Demographic Analysis and Clinico-Pathological Characteristics of Breast Cancer Presentation in Erbil, Iraq
Dr. Ahmed Abdulkadir Baban*
Department of Surgery, College of Medicine, Hawler Medical University, Erbil-Iraq
Received Date: 02/07/2024; Published Date: 17/10/2024
*Corresponding author: Dr. Ahmed Abdulkadir Baban, MBChB, MRCS, FRCS, FACS, Assistant Professor, Department of Surgery, College of Medicine, Hawler Medical University, Erbil, Iraq
Abstract
Background: Breast cancer is the commonest type of female malignant neoplasms worldwide, including Iraq. Its clinical presentation and diagnosis depend on various factors including the presence of screening program, level of education, socio-economic status, and rate of mutation in breast cancer associated genes.
Aim of the study: The aim of this study was to review the main demographic characteristics and clinico-pathological presentation of breast cancer in women in Erbil.
Patients and Methods: A hospital based cross-sectional study was conducted between January 2020 and January 2023 at the main Centre for early breast cancer detection and the Department of Surgery at Rizgary Teaching Hospital, Erbil, Iraq.
Results: Out of 1276 patients presented with breast symptoms, 357 (28%) were diagnosed with neoplastic breast abnormalities. One-third of the breast cancer patients were aged 40–50 years (Patients’ mean age was 48±5 Range: 28-83 years). 65.3% came from urban areas, and 71.2% were married. Positive family history was recorded in 21.7%. Patients self-presented with a lump in 79.5% of the cases. Accordingly, 49.8% of all patients were stage III and IV at presentation. The main histological type was Invasive Ductal Carcinoma, of which 56.5% was grade II and 30.7% was grade III.
Conclusion: These findings justify collaborative efforts in our region to establish breast screening and women-health education programs to detect and treat breast cancer at earlier stages.
Introduction
Breast cancer comprises the most commonly diagnosed cancer among women. In the United States approximately 182,000 women with breast neoplasm diagnosed annually, accounting for about 26% of all incident cancers among women [1]. In Iraq, breast cancer is the commonest type of female malignancy accounting for approximately one third of the registered female cancers according to the Iraqi Cancer Registry [2]. The aim of this study was to review the main demographic characteristics and clinic-pathological presentation of females diagnosed with breast cancer.
Patients and Methods
This hospital-based cross-sectional study was carried out at the main Centre for early breast cancer detection and the Department of Surgery at Rizgary Teaching Hospital in collaboration with the Department of Pathology. Clinical notes of all patients presented with breast symptoms between January 2017-January 2022 were reviewed.
The following data were captured: date of birth, marital status, permanent residence, pregnancies and lactation, use of contraceptive pills, and family history of breast cancer. Examination of tissue biopsies and nodal status were recorded. Carcinoma type was determined following the WHO classification [3] while the TNM (tumour, node, and metastasis) staging system of the American Joint Committee on Cancer (AJCC) was used in recording the clinical stage of the disease [4]. Ductal carcinoma was graded following the recommendations of Scarff, Bloom and Richardson [5] Staining of the HER2 protein; ERs and PRs were performed for all specimens as previously described [6,7].
Ethical considerations
The study was approved by the research Ethical Committee of Hawler Medical University/ College of Medicine-Erbil. An informed and verbal consent was taken from each participant.
Results
Among 1276 patients presented to the main Centre for early detection of breast cancer and Rizgary Teaching hospital in Erbil with breast lump, nipple discharge and skin changes, 357 patients (28%) revealed pathological diagnosis consistent with breast carcinomas. Table 1 illustrates the demographic characteristics of the 357 patients with breast carcinoma. The patient’s age ranged between 25 to 83 years with a mean age of 48±5 years. More than half (203, 56.9%) were 50 years or younger i.e., age groups (21-30, 31-40,41-50). Regarding the marital status of the patients, the distribution of married, unmarried, and widow or divorce conditions were as such: (234, 65.5%), (97, 21.2%), (26, 7.3%) respectively. Two hundred and ninety-four patients (82.4%) were from urban and the remaining were from rural areas. In relation to breastfeeding history, 301(84.3%) patients had given a history of breastfeeding. Sixty-seven patients (18.8%) had oral contraceptives at some stage. Fifty-three patients (14.8%) were smokers.
Family history of breast cancer was reported in 83 patients (23.2%); 57 of these (68.7%) had at least one first-degree relative with breast cancer. 341 patients (95.5%) presented with a self-detected lump. It was noted that 297 out of these 341 patients (87.1%) sought medical advice within the first month of detecting the lump, while 44 patients (12.9%) did so within one year.
Table 2 showed the clinicopathological characteristics of the primary tumor in the studied group. The majority of tumors were in the upper outer quadrant (212/375, 59.4 %) and 243/375 patients (68.1%) had axillary nodal involvement.
Applying the WHO classification, 303/357 (84.9%) were invasive ductal carcinoma, and 38/357 (10.6%) were invasive lobular.
According to the AJCC system, the distribution of cases and their frequencies were as such (43, 12.1 %), (185,51.8%), (104, 29.1%), and (25, 7%) for stages I, II, III, and IV respectively.
Scarff–Bloom–Richardson classification in terms of grading, (25,7%) were grade I, 219 (61.4%) grade II and the rest (31.7%) were grade III. Immunohistochemical staining for Her-2/neu protein over-expression was positive in 48.5% (173/357).
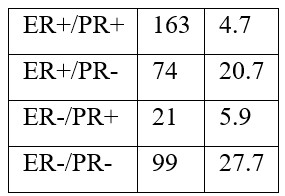
The distribution of ER/PR phenotype as illustrated in Table 3 was as such: ER+/PR+ 163/357(45.7%) ER+/PR- 74/357 (20.7%), ER-/PR+ 21/357 (5.9%), and ER- /PR- 99/357 (27.7%).
Table 1: Demographic characteristics of the 357 patients diagnosed with breast cancer.

Table 2: Clinico-pathological characteristics of the primary tumor (N=375).

Table 3: Distribution of hormone receptor (ER and PR) phenotypes in pathological specimens belonging to 357 breast cancer patients.
Discussion
According to WHO mortality database, cancer is the fourth ranked cause of death in the Eastern Mediterranean Region (EMR), after cardiovascular diseases, infectious/parasitic diseases and injuries [8]. In this current study, breast cancer was diagnosed in 28% of patients presenting with apparent breast lumps, and more than half of the patients (203/357, 56.9%) were 50 years or younger. A study by Alwan NAS [2] in Iraq demonstrated that breast cancer was diagnosed in 14.3% of patients presenting with apparent breast lumps, and one third of the patients had been diagnosed in their forties, where the peak frequency occurred, but an obvious decline was displayed after the age of 60 years.
The continuing trend for this disease to affect younger generations has been comprehensively illustrated in the Iraqi Cancer Registry [8]. WHO estimates revealed that approximately half of the cancers in the EMR occur before the age of 55 and that the age standardized incidence rates of all cancers in this region is expected to double as risk factor exposure increases [9]. Hacihasanoglu & Gozum, also reported the mean age of patients with breast cancer to be one decade lower in Turkey, compared to western countries [10]. Comparable to our study, Hashemian H et al 2016 showed in their study the highest incidence rate of breast cancer was observed in patients aged 40-49 years [11]. Similar observations were also reported by Suresh p [12] from North India, Kokiwar P [12] from South India and Acharya SC [13] from Nepal. Our findings was similar to studies from other African Centre were the mean age is 48 years and approximately two-thirds are premenopausal [14,15]. The increase in the incidence of breast cancer is most likely due to the adoption of a more “Westernized” lifestyle, like physical activity, adverse changes in diet, and fertility (Shin et al., 2010, Youden et al., 2014, Park et al., 2008, Porter, 2008). This effect has been greatest among younger women living in urban areas of lower and middle-income countries (Green and Raina, 2008).
This finding differs from that demonstrated in studies from Western and developed countries. In this regard, American Cancer Society (ACS) has suggested age to be the second leading risk factor for breast cancer following gender [16]. It is possible that the higher median age at diagnosis among women in Western countries could be partly explained by the population-based mammography breast-screening program that is widely available in these countries, which mainly targets women aged 50 years and over (Youlden et al., 2012) [17] which is not at all available in India neither in Erbil province. Another factor for the difference in age of onset of female breast cancer between Asian countries and Western countries can be attributed to differences in life expectancy, with a greater proportion of the population in the younger age groups for females in developing countries (United Nations, 2010).
The present study showed that family history of breast cancer was reported in 83 patients (23.2%); 57 of these (68.7%) had an affected first-degree relative. The study by Alwan N 2010 in Iraq documented that family history was positive in 16.2% of 721 females 2. Having a positive family history of breast cancer is a well-known risk factor for the disease. Women having one first degree relative with breast cancer have nearly two-fold increased risk of developing breast cancer [18]. Nevertheless, this result was not in congruence with the studies by Khameghian et al. (frequency: 2%) [19], and Gao (frequency: 3.7%) [20]. The prevalence of positive family history of breast cancer might vary based on the race/ethnicity of the patients, which could be the reason for this inconsistency in results. The relatively high incidence of positive family history noted in this study could be related to the customary consanguineous marriages, which is common in this region.
The findings from this study indicates an urgent cooperation between department of health, education and Early detection breast Centre to develop breast screening program in Erbil province and ideally include patients 40 years’ old, together with targeted education would help in early diagnosis and potentially reduce the current high incidence of stage III and IV breast cancer.
References
- Chasib TJ, Al-Hawaz M, Jasim NH. Evaluation of the estrogen and progesterone receptors in female breast Cancer in respect to age, grade, and stage. Bas J Surg, 2013; 19(2): 9-18.
- Alwan NA. Breast cancer: demographic characteristics and clinic-pathological presentation of patients in Iraq. East Mediterr Health J, 2010; 16(11): 1159-1164.
- Edge SB, et al. AJCC Cancer Staging Manual, 7th ed. New York, Springer–Verlag, 2010.
- American Joint Committee on Cancer. The breast. In: AJC cancer staging manual, 6th ed. New York, Springer, 2002: 171-180.
- Rosai J, ed. Rosai and Ackerman’s surgical pathology: breast, 9th ed, vol. II. St. Louis, Mosby, 2004: 1763–1839.
- Plesan DM, Georgescu M, Patrana N, Nina T, Plesan C. Immunohistochemical evaluation of hormone receptor with predictive value in mammary carcinoma Rom J histological grade in invasive breast cancers in a cohort of Saudi Arabia.Turk Patoloji Derj, 2012; 28(1): 38-43.
- Arafah M. Correlation of hormone receptors with HER2-neuprotein expression and the Morphol Embryol, 2011; 52(4): 1331-1336.
- Iraqi Cancer Board. Results of the Iraqi Cancer Registry 2004. Baghdad, Iraqi Cancer Registry Center, Ministry of Health, 2007.
- Rennert G. Breast cancer. In: Freedman L et al. Cancer incidence in four member counties (Cyprus, Egypt, Israel and Jordan) of the Middle East Cancer Consortium (MECC) compare to SEER. Bethesda, Maryland, NCI (NIH Pub. No. 06-5873), 2006; 73–81.
- Hacihasanoglu R, Gozum S. The effect of training on the knowledge levels and beliefs regarding breast self-examination on women attending a public education center. European Journal of Oncology Nursing, 2008; 12(1): 58-64.
- Hashemian M, Akbar Zade R, Khosroabadi AA, Asadi ZS, Saleh Abadi S, Hoseini BL, et al. A Ten-Year Study on the Prevalence and Frequency of Risk Factors for Breast Cancer in Sabzevar, Iran. Journal of Midwifery and Reproductive Health, 2016; 4(3): 673-678.
- Suresh P, Batra U, Doval DC. Epidemiological and clinical profile of triple negative breast cancer at a cancer hospital in North India. Indian J Med Paediatr Oncol, 2013; 34(2): 89-95.
- Acharya SC, Jha AK, Manandhar T. Clinical profile of patients presenting with breast cancer in Nepal. Kathmandu Univ Med J (KUMJ), 2012; 10(39): 3-7.
- Adesunkanmi ARK, Lawal OO, Adelusola KA, Durosimi MA. The severity, outcome and challenges of breast cancer in Nigeria. Breast, 2006; 15(3): 399-409.
- Rambau PF, Chalya PL, Manyama MM, Jackson KJ. Pathological features of Breast Cancer seen in Northwestern Tanzania: A nine years retrospective study. BMC Research Notes, 2011; page 214.
- Breast cancer statistics. Center for Disease Control and Prevention, 2010.
- Youlden DR, Cramb SM, Dunn NA, Muller JM, Pyke CM, Baade PD, et al. The descriptive epidemiology of female breast cancer: An international comparison of screening, incidence, survival and mortality. Cancer Epidemiology, 2012; 36: 237248. doi:10.1016/j.canep.2012.02.007.
- Kilfoy BA, Zhang Y, Shu XO, et al. Family history of malignancies and risk of breast cancer: prospective data from the Shanghai women's health study. Cancer Causes Control, 2008; 19: 1139-1145.
- Khamechian T, Mazouchi T. Frequency of positive family history of breast cancer in 100 breast cancer sufferers in Kashan. Journal of Kashan University of Medical Sciences, 2003; 7(4): 90-94.
- Gao YT, Shu XO, Dai Q, Potter JD, Brinton LA, Wen W, et al. Association of menstrual and reproductive factors with breast cancer risk: results from the Shanghai Breast Cancer Study. International Journal of Cancer, 2000; 87(2): 295-300.

