively and safely treated patients with ulcerative colitis [2]. The high-dose mesalazine (> 2.4 grams daily) was safe and well-tolerated as the low-dose of mesalazine (< 2.4 grams daily) for treatment of patients with ulcerative colitis and the high-dose of mesalazine was not associated with greater risk of adverse-effects [3]. Long-term treatment with mesalazine, administered at the daily dose of 4 grams for > 105 days, was more efficacious (P-value < 0.05) than the short-term treatment of mesalazine, administered at the daily dose of 4 grams for < 105 days, for treatment of patients with ulcerative colitis. Both long-term and short-term treatments of mesalazine were safe and well-tolerated [4]. It was compared the high-dose of 3 grams daily of mesalazine to the low-dose of 1.5 grams daily of mesalazine in treatment of patients with ulcerative colitis. The high-dose of mesalazine was more efficacious (P-value < 0.05) in preventing relapses of ulcerative colitis than the low-dose of mesalazine and the high-dose of mesalazine was safe and well-tolerated [5].
Treatment of patients with mesalazine
Four studies on the treatment of patients with mesalazine have been reported. Mesalazine suppository, administered at the dose of 1 gram at bedtime and 0.5 grams twice-daily in the morning, effectively treated patients with ulcerative colitis [6]. Mesalazine, administered at the daily dose of 4 grams, is an effective and well-tolerated treatment of patients with mid-to-moderate ulcerative colitis [7]. Mesalazine, administered at the daily dose of 2.4 grams, effectively treated patients with ulcerative colitis [8]. A mesalazine dose of < 2 grams daily was used in 15.3% of patients, a dose of 2.0 to 2.9 grams daily was used in 35.0% of patients, a dose of 3.0 to 3.9 grams daily was used in 29.5% of patients, and a dose ≥ 4 grams daily was used in the remaining 20.2% of patients. Patients had ulcerative colitis and all doses of mesalazine were well-tolerated and treated patients with ulcerative colitis [9].
Trials conducted with mesalazine
Four trials conducted with mesalazine have been reported. A double-blind, multicentre, randomized trial was conducted in patients with ulcerative colitis who received either 1 gram of mesalazine tablet or 0.5 grams of mesalazine tablet and the primary efficacy variable was the clinical remission of ulcerative colitis at 8 weeks of therapy. The oral treatment with 1 gram of mesalazine tablet was well-tolerated without providing adverse-effects. The treatment with 0.5 grams of mesalazine tablet was non-inferior to the treatment 1 gram of mesalazine tablet, and mesalazine safely treated ulcerative colitis [10]. A randomized, clinical trial was conducted to assess the effectiveness of mesalazine in improving symptoms of abdominal pain and in preventing diverticulitis in patients with symptomatic uncomplicated diverticular disease. The trial enrolled 1,021 patients: 526 patients (51.5%) received mesalazine and 495 patients (48.5%) received placebo. Mesalazine relieved abdominal symptom more effectively (P-value < 0.05) than placebo and the incidence of diverticulitis was lower (P-value < 0.05) in patients who received mesalazine. Mesalazine was effective in relieving abdominal symptom and in the prevention of diverticulitis in patients with symptomatic uncomplicated diverticular disease [11]. A randomized trial was conducted to compare the efficacy and tolerability of mesalazine administered at the daily dose of 4.8 grams versus those of mesalazine administered at the daily dose of 2.4 grams in 112 patients with ulcerative colitis. Fifty-six patients (50.0%) received mesalazine at the daily dose of 4.8 grams and 56 patients (50.0%) received mesalazine at the daily dose of 2.4 grams, both treatments lasted one year, and the patients had ulcerative colitis. At the end of treatment, the intention to treat analysis occurred in 42 patients (75.0%) who received mesalazine at the daily dose of 4.8 grams and in 36 patients (64.2%) who received mesalazine at the daily dose of 2.4 grams (P-value = 0.300). The daily dose of 4.8 grams of mesalazine reduced the ulcerative colitis in 90.5% of patients and the daily dose of 2.4 grams of mesalazine reduced the ulcerative colitis in 50.0% of patients (P-value = 0.0095, Fisher’s exact rest). In patients with ulcerative colitis, the daily dose of 4.8 grams of mesalazine caused a reduction of ulcerative colitis in greater amounts than the daily dose of 2.4 grams of mesalazine [12]. A randomized, controlled trial was conducted in 83 children, aged 4 to 18 years, with ulcerative colitis. Forty-three children (51.8%) received 1 gram of mesalazine once-daily and 40 children (48.2%) received 1 gram of mesalazine twice-daily. There was no difference in median ulcerative colitis index score between children who received mesalazine once-daily and children who received mesalazine twice-daily (P-value = 0.480). Response to treatment was seen in 25 children (58.1%) who received mesalazine once-daily and in 25 children (62.5%) who received mesalazine twice-daily (P-value = 0.780). Proportion of children with reduction of ulcerative colitis at 6 weeks of treatment was 30.0% of children who received mesalazine once-daily and was 40.0% of children who received mesalazine twice-daily (P-value = 0.350). The rate of adverse-effects was similar in children who received mesalazine once-daily and in children who received mesalazine twice-daily. The reduction of ulcerative colitis was similar in both treatments of mesalazine and the remission of ulcerative colitis was achieved in 35.0% of children who received mesalazine once-daily and twice-daily [13].
Metabolism of mesalazine
Lück et al. [14] observed that mesalazine undergoes extensive metabolism by N-acetylation in human liver. The N-acetylation of mesalazine is mostly performed by N-acetyltransferase type 1 whereas the N-acetyltransferase type 2 has no major effect on the N-acetylation of mesalazine. Hussain et al. [15] measured the pharmacokinetic parameters of mesalazine and N-acetyl mesalazine in the serum of 24 healthy volunteers aged 18 to 36 years. Mesalazine was administered orally at the dose of 0.4 and 0.8 grams thrice-daily and at the dose of 1.2 and 2.4 grams once-daily. Table 1 summarizes the pharmacokinetic parameters of mesalazine and N-acetyl mesalazine which have been obtained in the serum of 24 healthy volunteers.
Table 1: Pharmacokinetic parameters of mesalazine and N-acetyl mesalazine which have been obtained in the serum 24 healthy volunteers. Values are the median and (range), by Hussain et al. [15].
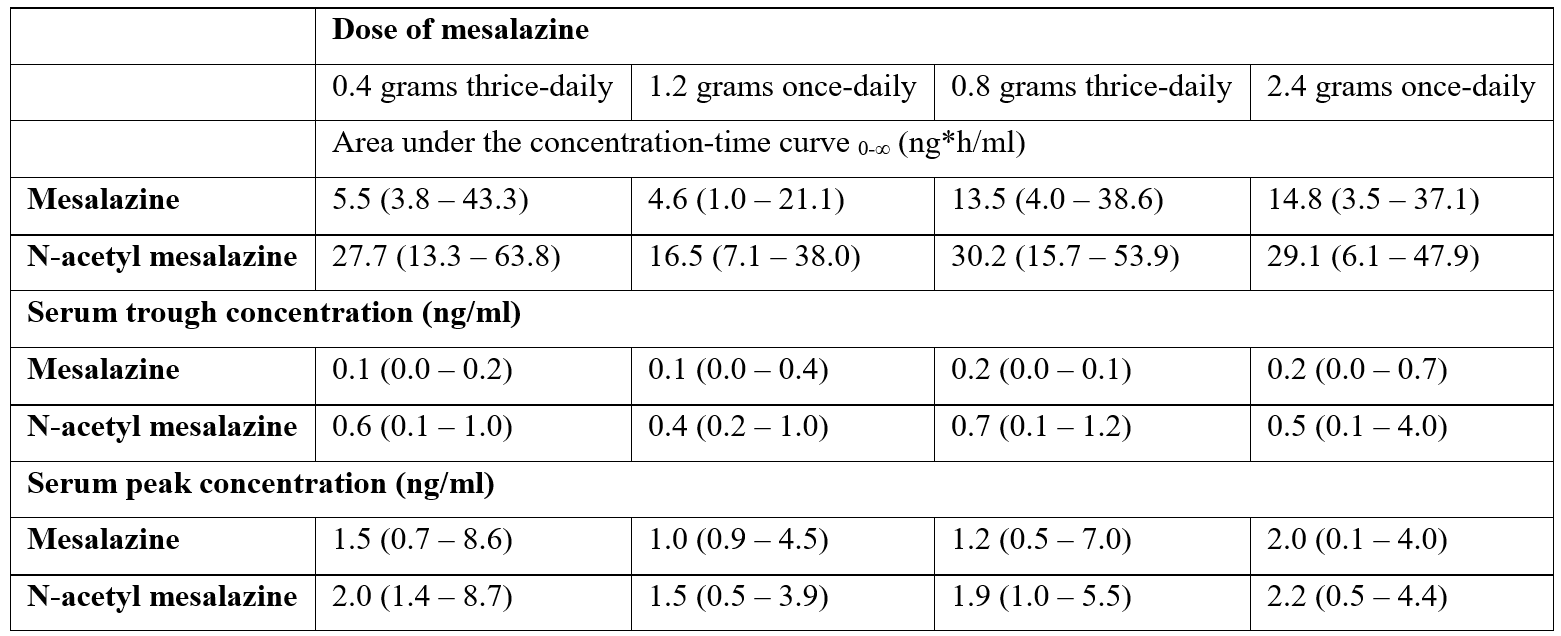
This table shows that the area under the concentration-time curve of N-acetyl mesalazine is higher than that of mesalazine suggesting that N-acetyl mesalazine accumulates in serum more extensively than mesalazine probably because N-acetyl mesalazine is cleared more slowly than mesalazine. The serum trough and peak concentrations of N-acetyl mesalazine are only a little higher than those of mesalazine. Kim et al. [16] observed that mesalazine induces human CYP1A2, CYP2B6, CYP2C9, and CYP3A4.
Pharmacokinetics of mesalazine
Cao et al. [17] studied the pharmacokinetics of mesalazine in the serum of 11 healthy volunteers, aged 27.6+2.6 years and with a body-mass-index of 21.8+1.4 kg/m2, who received mesalazine orally at the dose of 1 gram. The pharmacokinetic parameters of mesalazine were measured after the 1st, 5th, and 7th. Table 2 summarizes the pharmacokinetic parameters of mesalazine which have been obtained in the serum of 11 healthy volunteers who received mesalazine orally at the dose of 1 gram. The pharmacokinetic parameters were assessed after the 1st, 5th, and 7th dose of mesalazine.
Table 2: Pharmacokinetic parameters of mesalazine which have been obtained in the serum of 11 healthy volunteers who received mesalazine orally at the dose of 1 gram. Values are the mean+SD, by Cao et al. [17].
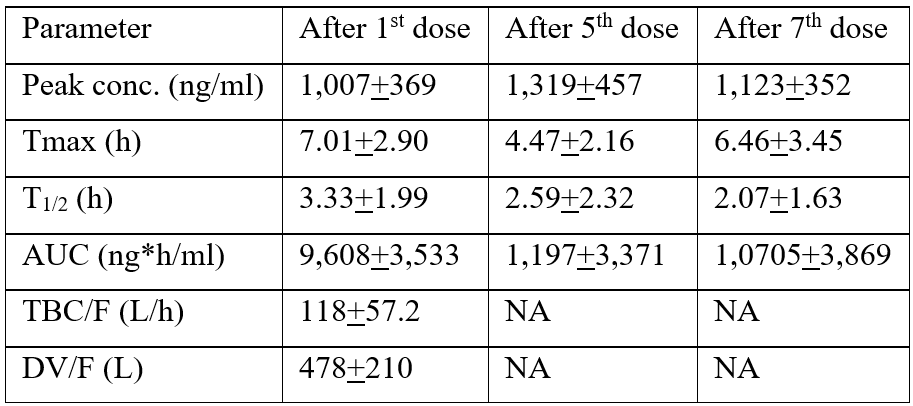
Tmax = time to reach the peak concentration. T1/2 = elimination half-life. AUC = area under the concentration-time curve. TBC = total body clearance. DV = distribution volume. F = bioavailability. NA = not available.
This table shows that mesalazine is slowly absorbed following oral administration as the time to reach the peak concentration ranges from 4.47 to 7.01 hours and mesalazine is rapidly eliminated as the elimination half-life ranges from 2.07 and 3.33 hours. The peak concentration and the area under the concentration-time curve of mesalazine measured after the 1st, 5th, and 7th dose are similar suggesting that mesalazine does not accumulate in serum.
Toxicity induced by mesalazine
The toxicity induced by mesalazine has been reported in eight studies. A 51-year-old woman with ulcerative colitis was treated with mesalazine and two weeks after the start of treatment she presented severe liver cholestatic injury [18]. A 57-year-old woman with ulcerative colitis was treated with mesalazine and she developed worsening respiratory distress and cough [19]. A 75-year-old woman with ulcerative colitis took mesalazine over a period of 2 years and 8 months and she presented a progressive shortness of breath [20]. A 56-year-old woman took mesalazine for ulcerative colitis and she had an elevated eosinophil count in the peripheral blood and in the bronchoalveolar lavage fluid [21]. A 32-year-old man with ulcerative colitis was treated with mesalazine and he had myocarditis and the diagnosis of myocarditis was confirmed with cardiac magnetic resonance imaging [22]. A 79-year-old man had ulcerative colitis and was treated with mesalazine and he developed potentially life-threatening cardiac injury [23]. A 20-year-old man took 14.5 grams of mesalazine rectally and orally for suicide purpose and a diffuse hyperaemia and oedema was observed [24]. A 24-year-old woman with ulcerative colitis was treated with mesalazine at the daily dose of 3 grams and she developed chest pain and dyspnoea [25].
Discussion
Mesalazine (5-aminosalicylic acid) is a salicylate and is used to treat ulcerative colitis. In adults, the oral daily dose of mesalazine is 2.4 to 4.0 grams and mesalazine is slowly absorbed following oral administration [1]. The efficacy and safely of mesalazine have been reviewed. Mesalazine, administered at the daily dose of 2 to 4 grams for 8 weeks, effectively and safely treats patients with ulcerative colitis [2], the high-dose of mesalazine (> 2.4 grams daily) is safe and well-tolerated as the low-dose of mesalazine (< 2.4 grams daily) for treatment of patients with ulcerative colitis and the high-dose of mesalazine is not associated with greater risk of adverse-effects [3], the long-term treatment with mesalazine, administered at the daily dose of 4 grams for > 105 days, is more efficacious (P-value < 0.05) than the short-term treatment of mesalazine, administered at the daily dose of 4 grams for < 105 days, for treatment of patients with ulcerative colitis and both treatments are safe and well-tolerated [4], and the high-dose of 3 grams of mesalazine is more efficacious (P-value < 0.05) that the low-dose of 1.5 grams of mesalazine in preventing relapses of ulcerative colitis and the high-dose of mesalazine is safe and well-tolerated [5]. These results indicate that mesalazine is efficacy and safe in treatment of patients with ulcerative colitis. The treatment of patients with mesalazine has been reviewed. Mesalazine suppository, administered at the dose of 1 gram at bedtime and 0.5 grams twice-daily in the morning, effectively treated patients with ulcerative colitis [6], mesalazine, administered at the daily dose of 4 grams, is an effective and well-tolerated treatment of patients with mild-to-moderate ulcerative colitis [7], mesalazine, administered at the daily dose of 2.4 grams, effectively treats patients with ulcerative colitis [8], and mesalazine, administered at different doses, effectively treats patients with ulcerative colitis and different doses of mesalazine are well-tolerated [9]. These results indicate that mesalazine, administered at different doses, effectively treats patients with ulcerative colitis. The trials conducted with mesalazine have been reviewed. A double-blind, multicentre, randomized trial was conducted in patients with ulcerative colitis who received either 1 gram of mesalazine tablet or 0.5 grams of mesalazine tablet. One gram of mesalazine tablet effectively treats patients with ulcerative colitis without providing adverse-effects. The treatment with 0.5 grams of mesalazine tablet is not inferior to the treatment with 1 gram of mesalazine tablet in patients with ulcerative colitis and mesalazine is well-tolerated [10], a randomized, clinical trial assessed the effectiveness of mesalazine in improving symptoms of abdominal pain and in preventing diverticulitis in patients with symptomatic uncomplicated diverticulitis disease. Patients received either mesalazine or placebo and mesalazine reliefs abdominal symptoms more effectively (P-value < 0.05) than placebo and the incidence of diverticulitis is lower (P-value < 0.05) in patients who received mesalazine. Mesalazine effectively reliefs abdominal pain and prevents diverticulitis in patients with symptomatic uncomplicated diverticulitis disease [11], a randomized trial assessed the efficacy and tolerability of mesalazine administered at the daily dose of 4.8 grams versus those of mesalazine administered at the daily dose of 2.4 grams in patients with ulcerative colitis and both treatments lasted one year. The intention to treat analysis occurs in 75.0% of patients who received mesalazine at the daily dose of 4.8 grams and in 64.2% of patients who received mesalazine at the daily dose of 2.4 grams (P-value = 0.300). The daily dose of 4.8 and 2.4 grams of mesalazine reduces the ulcerative colitis in 90.5% and in 50.0% (P-value = 0.0095), respectively. In patients with ulcerative colitis, the daily dose of 4.8 grams of mesalazine reduces the ulcerative colitis in greater amounts than the daily dose of 2.4 grams of mesalazine [12], and a randomized, controlled trial assessed the efficacy of mesalazine in treating children with ulcerative colitis. Children received either 1 gram of mesalazine once-daily or 1 gram of mesalazine twice-daily. Response to treatment is seen in 58.1% and in 62.5% of children who received mesalazine once-daily and twice-daily (P-value = 0.780), respectively, and the proportion of children with reduction of ulcerative colitis at 6 weeks of treatment is 30.0% and 40.0% (P-value = 0.350) in children who received mesalazine once-daily and twice-daily, respectively. The rate of adverse-effects and the reduction of ulcerative colitis are similar in children who received mesalazine once-daily or twice-daily [13]. The metabolism of mesalazine has been reviewed. Mesalazine is N-acetylated in human liver and the N-acetylation of mesalazine is mostly due to N-acetyltransferase type 1 whereas the N-acetyltransferase type 2 has no major effect on the N-acetylation of mesalazine [14]. Hussain et al. [15] measured the pharmacokinetic parameters of mesalazine and N-acetyl mesalazine in the serum of healthy volunteers and observed that the area under the concentration-time curve of N-acetyl mesalazine is higher than that of mesalazine suggesting that N-acetyl mesalazine accumulates in serum probably because it is cleared more slowly than mesalazine. Kim et al. [16] observed that mesalazine induces the human CYP1A2, CYP2B6, CYP2C9, and CYP3A4. Cao et al. [17] studied the pharmacokinetics of mesalazine in healthy volunteers and the pharmacokinetic parameters of mesalazine were measured after the 1st, 5th, and 7th oral dose. Mesalazine is slowly absorbed as the time to reach the peak concentration ranges from 4.47 and 7.01 hours and mesalazine is rapidly eliminated as the elimination half-life of mesalazine ranges from 2.07 to 3.33 hours. The peak concentration and the area under the concentration-time curve of mesalazine are similar after the 1st, 5th, and 7th dose suggesting that mesalazine does not accumulate is serum. The toxicity induced by mesalazine has been reviewed. All reported cases of toxicity consist in patients with ulcerative colitis who were treated with mesalazine. Mesalazine induces severe liver cholestatic injury [18], induces respiratory distress and cough [19], induces progressive shortness of breath [20], induces elevated eosinophil count in the peripheral blood and in the bronchoalveolar lavage fluid [21], induces myocarditis [22] and cardiac injury [23], induces diffuse hyperaemia and oedema [24], and induces chest pain and dyspnoea [25]. These results indicate that mesalazine may induce hepatic, lung, and cardiac toxicity.
Conclusion
Mesalazine (5-aminosalicylic acid) is a salicylate and in adult the oral daily dose of mesalazine is 2.4 to 4.0 grams. The efficacy and safely of mesalazine, the treatment of patients with mesalazine, and the trials conducted with mesalazine have been reviewed. Mesalazine is N-acetylated in the human liver, the N-acetylation of mesalazine is mostly due to N-acetyltransferase type 1, and N-acetyl mesalazine is cleared from serum more slowly than mesalazine. Mesalazine induces human CYP1A2, CYP2B6, CYP2C9, and CYP3A4. The pharmacokinetics of mesalazine have been studied in healthy volunteers after the 1st, 5th, and 7th oral dose and mesalazine is slowly absorbed as the time to reach ranges from 4.47 to 7.01 hours and mesalazine is rapidly eliminated as the elimination half-life of mesalazine ranges from 2.07 to 3.33 hours. The peak concentration and the area under the concentration-time curve of mesalazine are similar after the 1st, 5th, and the 7th dose suggesting that mesalazine does not accumulate is serum. The toxicity induced by mesalazine has been reviewed and mesalazine may cause hepatic, lung, and cardiac toxicity. The aim of this study is to review the clinical pharmacology of mesalazine.
Conflict of interests: The authors declare no conflicts of financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employments, gifts, and honoraria.
This article is a review and drugs have not been administered to men or animals.
Acknowledgments: The author thanks Dr. Patrizia Ciucci and Dr. Francesco Varricchio, of the Medical Library of the University of Pisa, for retrieving the scientific literature.
References
- Grosser T, Ricciotti E, FitzGerald GA. Pharmacotherapy of inflammation, Fever, Pain, and Gout, In Goodman@Gilman’s. The Pharmacological Basis of Therapeutics. Brunton LL, Knollmann BC editors. Mc Graw Hill, 14th 25023: pp. 829-56.
- Paridaens K, Fullarton JR, Travis SPL. Efficacy and safety of oral Pentasa (prolonged-release mesalazine) in mild-to-moderate ulcerative colitis: a systematic review and meta-analysis. Curr Med Res Opin, 2021; 37(11): 1891-1900.
- Sehgal P, Colombel J-F, Aboubakr A, Narula N. Systematic review: safety of mesalazine in ulcerative colitis. Aliment Pharmacol Ther, 2018; 47(12): 1597-1609.
- Takeshima F, Matsumura M, Makiyama K, Ohba K, Yamakawa M, Nishiyama H, et al. Efficacy of long-term 4.0 g/day mesalazine (Pentasa) for maintenance therapy in ulcerative colitis. Med Sci Monit, 2014; 20: 1314-1318. doi: 10.12659.
- Fockens P, Mulder CJ, Tytgat GN, Blok P, Ferwerda J, Meuwissen SG, et al. Comparison of the efficacy and safety of 1.5 compared with 3.0 g oral slow-release mesalazine (Pentasa) in the maintenance treatment of ulcerative colitis. Dutch Pentasa Study Group. Eur J Gastroenterol Hepatol, 1995; 7(11): 1025-1030.
- Kato S. Ishibashi A, Kani K, Yakabi K. Optimized Management of Ulcerative Proctitis: When and How to Use Mesalazine Suppository. Digestion, 2018; 97(1): 59-63.
- Schroeder KW. Role of mesalazine in acute and long-term treatment of ulcerative colitis and its complications. Scand J Gastroenterol Suppl, 2002; (236): 42-47.
- Ye B, van Langenberg DR. Mesalazine preparations for the treatment of ulcerative colitis: Are all created equal? World J Gastrointest Pharmacol Ther, 2015; 6(4): 137-144.
- Algaba A, Guerra I, de Paredes AGG, Tejero MH, Ferre C, Bonillo D, et al. What is the real-life maintenance mesalazine dose in ulcerative colitis? Rev Esp Enferm Dig, 2017; 109(2): 114-121.
- Dignass A, Schnabel R, Romatowski J, Pavlenko V, Dorofeyev A, Derova J, et al. Efficacy and safety of a novel high-dose mesalazine tablet in mild to moderate active ulcerative colitis: a double-blind, multicentre, randomised trial. United European Gastroenterol J, 2018; 6(1): 138-147.
- Picchio M, Elisei W, Brandimarte G, Di Mario F, Malfertheiner P, Scarpignato P, et al. Mesalazine for the Treatment of Symptomatic Uncomplicated Diverticular Disease of the Colon and for Primary Prevention of Diverticulitis. A Systematic Review of Randomized Clinical Trials. J Clin Gastroenterol, 2016; 50(10): S64-S69.
- Pica R, Cassieri C, Cocco A, Zippi M, Marcheggiano A, De Nitto D, et al. A randomized trial comparing 4.8 vs. 2.4 g/day of oral mesalazine for maintenance of remission in ulcerative colitis. Digestive Liver Dis, 2015; 47(11): 933-937.
- Turner D, Yerushalmi B, Kori M, Broide E, Mozer-Glassberg Y, Shaoul R, et al. Once- Versus Twice-daily Mesalazine to Induce Remission in Paediatric Ulcerative Colitis: A Randomised Controlled Trial. J Crohns Colitis, 2017; 11(5): 527-533.
- Lück H, Kinzig M, Jetter A, Fuhr U, Sörgel F. Mesalazine pharmacokinetics and NAT2 phenotype. Eur J Clin Pharmacol, 2009; 65(1): 47-54.
- Hussain FN, Ajjan RA, Kapur K, Moustafa M, Riley SA. Once versus divided daily dosing with delayed-release mesalazine: a study of tissue drug concentrations and standard pharmacokinetic parameters. Aliment Pharmacol Ther, 2001; 15(1): 53-62.
- Kim Y-H, Bae Y-J, Kim HS, Cha H-J, Yun J-S, Shin J-S, et al. Measurement of Human Cytochrome P450 Enzyme Induction Based on Mesalazine and Mosapride Citrate Treatments Using a Luminescent Assay. Biomol Ther (Seoul), 2015; 23(5): 486-492.
- Cao Y, Wang J, Tang X, Tian Y, Yu J, Liang H, et al. Pharmacokinetic and safety profiles of mesalazine enema in healthy Chinese subjects: A single- and multiple-dose study. Plos One, 2024; 19(2): e0296940.
- Garrido I, Santos AL, Lopes J, Lopes S, Macedo G. Hepatotoxicity in inflammatory bowel disease: mesalazine, the forgotten drug. Eur J Gastroenterol Hepatol, 2021; 33(Suppl 1): e1067-1070.
- Pereira FM, Marques C, Boncoraglio T, Esteves J, Silva M, Braga J, et al. Mesalazine-induced Hypersensitivity Pneumonitis. Eur J Case Rep Intern Med, 2021; 8(1): 002194. doi: 10.12890.
- Huang P-H, Kuo C-J, Lin C-W, Cheng Y-M, Hu H-C, Lin C-Y, et al. Mesalazine-related lung disease in a patient with ulcerative colitis: A case. Medicine (Baltimore), 2018; 97(48): e13242. doi: 10.1097.
- Zhang Y, Luo L, Wang X, Liu X, Wang X, Ding Y. Mesalazine-induced eosinophilic pneumonia with bone marrow infiltration: a case report and literature review. Ther Clin Risk Manag, 2016; 12: 975-81. doi: 10.2147.
- Kyriakou M, Sakellaropoulos S, Constantinides T, Chatzantonis G, Avraamides P, Mitsisa A. Myocarditis Related to the Use of Mesalazine. J Med Cases, 2023; 14(7): 237-243.
- Andrei V, D'Ettore N, Scheggi V, di Mario C. Mesalazine-induced myopericarditis: a case series. Eur Heart J Case Rep, 2023; 7(9): ytad424. doi: 10.1093.
- Koseoglu Z, Satar S, Kara B, Sebe A, Kosenli O. An unusual case of mesalazine intoxication: oral and rectal overloading of the rectal suppository form. Hum Exp Toxicol, 2011; 30(7): 772-776.
- Costa JM, Carvalho SD, Soares JB. Mesalazine-induced Bronchiolitis Obliterans with Organising Pneumonia in a Young Patient with Ulcerative Colitis. J Crohns Colitis, 2018; 12(11): 1377-1378.

