Comparative Study of Adjunct Misoprostol and Oxytocin Versus Oxytocin Alone on Side Effects Profile and Need for Additional Uterotonics in The Prevention of Postpartum Hemorrhage, A Randomized Control Trial
Mube WA1,*, Gbaranor KB2, Ozims SJ3, Eberendu IF3, Olaka EE1, Ikpeazu O4 and Nwogu HC5
1Department of Obstetrics and Gynaecology, University of Port Harcourt Teaching Hospital, Rivers State, South-South, Nigeria
2Department of Human Physiology, College of Medical Sciences, Rivers State University, Rivers State, South-South, Nigeria
3Department of Public Health, Faculty of Health Sciences, Imo State University, Imo State, South-East, Nigeria
4Department of Family Medicine, College of Medical Sciences, Rivers State University, Rivers State, South-South, Nigeria
5Department of Anesthesia, College of Medical Sciences, Rivers State University, Rivers State, South-South, Nigeria
Received Date: 28/11/2023; Published Date: 18/04/2024
*Corresponding author: Gbaranor KB, Department of Human Physiology, College of Medical Science, Rivers State University, Rives State, South-South, Nigeria
Abstract
Due to the synergistic activity of misoprostol with oxytocin in preventing postpartum hemorrhage, it can serve as an adjunct because of its good uterine contractility, availability, convenience of storage, and comparatively cheaper cost, among other reasons. The primary aim of this research was to evaluate the efficacy of oxytocin alone vs oxytocin plus misoprostol in reducing the risk of postpartum bleeding, comparing their side effect profile and need for additional uterotonics and blood transfusion. After giving birth, some women in the M+O group were given 400ug of misoprostol orally along with 10IU of oxytocin intramuscularly, while others in the O+P group were given 400mg of white vitamin C (placebo) together with 10IU of oxytocin. The need for additional uterotonics and blood transfusion was recorded and the side effects from both groups were also observed and recorded. Data was analyzed using SPSS version 25 with the significance level set at 0.05. From the findings, additional oxytocics were required more frequently in the oxytocin group compared to the misoprostol group (38.5% vs. 6%). Nausea (44%) and vomiting (8%) were the side effects noticed in the oxytocin group, while fever (60%) and shivering (32%) in addition to nausea (56%) and vomiting (20%) were noticed in the oxytocin + misoprostol group. The overall incidence of side effects was higher in the misoprostol group (88.0% and 44.0%) respectively, with a higher risk of side effects in the misoprostol group (RR: 2.000, p <0.001). The oxytocin group required blood transfusion more than the misoprostol group (10% vs 6%) respectively.
Keywords: Comparative; Misoprostol; Oxytocin; Effects; Prevention Postpartum- Haemorrhage
Introduction
Postpartum hemorrhage (PPH) is the leading cause of maternal mortality in the world, accounting for one-third of all maternal deaths worldwide (Derman, 2006). PPH causes up to 60% of all maternal deaths in developing countries. The majority of these deaths occur within 4 hours of delivery, indicating they are a consequence of events in the third stage of labour (Ramanthan, 2006: 967).
Preventive ways of post-partum haemorrhage have been instituted by the World Health Organization as the active management of the third stage of labour and appropriate use of uterotonics [1]. The use of uterotonics in labour have reduced the prevalence of postpartum haemorrhage according to different scholars [2,3], (Westhoff, 2013). The active management of third stage of labour is an intervention package that comprises of the administration of an oxytocic immediately after the delivery of the baby by any route, delayed clamping of the cord and delivery of the placenta by controlled cord traction and assessment of the uterus for its tonicity. Oxytocin at a dose of 10 IU administered either intravenous or intramuscular is the recommended uterotonic drug for the prevention of PPH. However, researchers have identified other common uterotonics used in the third stage of labour as syntometrine (IM), ergometrine (IV or IM), misoprostol (IM) and carbetocin [2,3], (Liabsuetrakul 2018; McDonald 2007b; Su 2012; Westhoff 2013). Recent guidelines from the World Health Organization (WHO), the International Federation of Gynecology and Obstetrics (FIGO), the International Society for Clinical Endocrinology and Metabolism (ISCEM), and the National Institute for Health and Care Excellence [1], (ICMFIGO 2003; NICE 2014) also advocates the use of uterotonics in the management of third stage of labour however, their dosages and routes of administration is up for debate.
Regarding the use of oxytocin and misoprostol, there is still controversy regarding the ideal dose, infusion rates and mode of therapy, and so far, the literature reports variable outcomes [3,5]. Studies have reported a higher efficacy of sublingual misoprostol in controlling blood loss. Misoprostol is a useful medicine since it may be administered in a variety of ways (including orally, sublingually, buccally, vaginally, and rectal). Moreover, the price of misoprostol is inexpensive. It maintains its integrity even in the scorching heat typical of Nigeria and other tropical nations. Nausea, chills, and vomiting are common adverse reactions, which are often self-limiting. When compared to the sublingual method, plasma levels are sustained for a longer time after a rectal delivery [6] (Hofmeyr et al., 2005). Due to the lower peak plasma concentration achieved with rectally given medication, it is also linked with fewer and more manageable adverse effects [7,8].
Several studies have compared the efficacy of oxytocin alone and oxytocin plus adjunct misoprotol in the management of post-partum haemorrhage and reported a higher effect gotten from the misoprostol group [9,10], (Fawole et al. 2011; Anita et al. 2018)). [11] conducted an analysis to evaluate the effects of sublingual misoprostol and oxytocin to those of oxytocin alone. They found that the combination therapy significantly reduced blood loss compared to the control group. On the need for additional uterotonics, studies have also reported that groups receiving oxytocin alone had higher rates of needing extra uterotonic drugs, compared to patients who received adjunct misoprostol plus oxytocin [11-14].
Owing to the importance of reducing maternal mortality and preventing postpartum haemorrhage, this study seeks to investigate the side effects and need for additional uterotonics associated with the oxytocin and misoprostol. The commonest cause of maternal mortality in the world especially in developing and underdeveloped nations according to the WHO, is postpartum haemorrhage (Kumar, 2019). Postpartum haemorrhage was identified as the most prevalent cause of maternal mortality in different parts of Nigeria. In Kaduna, it accounted for 25% of all maternal deaths (Ujah and Ejeh, 2006). Another study from the Southern Nigeria reported a prevalence of 4-28% of postpartum haemorrhage [15].
From the above background, this study seeks to achieve the following objectives:
- To ascertain the side effects in patients who received misoprostol plus oxytocin and oxytocin alone for prevention of post-partum haemorrhage.
- To determine the need for additional uterotonics and blood transfusion in patients who received misoprostol plus oxytocin and oxytocin alone for prevention of post-partum haemorrhage.
Methodology
This prospective randomized control trial was conducted in University of Port Harcourt Teaching Hosptial from November 2021 to January 2022 after obtaining clearance from the Hospital’s ethical committee.
100 low risk gravid women admitted in the labor room having single live fetus presenting by vertex, at term and about to have vaginal delivery, were recruited in the study after obtaining informed consent and performing history taking, general and obstetric examination as well as reviewing of antenatal records with investigations.
Women below 18 and over 40 years, women with hypertensive disorders, diabetic conditions, antepartum haemorrhage, multiple pregnancy, polyhydramnios, oligohydramnios, anaemia complicating pregnancy, heart disease complicating pregnancy, malpresentations, patients undergoing assisted vaginal deliveries, patients undergoing caesarean section, vaginal birth after caesarean section patients, associated with any medical or surgical disorder and patients with coagulative disorders were excluded from the study.
The sample size was determined using statistical formula for comparing two proportions with accepting a study power of 90%, confidence interval of 95%, study/control of 1:1 and an acceptable dropout rate of 10%. A total of 110 subjects were needed to make the study statistically significant.
All women were monitored closely. The first group M+O will receive 400ug of misoprostol orally plus 10IU of oxytocin intramuscularly and the second group O+P received 400mg of white vitamin C(placebo) plus 10IU of oxytocin immediately after delivery of the baby with the use of sealed, opaque, and sequentially numbered packets. The pre-weighed delivery mat and other pre-weighed vulva pads used with one hour after delivery of the baby were re-weighed to calculate the blood loss using the gravimetric blood measurement method by the researcher. The duration of the third stage of labour was measured using a stopwatch from the delivery of the baby to delivery of the placenta.
In the course of this research work, the following parameters were assessed; participants who had additional uterotonic and or blood transfusions in each arm of the study, noticeable side effects of the medications in each arm, estimated blood loss, duration of third stage of labour and the deficit in the haemoglobin level in both arms of the study.
The Women were followed up to 48 hours of birth. Admission to intensive care unit or mortality was recorded if any.
Data was retrieved in the proforma and computed using Microsoft Excel 2019 version. The data was analyzed with SPSS version 25. Descriptive statistics were presented in frequency and percentages for categorical data with results presented in tables and charts, while continuous data was shown in means and standard deviations. Student t-test was used for two mean comparisons, Mann-Whitney U test for associating two medians. Categorical variables were analyzed by Chi square test with p<0.05 considered as significant. Risk association for categorical variables was done using Odds Ratio (OR) and Relative risk (RR). All ORs were reported with their 95% CI and corresponding P-values.
Results
A total number of One hundred (100) consenting pregnant women who had vaginal delivery in the hospital were recruited for the study. Fifty (50) of the study group (A) had oxytocin and a placebo (white vitamin c) while the 50 in the other group (B) received misoprostol and oxytocin.
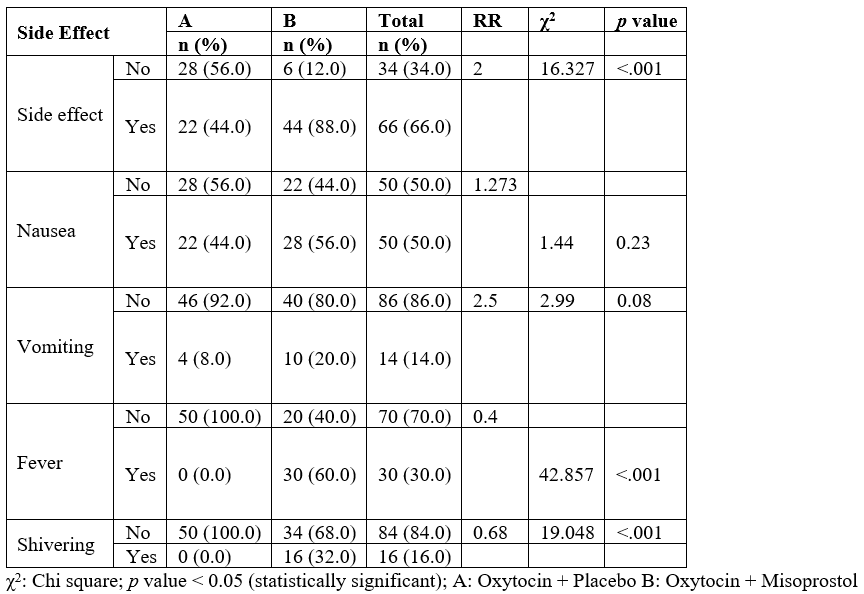
From the socio-demographic characteristics of the study population shown in table 1, the mean age for the two groups were 28.92 ± 3.20 for group A and 30.26 ± 3.64 for group B respectively. Most of the women (93.9%) in both groups were married, majority (52.0%) had secondary education, and majority (72.4%) were involved in business as an occupation. Half of the women (51.1%) were from the Igbo tribe. A large percentage (83.5%) of the women had a gestational age of 37 – 40 weeks, with a modal parity of and 2 – 4.
Table 2 shows the blood transfusion requirement and the need for additional uterotonic and the rate of PPH in both study groups. From the table, most of the women in both groups (92%) had no requirement for blood transfusion with an Odds ratio of 0.574. Women in the oxytocin plus misoprostol group (6%) had less need for additional uteretonics as compared to the group (38.5%) which is statistically significant (χ2 = 15.312, p = .02 OR = 0.106).
Table 3 shows a comparison of the side effects between the oxytocin group and oxytocin + misoprostol group. From the results, more side effects were noticed in the oxytocin + misoprostol group (88.0% and 44.0%) respectively. Nausea (44.0%) was the commonest side effects in the oxytocin group, while fever (60.0%) accounted for the common side effects in the oxytocin + misoprostol group. The presence of side effects among the groups was statistically significant (χ2 = 16.327, p = .00 and RR = 2.000).
Table 1: Socio Demographic Characteristics of the Study Population.
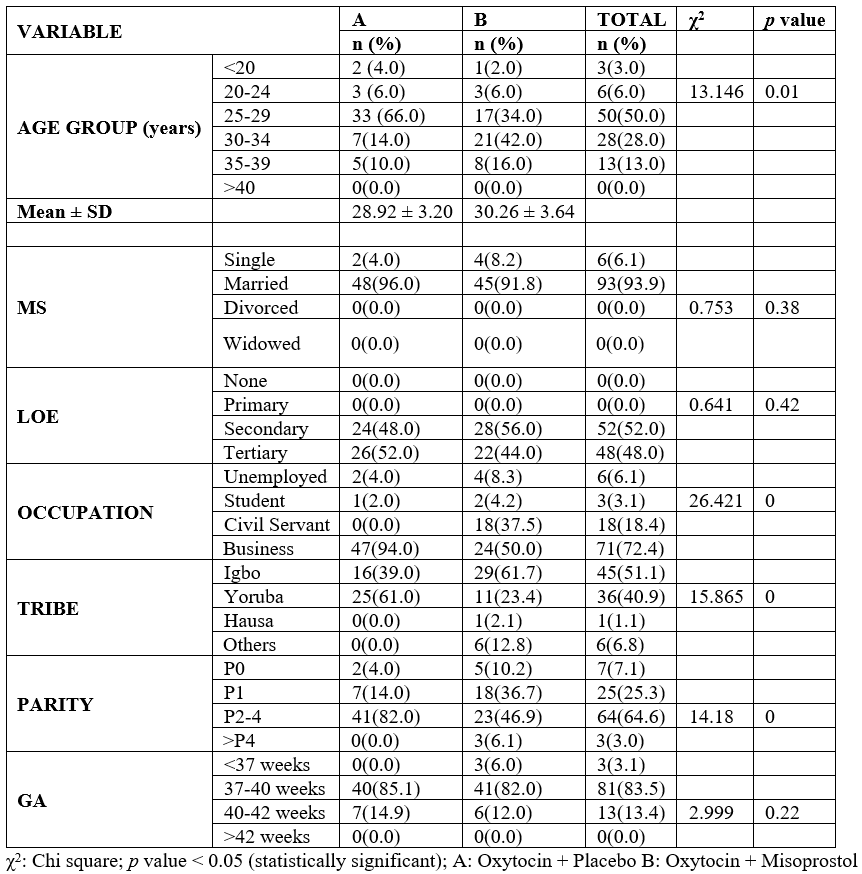
Table 2: Blood transfusion and additional oxytocic use as a measure of efficacy in the study groups.
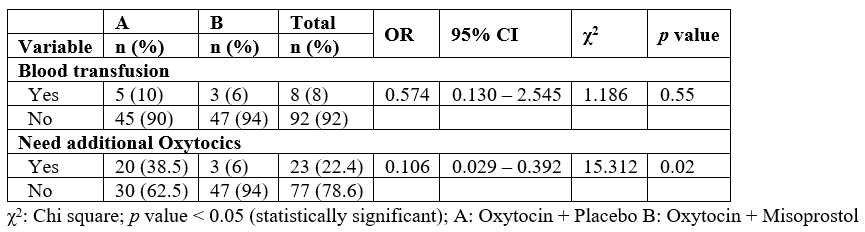
Table 3: Comparison of side effects between Oxytocin and Misoprostol groups.
Summary of Findings
i. The side effects in patients who received misoprostol plus oxytocin were nausea, vomiting, fever and shivering while patients who received oxytocin alone was nausea and vomiting.
ii. There was a 2 times risk of experiencing side effects in the oxytocin plus misoprostol group (RR = 2.000) and a significant difference between the occurrence of side effects in the two groups (RR= 2.000 p <0.001). The risks of not having fever and shivering as a side effect among the misoprostol plus oxytocin group was low (RR = 0.4000 and 0.680 respectively p <0.001).
iii. There was an increased need for additional uterotonics and blood transfusion among the oxytocin group compared to the misoprostol group.
Discussion
From the findings of this study, the oxytocin plus misoprostol group had more side effects compared to patients who received oxytocin alone. The prevalent side effects were nausea, vomiting, fever and shivering among the oxytocin plus misoprostol group, while patients who received oxytocin alone only experienced nausea and vomiting. The participants in the oxytocin plus misoprostol group had 2.0 times more chance of having side effects which was significant (p = <0.001, RR = 2.000). There was a significant difference between the fever and shivering side effects experienced by the participants in the Arm B, as none of the participants in oxytocin alone group experienced fever or shivering as a side effect. The participants in the oxytocin plus misoprostol group had a very less risk of not developing fever and shivering (RR = 0.400 and 0.680 respectively, p = <0.001). The side effects of these two agents play a synergistic action when combined. Mousa (2014) noted the major side effects of misoprostol as fever, nausea and shivering while WHO and UNICEF studies have shown that shivering and fever does not exist as side effects of oxytocin. This accounts for the fever and shivering recorded in the misoprostol plus oxytocin group. A study conducted in six Nigerian hospitals by [4] and another study by Ahmed (2017), also noted fever and shivering as side effects experienced by women who received misoprostol; however, the side effects were temporary and self-limiting. Another study by [11] also reported a significant effect of shivering and fever among patients who received misoprostol plus oxytocin versus those who received oxytocin alone (p = 0.007; p < 0.001, respectively). Bilgin and Kömürcü (2019) also noted substantially greater rate of drug-induced shivering, nausea, and increased body temperature among patients who received adjunct misoprostol than the oxytocin and placebo groups (p 0.05). The side effects increased with increasing dose of misoprostol in the study, with sublingual misoprostol 600 mg group experiencing the highest frequency of shivering (56.4%).
In a Nigerian Study by Uthman et al. (2013) to determine the side effects and tolerance of intravenous oxytocin 10 IU and oral misoprostol 600ug for the prevention of postpartum hemorrhage during the third stage of labor, side effects recorded were shivering (33.9% vs. 0.0%; p 0.001) and fever (19.7% vs. 1.8%; p 0.001), which were more prevalent in the misoprostol group, while abdominal pain was more common in the oxytocin group (7.1% vs. 0.0%; p 0.001). The occurrence of other unpleasant effects, such as vomiting and nausea, did not differ significantly. There was no statistically significant difference (p > 0.05) in the acceptance rates of injectable oxytocin (97.3%) and oral misoprostol (98%). Those who received oxytocin for PPH prophylaxis experienced stomach pains and headaches, whereas those who took misoprostol reported chills and a high fever [16-19].
Women in the oxytocin group had more need for additional uterotonics to control post-partum blood loss, and also had indications for more blood transfusion, compared to the misoprostol group which had a less need for additional uterotonics and blood transfusion. The need for additional uterotonics differed significantly among the two groups (p = 0.02), which means that participants in the misoprostol group had a significantly lower odd for needing additional uterotonics (OR: 0.106). This agrees with studies of [11], who reported a statistically significant difference in the need for additional uterotonics among oxytocin and misoprostol group (p <0001). However, the need for blood transfusion did not differ significantly among both groups (p >0.05) These findings are similar to findings from Nigerian research (Ahmed, 2014) where oxytocin group required more blood transfusion compared to misoprostol group (5.3% vs 1.3%), and not statistically significant. The participants in the above study received higher doses of oxytocin and misoprostol (20 IU and 600µg respectively), which accounts for a lower blood loss and need for transfusion [20-22].
Conclusion
Postpartum haemorrhage is a major cause of maternal mortality in Nigeria, owing to the complications from labour. In a bid to reduce the incidence of PPH, the active management of third stage of labour was advocated. The demerits of oxytocin use in African region also advocated the need for adjunct therapy for effective management of PPH. This study evaluated the adjunct therapy of misoprostol plus oxytocin versus oxytocin alone in the prevention of PPH, considering the side effect profile and need for additional uterotonics and blood transfusion among both groups. Findings from the study revealed more side effects from the combination with misoprostol, but a reduction in the need for additional uterotonics and blood transfusion. Therefore, the use of misoprostol and oxytocin in management of labour has a higher potential for reducing blood loss and reducing the incidence of maternal mortality, with self-limiting side effects. This study therefore recommends the introduction of this adjunct therapy as a standard for managing labour in Nigeria.
References
- World Health Organization. WHO recommendations for the prevention and treatment of postpartum haemorrhage. World Health Organization, 2012.
- Gallos ID, Papadopoulou A, Man R, Athanasopoulos N, Tobias A, Price MJ, et al. Uterotonic agents for preventing postpartum haemorrhage: a network meta‐analysis. Cochrane Database of Systematic Reviews, 2018; 12.
- Tuncalp O, Hofmeyr GJ, Gulmezoglu AM. Prostaglandins for preventing postpartum haemorrhage. Cochrane Database Syst Rev, 2012; 8: CD000494. doi:10.1002/14651858.CD000494.pub4
- Fawole AO, Sotiloye OS, Hunyinbo KI, Umezulike AC, Okunlola MA, Adekanle DA, et al. A double‐blind, randomized, placebo‐controlled trial of misoprostol and routine uterotonics for the prevention of postpartum hemorrhage. International Journal of Gynecology & Obstetrics, 2011; 112(2): 107-111.
- Chu CS, Brhlikova P, Pollock AM. Rethinking WHO guidance: review of evidence for misoprostol use in the prevention of postpartum haemorrhage. J R Soc Med, 2012; 105: 336–347.
- Tang OS, Gemzell-Danielsson K, Ho PC. Misoprostol: pharmacokinetic profiles, effects on the uterus and side-effects. International Journal of Gynecology & Obstetrics, 2007; 99: S160-S167.
- Breathnach F, Geary M. Uterine atony: definition, prevention, nonsurgical management, and uterine tamponade. In Seminars in perinatology,2009; 33(2): pp. 82-87.
- Khan RU, El-Refaey H. Pharmacokinetics and adverse-effect profile of rectally administered misoprostol in the third stage of labor. Obstetrics & Gynecology, 2003; 101(5): 968-974.
- Chaudhuri P, Majumdar A. A randomized trial of sublingual misoprostol to augment routine third-stage management among women at risk of postpartum hemorrhage. International Journal of Gynecology & Obstetrics, 2016; 132(2): 191-195.
- Pakniat H, Khezri MB. The effect of combined oxytocin–misoprostol versus oxytocin and misoprostol alone in reducing blood loss at cesarean delivery: a prospective randomized double-blind study. The Journal of Obstetrics and Gynecology of India, 2015; 65(6): 376-381.
- Ugwu IA, Enabor OO, Adeyemi AB, Lawal OO, Oladokun A, Olayemi O. Sublingual misoprostol to decrease blood loss after caesarean delivery: a randomised controlled trial. Journal of Obstetrics and Gynaecology, 2014; 34(5): 407-411.
- Owonikoko KM, Arowojolu AO, Okunlola MA. Effect of sublingual misoprostol versus intravenous oxytocin on reducing blood loss at cesarean section in Nigeria: a randomized controlled trial. Journal of Obstetrics and Gynaecology Research, 2011; 37(7): 715-721.
- Sood AK, Singh S. Sublingual misoprostol to reduce blood loss at cesarean delivery. The Journal of Obstetrics and Gynecology of India, 2012; 62(2): 162-167.
- Vimala N, Mittal S, Kumar S. Sublingual misoprostol versus oxytocin infusion to reduce blood loss at cesarean section. International Journal of Gynecology & Obstetrics, 2006; 92(2): 106-110.
- Green KI, Ojule JD, Mmom CF. Primary Postpartum Haemorrhage at the University of Port Harcourt Teaching Hospital: Prevalence and Risk Factors. The Nigerian Health Journal, 2016; 15(3): 111.
- Chaudhuri P, Biswas J, Mandal A. Sublingual misoprostol versus intramuscular oxytocin for prevention of postpartum hemorrhage in low-risk women. International Journal of Gynecology & Obstetrics, 2012; 116(2): 138-142.
- Fazel MR. A comparison of rectal misoprostol and intravenous oxytocin on hemorrhage and homeostatic changes during cesarean section. Middle East journal of anaesthesiology, 2013; 22(1): 41-46.
- Frolova AI, Stout MJ, Tuuli MG, López JD, Macones GA, Cahill AG. Duration of the third stage of labor and risk of postpartum hemorrhage. Obstetrics & Gynecology, 2016; 127(5): 951-956.
- Gallos I, Williams H, Price M, Pickering K, Merriel A, Tobias A, et al. Uterotonic drugs to prevent postpartum haemorrhage: a network meta-analysis, 2019.
- McDonald SJ. Prophylactic ergometrine‐oxytocin versus oxytocin for the third stage of labour. Cochrane database of systematic reviews, 2004; (1).
- Owa OO, Lemadoro AS, Temenu BA, Ayeyemi JA, Loto OM. Misoprostol versus oxytocin in preventing postpartum hemorrhage: A randomized controlled trial. Tropical Journal of Obstetrics and Gynaecology, 2019; 36(2): 196-199.
- World Health Organization. Trends in maternal mortality 2000 to 2017: estimates by WHO, UNICEF, UNFPA, World Bank Group and the United Nations Population Division, 2019.

