Soybean Phytoestrogen-Rich Extract Lowers Total Lipids and Lipid Peroxidation Index in 4-Vinyl Cyclohexane Diepoxide –Induced Menopause in Female Wistar Rats
Mathias Abiodun Emokpae1,*, Edusola Juliana Olaniyan1 and Henry Osamuyi Uwumarongie2
1Department of Medical Laboratory Science, School of Basic Medical Sciences, College of Medical Sciences, University of Benin, Nigeria
2Department of Pharmacognosy, Faculty of Pharmacy, University of Benin, Benin City, Nigeria
Received Date: 10/06/2024; Published Date: 07/10/2024
*Corresponding author: Emokpae MA, Department of Medical Laboratory Science, School of Basic Medical Sciences, College of Medical Sciences, University of Benin, Benin City, Nigeria
Abstract
Background: Menopause is characterized by increased oxidative stress and may be due to a decline in the levels of estrogen (a natural antioxidant by itself). It can cause changes in the lipid composition of the blood and tissues, leading to enhanced lipid peroxidation. Oxidative stress and lipid peroxidation play a major role in the pathogenesis of diseases associated with menopause.
Objective: To assess the potential of Soybean phytoestrogen-rich extract to reduce the lipid peroxidation index in 4-vinyl cyclohexane diepoxide (VCD)-induced menopause in Wistar rats.
Materials and Methods: Serum lipid profile, malondialdehyde (MDA), and total antioxidant status (TAS) were determined in VCD-induced menopause in Wistar rats, following the administration of varying concentrations of Soybean phytoestrogen-rich extract. Total lipids and lipid peroxidation index were calculated and compared with controls.
Results: Serum MDA levels increased while TAS levels decreased in VCD-induced menopause rats without Soybean phytoestrogen supplementation. In groups of rats that received supplementation, serum MDA levels decreased while TAS increased in a dose-dependent manner from group 3 which received 200 mg/kg, to group 4 which received 400 mg/kg, and group 5 which received 600 mg/kg. The differences in the mean were statistically significant (p<0.001) compared with the control. The lipid peroxidation index was significantly higher in rats in group 2 (positive control) compared with group 1 (negative control). The lipid peroxidation index decreased with increasing concentrations of Soybeans phytoestrogen-rich extract administered. The total lipids concentrations were also reduced in a dose-dependent manner, and the differences in the mean total lipids were statistically significant (p<0.001) compared with negative control (group 1) and positive control (group 2).
Conclusion: The supplementation of Soybean phytoestrogen-rich extract to VCD-induced menopause in Wistar rats increased the levels of serum TAS, decreased MDA concentrations, and lowered the lipid peroxidation index. This experiment can be replicated in humans to fully assess the potential of Soybean phytoestrogen-rich in preventing menopause-associated oxidative stress and lipid peroxidation.
Keywords: Female Wistar rats, Phytoestrogens, Lipid Peroxidation, 4-vinyl-1-cyclohexene dioxide, Lipids, Menopause
Introduction
Changes in lipids and lipid peroxidation among postmenopausal women are common due to hormonal changes compared to their reproductive years. Longer time since menopause was associated with an atherogenic lipid profile and marginally low levels of high-density lipoprotein concentrations among Chinese women [1]. Although the frequency of Cardiovascular Diseases (CVDs) is high with increasing age for both males and females, the risk of CVD is higher in postmenopausal women than in men of the same age and premenopausal women [2]. Menopause is a natural aging condition characterized by permanent cessation of ovarian follicular function, due to a decline in the secretion of estrogen and often exacerbates the risk of CVDs [3]. More so, most women now live about one-third of their lifespan after menopause [1]. Given that dyslipidemia is an important risk factor for CVDs, prevention and practical management of dyslipidemia are desirable to improve the quality of life of post-menopausal women.
Menopause is characterized by increased oxidative stress [4] and may be due to a decline in estrogen levels (a natural antioxidant by itself). It can cause changes in the lipid composition of the blood and tissues, leading to enhanced lipid peroxidation [5]. Several studies have indicated that oxidative stress plays a major role in the pathogenesis of diseases associated with menopause [3,4,6,7].
The chemical 4-Vinyl Cyclohexene diepoxide (VCD) induces selective destruction of small ovarian pre-antral (primordial and primary) follicles in Wistar rats and mice. It chemically expedites the natural, apoptotic process of atresia to bring about the gradual ovarian failure and loss of estrogen production. In-vivo exposure of Wistar rats to VCD depletes the primordial and primary follicles, by directly interacting with the oocyte-associated c-kit receptor and preventing its auto-phosphorylation thereby causing pre-matured menopause. This model is believed to produce menopause similar to menopause in women [8,9].
Malondialdehyde (MDA) is derived from the peroxidation of polyunsaturated fatty acids and is used as a marker to measure oxidative stress in biological fluids. Since the body's antioxidants are highly devised, measurement of total antioxidant status (TAS) is a suitable and reliable method of assessing extracellular non-enzymatic antioxidants in biological fluids [10].
The health system in our setting pays more attention to women of the reproductive age group than postmenopausal women, who are more or less neglected even though the prevalence and severity of menopausal symptoms are very high in both urban and rural centers [11]. The orthodox treatment for menopausal symptoms is Hormone Replacement Therapy (HRT), unfortunately, this treatment is not only expensive but may complicate clinical issues that require an in-depth risk and benefit assessment. Therefore, other sources of supplements containing phytoestrogen are suggested for the ease of transition from perimenopause to post-menopause, and the amelioration of post-menopausal symptoms [7]. Some authors have suggested the consumption of phytoestrogens as an important action plan to minimize or prevent menopause-associated low-grade chronic inflammation and oxidative stress [6]. This study aimed to assess the potential of Soybean phytoestrogen-rich extract to reduce the lipid peroxidation index in VCD-induced menopause in Wistar rats.
Materials and Methods
Ethical Consideration and Induction of Menopause
The study protocol was approved by the Animal Studies Ethics Review Committee, Faculty of Pharmacy, University of Benin, Benin City.
Menopause was chemically induced in sexually matured female Wistar rats (age 10 weeks). The rats were separated into 4 groups of three rats each. Group 1 to group 4 were administered 0 mg/kg, 40 mg/kg, 80 mg/kg, and 160 mg/kg respectively with VCD intraperitoneally (V3630; Sigma-Aldrich, St. Louis, MO) for 15 consecutive days. Wistar rats in group 1 (controls) were given Sesame oil, which is the solvent used for VCD as previously described [12]. On the 16th day, the estrous cycles were kept track of using vaginal cytology to know when cycling stopped. This is an indication of ovarian failure. The animals were observed to be acyclic after 15 consecutive days in continuous diestrus. Thereafter, blood was collected by cardiac puncture following chloroform anesthesia for estradiol, follicle-stimulating hormone, and anti-Mullerian hormone determinations. The ovaries were processed for histomorphological examination by counting the follicles to confirm the onset of menopause in the rats as previously described [8].
Experimental design
Animal models of VCD-induced menopause in Wistar rats were separated into six (6) groups of five rats per group. Menopause was induced using 80 mg/kg VCD intraperitoneally as described above.
Group 1: Negative control received Sesame oil only
Group 2: Positive control was given 80 mg/kg VCD only
Group 3: Given 80 mg/kg VCD + 200 mg/kg Soyabeans phytoestrogen-rich extract
Group 4: Given 80 mg/kg VCD + 400 mg/kg of Soyabeans phytoestrogen-rich extract
Group 5: Given 80 mg/kg VCD + 600 mg/kg of Soyabeans phytoestrogen-rich extract
Group 6: Given 80 mg/kg VCD + 14 μg/100g estrogen
Preparation and Extraction of Soyabean flour
Following the authentication of the soybeans by a plant taxonomist at the Department of Plant Biology and Biotechnology, University of Benin, Benin City, it was assigned a voucher number (UBH-G628). The Soybean seeds were manually picked to remove debris and rinse in water. The bean chaffs were removed after soaking overnight by washing and water draining manually. The Soybeans were then dried in the sun and fried using a frying pan over medium heat until they turned brown with continuous stirring. The seeds were blended immediately with a chicken blender. The quick transfer from a hot frying pan into the blender facilitates the grinding of the seeds to smooth powder. The extraction of the Soyabean flour to obtain the phytoestrogen-rich extract was made as previously described [7].
Biochemical analyses
Serum levels of follicle-stimulating hormone (FSH), Estradiol, and Anti-mullerian hormone were assayed by Enzyme-Linked Immunosorbent Assay (ELISA) technique using reagents supplied by Calbiotech Diagnostic Products Monobind Inc. Lake Forest, USA. Serum total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDLc), malondialdehyde (MDA), and total antioxidant status (TAS) were determined by a spectrophotometric method using reagents supplied by Randox Laboratories Ltd, UK. The Freidewald formula was used to calculate the plasma LDLc [13], and total lipids were calculated using the formula: Total lipids (mg/dL)= 2.27 x total cholesterol value + triglyceride value +62.3 mg/dL [14].
Statistical analysis
The data analyses were done using SPSS Software (IBM) version 23.0 and results were expressed as Mean ± Standard Error of Mean (SEM). The mean differences between the groups were ascertained using a pair Student’s T-test and one-way ANOVA. A P-value less than 0.05 was considered statistically significant.
Results
Table 1 shows the mean levels of serum estradiol, FSH, and anti-mullerian hormone (AMH) in VCD-induced menopause in Wistar rats. Serum estradiol was not detected in rats in Group 2 and Group 3 but was slightly detected in rats in Group 1. Similarly, AMH was not detected in rats in Group 2 and Group 3 but minute concentration was detected in rats in Group 1. The mean differences were statistically significant (p<0.05) compared with untreated rats in group 4. Serum FSH was not significantly altered between untreated and VCD-treated Wistar rats (p>0.05).
Table 1: Mean±SEM values of measured reproductive hormones in 4-vinyl cyclohexane diepoxide–induced menopause in female Wistar rats.
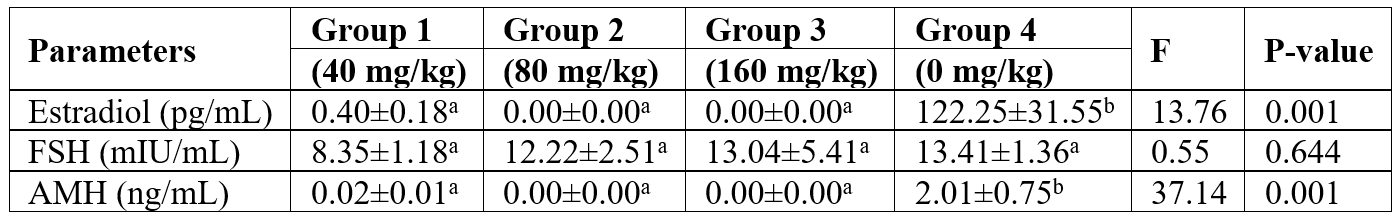
The values with different superscripts indicate significant differences from each other (p<0.05) while values with the same superscript are not significantly different from each other (p>0.05). Group 1: 40 mg/kg VCD, Group 2: 80 mg/kg VCD, Group 3: 160 mg/kg VCD, Group 4: Control, FSH- Follicle Stimulating Hormone, AMH- Anti-Mullerian Hormone.
Table 2 revealed that serum MDA was significantly elevated (p<0.001) in group 2 rats (Positive control) compared with group 1 (negative control) rats. In VCD-induced menopause rats that received Soybean phytoestrogen-rich extract supplementation, serum MDA decreased with increasing concentrations of Soybean phytoestrogen-rich extract administered from group 3 to group 5. The MDA concentrations in rats that received 600 mg/kg Soybean phytoestrogen-rich extract (group 5) and the rats that received VCD + 14 µg Estrogen injection (group 6) were the same. The TAS was significantly decreased in rats in group 2 compared to group 1 (p<0.001). The serum TAS gradually increased with increasing concentrations of Soybean phytoestrogen-rich extract, from group 3 to group 5, and the levels were higher than in rats given 14 µg Estrogen. The differences in the mean were statistically significant (p<0.001) compared with control. The lipid peroxidation index was significantly higher in rats in group 2 compared with the control (group 1). The lipid peroxidation index decreased with increasing concentrations of Soybean phytoestrogen-rich extract administered. The lipid peroxidation index in rats in group 5 that received 80 mg VCD + 600 mg/kg Soybean phytoestrogen-rich extract was lower than in rats in group 6 that received 80 mg VCD + 14 µg Estrogen.
Table 2: Levels of serum malondialdehyde, total antioxidant status, and lipid peroxidation index in VCD-induced menopause in Wistar Rats.
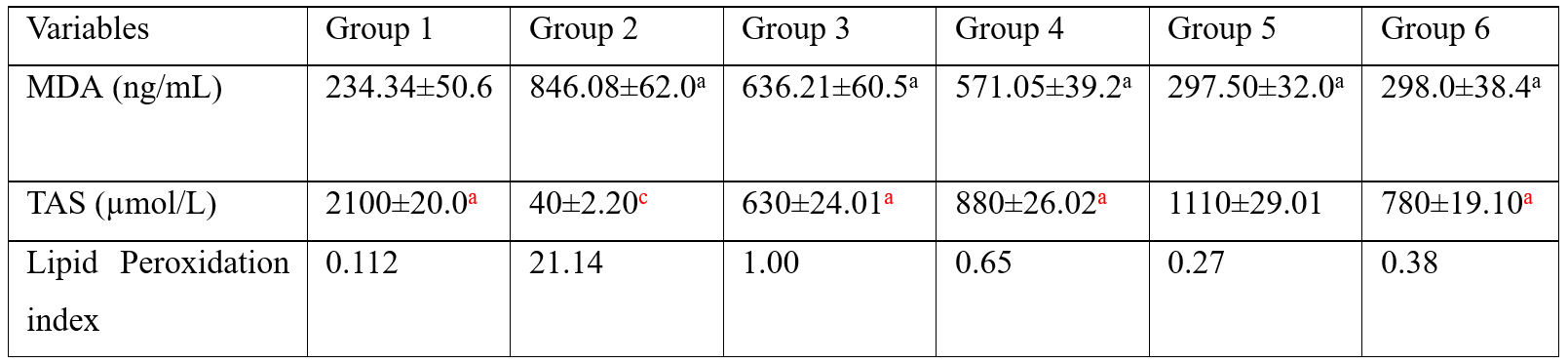
MDA=malondialdehyde; TAS=total antioxidant status. Group 1 - control (untreated); Group 2 - Positive control (given 80 mg/kg VCD only); Group 3 - given 80 mg/kg VCD + 200 mg/kg Soyabean Phytoestrogen extract; Group 4-given 80 mg/kg VCD+400 mg/kg Soybean Phytoestrogen extract; Group 5 - 80 mg/kg VCD + 600 mg/kg Soybean Phytoestrogen extract; Group 6 - given 80 mg VCD + 14 μg Estrogen; The value with different superscript indicate significant difference from each other (p<0.05) while value with same superscript are not statistically difference from each other (p>0.05)
Table 3: The total lipids concentration also decreased with increasing concentrations of Soybean Phytoestrogen-rich extract, the differences in the mean total lipids were significantly different compared with control group 1 and positive control group 2. Whereas the concentrations of TC, TG, and LDLc increased with increasing concentrations of Soybean phytoestrogen-rich extract administered, the levels of HDLc increased with increasing concentration of the extract administered.
Table 3: Serum levels of lipid profile and calculated Total Lipids in VCD-induced menopause in Wistar Rats.
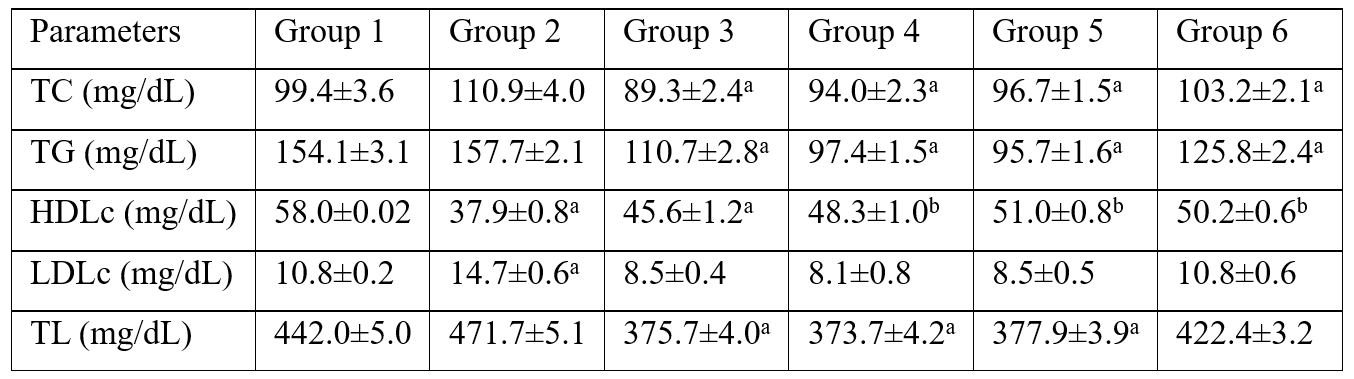
TC=total cholesterol, TG=triglycerides, HDLc=high density lipoprotein cholesterol, LDLc=low density lipoprotein cholesterol, TL=total lipids. Group 1 - control (untreated); Group 2 - Positive control (given 80 mg/kg VCD only); Group 3 - given 80 mg/kg VCD + 200 mg/kg Soyabean Phytoestrogen extract; Group 4-given 80 mg/kg VCD+400 mg/kg Soybean Phytoestrogen extract; Group 5 - 80 mg/kg VCD + 600 mg/kg Soybean Phytoestrogen extract; Group 6 - given 80 mg VCD + 14 μg Estrogen; The value with different superscript indicate significant difference from each other (p<0.05) while value with same superscript are not statistically difference from each other (p>0.05).
Discussion
This study evaluated the concentrations of serum MDA, and TAS as biomarkers of oxidative stress. Also, it assessed the possible effects of soybean phytoestrogen-rich extract consumption on lipid peroxidation index in VCD-induced menopause in Wistar rats. Menopause is associated with oxidative stress due to the lopsidedness in the rate of free radical generation and available antioxidant activity in the body. This is considered a risk factor for the development and exacerbation of several cardiovascular diseases [4]. The decline in estrogen concentration may lead to an alteration in lipid profile and enhanced lipid peroxidation [5]. Studies have indicated that oxidative stress plays a major role in the pathogenesis of several menopause-related diseases [4,7,15-17].
In this study, the serum MDA was significantly increased in VCD-induced menopause Wistar rats when compared with negative control rats, that did not receive VCD. This is an indication that menopause induced increased secretion of MDA. This aligned with a human study conducted to compare premenopausal and postmenopausal women [4,18]. MDA is an end product of lipid peroxidation, which is used as a biomarker for assessing lipid peroxidation in the investigation of several diseases [19]. It is harmful to the cells because, once formed, MDA can be metabolized by some enzymes, especially in the mitochondrial by aldehyde dehydrogenase, or covalently react with proteins and nucleic acids to form DNA-protein crosslink and several adducts responsible for the damage of biomolecules [20]. The decline in estrogen levels in menopause can lead to oxidative stress since the follicular activity of the ovary is terminated. During this period, the production of extra ovarian estrogen predominates. The predominant estrogen is estrone, which is not as effective as estrogen as an antioxidant. There are also low levels of nitric oxide, which protects the heart and inhibits smooth muscle multiplication in heart disease, as well as increased proliferation of free fatty acids. These increase the likelihood of exacerbating lipid peroxidation and development of CVDs [15,17]. The unpleasant accumulation of lipid peroxidation products in biological fluids might lead to alterations in the metabolism of proteins, fats, carbohydrates, nucleic acids, water, and electrolyte metabolism. These could result in severe tissue damage and organism adaptive capacity reduction [21]. The administration of Soybean phytoestrogen-rich extract resulted in the reduction in lipid peroxidation index in a dose-dependent manner. This is an indication that Soybean phytoestrogen-rich extract may prevent or/and ameliorate these biochemical sequelae.
Dyslipidaemia, higher MDA levels, Oxidative stress, and lipid peroxidation have been reported among postmenopausal women with insomnia [21]. It was suggested that oxidative stress observed among menopausal women may be due to sleep disorder since cerebral free radicals accumulate during wakefulness and are scavenged during sleep. That sleep enhances the efficiency of free radical removal by endogenous antioxidant mechanism [21]. Therefore, sleep disorders as occur in menopause may lead to increased levels of free radicals in the body. The brain in particular may be susceptible to oxidative damage due to the preponderance of polyunsaturated fatty acids and the presence of a low antioxidant capacity [22]. Contrary to the observation above, an earlier study on the animal model which was deprived of 1-2 weeks of sleep did not observe any alteration in rats' brains, and other tissues' proteins' free radical oxidation and lipids concentrations [23].
The serum TAS in VCD-induced menopause Wistar rats was significantly reduced compared to negative control rats. This is consistent with previous studies among postmenopausal and premenopausal women, in which enzymatic and non-enzymatic antioxidants were compared between the participants. It was reported that, in postmenopausal women, enzymatic antioxidants and nonenzymatic antioxidants such as vitamin C, α-tocopherol, and retinol were significantly decreased suggesting the presence of oxidative stress in cells [4,24]. Again, the administration of Soybean phytoestrogen-rich extract increased the concentrations of TAS in a dose-dependent manner. The assessment of TAS indicates the concentration of non-enzymatic antioxidants in the body.
Although some authors have suggested dietary supplementations of at least 40 mg of phytoestrogens per day, others have not reported a specific dosage of phytoestrogens needed to effect diverse outcomes [25]. Findings from this study suggest that the supplementation of Soybean phytoestrogen-rich extract lowered the concentrations of total lipid and lipid peroxidation index in a dose-dependent manner. It was reported that Soyabean phytoestrogen-rich extract supplementation is safe without side effects, and can be taken for a couple of months [25]. The need for support programs for postmenopausal women to facilitate prompt and appropriate treatment of menopause-associated ailments was suggested, which may include the possible use of Soybean phytoestrogen-rich extract [7]. This is particularly important in resource-limited settings like ours where estrogen replacement treatment is beyond the reach of the general population. Soybean products have long been accepted for their phytoestrogen-rich contents and their possible role in reducing CVD risks [26]. The potential to reduce oxidative stress and lower lipid peroxidation has been confirmed in this study.
Menopause affects every woman and forms a major health challenge for women, especially in developing countries of the world where about 76% of menopausal women are affected [27]. Therefore, it is of public health importance to pay attention to this vulnerable population, since a study has reported that a longer duration since menopause among menopausal women may be associated with adverse changes in the lipid profile [[1] and peroxidation index.
In conclusion, the increased oxidative stress and decreased antioxidant defense after the induction of menopause in Wistar rats may cause oxidative damage in the cells. The supplementation of Soybean phytoestrogen-rich extract to VCD-induced menopause in Wistar rats increased the serum TAS, decreased MDA concentrations, and lowered the lipid peroxidation index. This suggests that Soybean phytoestrogen-rich extract has the potential to lower oxidative stress and lipid peroxidation index in VCD-induced menopause in Wistar rats. This experiment can be replicated in humans to fully assess the potential of Soybean phytoestrogen-rich extract in the prevention of menopause-associated oxidative stress and lipid peroxidation.
Acknowledgments: We appreciate the support of all staff of the Departments of Medical Laboratory Science and Pharmacognosy, at the University of Benin for their roles in ensuring the success of this study.
Author contributions: This study was conducted and approved by all authors. MAE designed the study MAE, and EJO wrote the protocol, MAE, EJO and HOU sourced for the funds, MAE, EJO, HOU contributed to the literature search, conducted the data gathering, laboratory analysis, statistical analysis, MAE,HOU drafted the manuscript, MAE supervised the study, MAE, EJO, HOU review the draft and proofread the final manuscript.
Conflict of interest: The authors declare that they have no conflict of interest.
Funding: Tetfund IBR batch 18/40 for reagents.
Ethical statement: The protocol used in this research was reviewed and approved by the institutional review board for Animal studies of the Faculty of Pharmacy, University of Benin.
Data availability: The data was from PhD project work.
References
- Lou Z, Huang Y, Lan Y, Li C, Chu K, Chen P, et al. Relationship between years since menopause and lipid variation in postmenopausal women: A cross-sectional study. Medicine (Baltimore), 2023; 102(2): e32684. doi: 10.1097/MD.0000000000032684.
- Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, et al. heart disease and Stroke Statistics-2022 Update: A Report from the American Heart Association. Circulation, 2022; 145(8): e153-e639. doi: 10.1161/CIR.0000000000001052.
- Leanza G, Conte C, Cannata F, Isgrò C, Piccoli A, Strollo R, et al. Oxidative Stress in Postmenopausal Women with or without Obesity. Cells, 2023; 12: 1137. https://doi.org/10.3390/cells12081137
- Zovari F, Parsian H, Bijani A, Moslemnezhad A, Shirzad A. Evaluation of Salivary and Serum Total Antioxidant Capacity and Lipid Peroxidation in Postmenopausal Women, Hindawi Int J Dentistry, 2020; 2020 Article ID 8860467, 5 pages. https://doi.org/10.1155/2020/8860467.
- Lizcano F, Guzmán G. Estrogen Deficiency and the Origin of Obesity during Menopause. Biomed Res Int, 2014; 2014: 757461. doi: 10.1155/2014/757461.
- Olaniyan EJ, Emokpae MA, Oyakhire FO, Ahmed LA, Esezobor IK, Olaniyan SO. Evaluation of Antioxidant Properties of Soybean Phytoestrogen-Rich Extract In 4-Vinylcyclohexane Diepoxide-Induced Menopausal Rats. Eur J Pharm Med Res, 2023; 10(10): 28-34.
- Emokpae MA, Uwumarongie HO, Olaniyan JE. Use of atherogenic indices in evaluating the potential cardio-protective effect of soybean phytoestrogen-rich extract in 4-vinyl cyclohexane diepoxide-induced menopause in Wistar rats. Open J Clin Med Case Rep, 2024; 2206.
- Konhilas JP, Sanchez JN, Regan JA, Constantopoulos E, Lopez-Pier M, Cannon DK, et al. Using 4-vinylcyclohexene diepoxide as a model of menopause for cardiovascular disease. Am J Physiol Heart Circ Physiol, 2020; 318(6): H1461-H1473. doi: 10.1152/ajpheart.00555.2019.
- Zucon Bacelar AC, Momesso NR, Pederro FHM, Gonçalves A, Ervolino E, Chaves-Neto AH, et al. Aged and induced-premature ovarian failure mouse models affect diestrus profile and ovarian features. PLoS One, 2023; 18(12): e0284887. doi: 10.1371/journal.pone.0284887.
- Silvestrini A, Meucci E, Ricerca BM, Mancini A. Total Antioxidant Capacity: Biochemical Aspects and Clinical Significance. Int J Mol Sci, 2023; 24(13): 10978. doi: 10.3390/ijms241310978.
- Akindele RA, Omopariola SO, Adeyemo AT, Adeyemo AT, Afolabi BA, Folami EO, et al. Prevalence of Menopausal Symptoms in Osogbo, South-West, Nigeria Niger J Med, 2023; 32(2): 155-160. DOI: 10.4103/NJM.NJM_31_23
- Kappeler CJ, Hoyer PB. 4-vinylcyclohexene diepoxide: a model chemical for ovotoxicity. Syst Biol Reprod Med, 2012; 58(1): 57-62. doi: 10.3109/19396368.2011.648820.
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem, 1972; 18: 499–502.
- Bernert JT, Turner WE, Patterson DG Jr, Needham LL. Calculation of serum “total lipid” concentrations for the adjustment of persistent organohalogen toxicant measurements in human samples. Chemosphere, 2007; 68(5): 824-831. https://doi.org/10.1016/j.chemosphere.2007.02.043
- Xiang D, Liu Y, Zhou S, Zhou E, Wang Y. Protective Effects of Estrogen on Cardiovascular DiseaseMediated by Oxidative Stress. Hindawi Oxidative Medicine and Cellular Longevity, 2021; 2021; Article ID 5523516: 15 pageshttps://doi.org/10.1155/2021/5523516.
- Heravi AS, Michos ED, Zhao D, Ambale-Venkatesh B, Doria De Vasconcellos H, Lloyd-Jones D, et al. Oxidative Stress and Menopausal Status: The Coronary Artery Risk Development in Young Adults Cohort Study. J Womens Health (Larchmt), 2022; 31(7): 1057-1065. doi: 10.1089/jwh.2021.0248.
- Kamińska MS, Schneider-Matyka D, Rachubińska K, Panczyk M, Grochans E, Cybulska AM. Menopause Predisposes Women to Increased Risk of Cardiovascular Disease. Journal of Clinical Medicine, 2023; 12(22): 7058. https://doi.org/10.3390/jcm12227058.
- Sanchez-Rodr´guez MA, Zacar´ıas-Flores M, Arronte-Rosales A, Correa-Muñoz E, Mendoza-N´uñez VM. Menopause as risk factor for oxidative stress, Menopause: J North Am Menopause Soc, 2012; 19(3): 361–367. doi: 10.1097/gme.0b013e318229977d.
- Cakir T, Goktas B, Mutlu MF, et al. Advanced oxidation protein products and malondialdehyde - the new biological markers of oxidative stress - are elevated in postmenopausal women. Ginekologia Polska, 2016; 87(5): 321–325. doi: 10.5603/gp.2016.0001.
- Cordiano R, Di Gioacchino M, Mangifesta R, Panzera C, Gangemi S, Minciullo PL. Malondialdehyde as a Potential Oxidative Stress Marker for Allergy-Oriented Diseases: An Update. Molecules, 2023; 28(16): 5979. doi: 10.3390/molecules28165979.
- Semenova NV, Madaeva IM, Kolesnikov SI, Solodova EI, Kolesnikova LI. Insomnia in Peri- and Postmenopausal Women: Plasma Lipids, Lipid Peroxidation and Some Antioxidant System Parameters, Neuropsychiatry (London), 2018; 8(4): 1452–1460. doi: 10.4172/Neuropsychiatry.1000477.
- Süer C, Dolu N, Artis AS, Sahin L, Yilmaz A, Cetin A. The effects of long-term sleep deprivation on the long-term potentiation in the dentate gyrus and brain oxidation status in rats. Neurosci Res, 2011; 70(1): 71-77. doi: 10.1016/j.neures.2011.01.008.
- Gopalakrishnan A, Ji LL, Cirelli C. Sleep deprivation and cellular responses to oxidative stress. Sleep, 2004; 27(1): 27-35. doi: 10.1093/sleep/27.1.27.
- Ansar S, Alhefdhi T, Aleem AM. Status of trace elements and antioxidants in premenopausal and postmenopausal phase of life: a comparative study. Int J Clin Experiment Med, 2015; 8(10): 19486–19490.
- Terzic M, Micic J, Dotlic J, Maricic S, Mihailovic T, Knezevic N. Impact of Phytoestrogens on Serum Lipids in Postmenopausal Women. Geburtshilfe Frauenheilkd, 2012; 72(6): 527-531. doi: 10.1055/s-0031-1298624.
- Azadbakht L, Kimiagar M, Mehrabi Y, Esmaillzadeh A, Hu FB, Willett WC. Dietary soya intake alters plasma antioxidant status and lipid peroxidation in postmenopausal women with the metabolic syndrome. Br J Nutr, 2007; 98(4): 807-813. doi: 10.1017/S0007114507746871.
- Vincent J, Inassi J. Comparison of oxidative stress between premenopausal and postmenopausal women. Natl J Physiol Pharm Pharmacol, 2020; 10(05): 359-362. DOI: 10.5455/njppp.2020.10.02051202002032020.

