Intra Incisional Bupivacaine Infiltration versus Meloxicam Infiltration on Post Cesarean Section Pain
Alaa Helmy Sherkawi Louza*
Registrar obstetrics and gynecology, Al Kawther Hospital, Hurghada, Red Sea, Egypt
Received Date: 09/11/2023; Published Date: 24/11/2023
*Corresponding author: Alaa Helmy Sherkawi Louza, Registrar obstetrics and gynecology, Al Kawther Hospital, Hurghada, Red Sea, Egypt
Abstract
Objective: To compare the Intra incisional Bupivacaine Infiltration versus Meloxicam Infiltration on Post Cesarean Section Pain.
Methods: A prospective randomized study on 105 term pregnant female was conducted at department of obstetrics and gynecology, Hurghada General Hospital during June 2022 till March 2023.
Results: VAS score was not significantly different between the three studied groups at 2, 12 and 24 hours. While, at 4- and 6-hours VAS were significantly lower among Bupivacaine group than Meloxicam and placebo groups. Also, VAS score was significantly lower in Bupivacaine and Meloxicam groups as compared placebo group at 8 hours. there were no statistically significant differences between the studied groups regarding complications (p=0.211) and drug side effects (p=0.859),
Conclusion: Local infiltration with either bupivacaine or meloxicam decreases pain score at 6, 12, and 24 hours and decreases need of postoperative diclofenac requirements. Bupivacaine is well tolerated and effective local anesthetic that can be used to reduce pain scores and postoperative analgesic use if infiltrated in surgical wound site in women after cesarean section deliveries and is more effective than meloxicam local infiltration in the same way.
Keywords: Bupivacaine Infiltration; Meloxicam Infiltration; Cesarean Section; Postoperative pain
Introduction
Cesarean Section (CS) rates have been increasing in various world regions. Although effective analgesia has been shown to reduce postoperative complications [1]. numerous studies show that inadequate postoperative pain control is frequent in CS patients [2,3]. Moreover, the most considerable concern of women undergoing CS is postoperative pain, which might hinder the bonding with the newborn and initiation of early breastfeeding [4].
On the other hand, insufficient pain relief would delay postoperative ambulation and, thus, increase the risk of thromboembolic events, which may increase postoperative maternal morbidity or mortality, give rise to prolonged hospital stay, and add up to the financial burden associated with CS. Acute pain following childbirth has also been demonstrated to impose ancreased risk for persistent pain and postpartum depression [5].
Hence, any intervention that improves postoperative pain alleviation would positively influence maternal and neonatal health as well as diminish the complications and costs. The standard mainstay of pain relief in the postoperative period are opioids; however, they are known for their high-rate transfer into breast milk and thus, sedative effects on the newborn in addition to decreased mentation and prolonged return of bowel function in mother [4].
To prevent the potential adverse effects of opioids, a variety of approaches, including local anesthetic agent wound infiltration, have been described for pain management after CS [6]. Cochrane Database systematic review also indicated local analgesic infiltration to be of benefit in cesarean section. Intra incisional infiltration of bupivacaine is a commonly used postoperative analgesic regimen to alleviate post-cesarean pain [7].
Meloxicam, an enol-carboxamide non-steroidal anti-inflammatory drug (NSAID) related to piroxicam, has long been used to treat acute pain and inflammation. In contrast to other NSAIDs, it has a greater inhibitory activity against the inducible isoform of cyclooxygenase (COX-2) than against the constitutive isoform (COX-1), [8]. COX-1 induces the synthesis of prostacyclin, which is responsible for vascular homeostasis, platelet aggregation, renal function, and gastric cytoprotection. The expression of COX-2 isoform increases during inflammation. Consequently, although meloxicam's anti-inflammatory and analgesic properties are like non-selective NSAIDs, it has both gastric mucosal and renal protective properties [9].
Patients and Methods
A prospective randomized study on 105 term pregnant female was conducted at department of obstetrics and gynecology, Hurghada General Hospital during June 2022 till March 2023.
According to result from previous study [17] compared effectiveness of local infiltration of levobupivacaine versus meloxicam on post cesarean pain and assumed that the VAS was significantly lower in the levobupivacaine group (3.3±0.8) compared to the other two groups (3.7±0.6 and 4.3±0.8). Based on this, using SATA program with setting alpha error at 5% and power at 90%, the needed sample was 35 cases for each group.
Inclusion criteria: Elective C.S at term Age between 21-40 years have no medical disorders, Cesarean section done by senior obstetrician and Spinal anesthesia.
Exclusion criteria: Extreme of reproductive age, Allergy to local anesthetic infiltration agent, Any medical disorders such as cardiac disease, bronchial asthma or obstetrical complications as ante partum hemorrhage and pre-eclampsia.
All patients included in this study were subjected to the following: Personal history: name, age, address, occupation, special habits, Obstetric history: gravidity, parity, operative delivery, hypertension with pregnancy, DM. Past and Family history: family history of type2 diabetes, previous GDM, systemic or organ disease and previous or present pharmacological therapy. Vital signs: blood pressure, pulse, temperature. Abdominal examination: for assessment of gestational age, fundal level, fetal lie, presentation and fetal heart sounds, uterine contraction, and scar of previous operation if relevant.
Trans-abdominal ultrasound: For gestational age, placental site, amount of liquor and fetal weight.
Randomization was done using Statistical package for Social Science (SPSS) program for choosing participants in every group. Group allocation was concealed in sealed, opaque envelopes. Each woman received a disposable syringe containing a medication corresponding to her order of participation in the trial. Neither the surgeon nor the investigator or woman knew of the type of the drug in the syringe.
Group A (bupivacaine group): included 35 patients in which subcutaneous tissue (upper and lower flaps) were infiltrated with 20 ml of 0.25% bupivacaine hydrochloride not diluted. (Marcaine, 0,25% vial, 50 ml Astra Zeneca).
Group B (meloxicam group): included 35 patients in which subcutaneous tissue (upper and lower flaps) were infiltrated with meloxicam 15mg (mobitil ampoule 15mg/3ml, Delta Pharma). diluted in 20 ml of 0.9% saline.
Group C (placebo group): included 35 patients in which skin and subcutaneous tissue was infiltrated with 20 ml 0.9% saline.
Wound infiltration:
All patients received spinal anesthesia and had lower segment cesarean section through pfannestiel incision by senior obstetrician, CS done through Pfannenstiel incision, opening of uterus by lower segment transverse incision, closure of uterus in two layers, closure of visceral and parietal peritoneum, closure of muscle and sheath, closure of subcutaneous tissue then closure of skin by subcuticular sutures.
The subcutaneous tissue and skin on each of upper and lower edges of the incision were infiltrated by one of the solutions mentioned before according to the randomization plan then closure of the skin was done. Injection of large volumes into the fatty layers, which are relatively devoid of nerve supply, was avoided to limit the total dose of local anesthetic needed. No preoperative or intraoperative analgesia was given. Post-operative pain assessment was done using a 10-point visual analogue scale (VAS) at 2,4,6, 12 and 24 hours postoperatively.
Results
This study included 105 term pregnant female attended to Hurghada General Hospital during June 2022 till March 2023. There was no significant difference between the studied groups regarding age, operative time, and previous cesarean section (p≥0.05), (Figure 1a, b, c).

Figure 1: Age (a), operative time (b) and previous cesarean section (c) among the studied groups.
The current study showed that VAS score was not significantly different between the three studied groups at 2, 12 and 24 hours. While, at 4- and 6-hours VAS were significantly lower among Bupivacaine group than Meloxicam and placebo groups. Also, VAS score was significantly lower in Bupivacaine and Meloxicam groups as compared placebo group at 8 hours (Figure 2).

Figure 2: Pain assessment distribution using VAS score among the studied groups.
There was statistically significant difference between the studied groups regarding addition dose of diclofenac. Bupivacaine group had significantly lower post-operative Consumption of diclofenac at 16, 18, 20 and 24 hrs. than Meloxicam and placebo groups, (Figure 3).

Figure 3: Diclofenac consumption distribution among the studied groups.
In our study, Bupivacaine and Meloxicam groups had significantly higher of satisfaction than placebo group (P<0.001).
Table 1: Patient satisfaction among the studied groups.

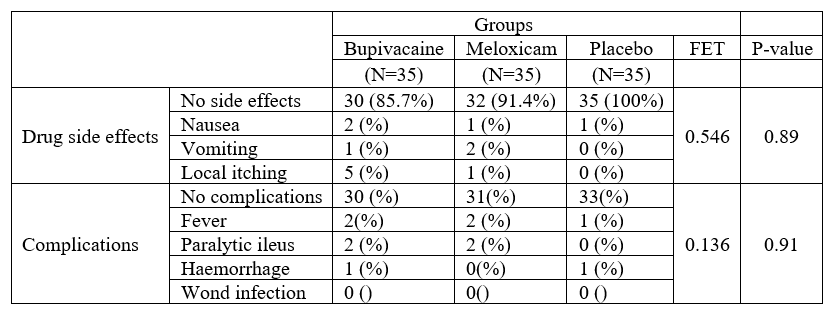
In the current study, there were no statistically significant differences between the studied groups regarding complications (p=0.211) and drug side effects (p=0.859), (Table 2).
Table 2: Complications and drug side effects among the studied groups.
Discussion
The current study showed that VAS score was not significantly different between the three studied groups at 2, 12 and 24 hours. While, at 4- and 6-hours VAS were significantly lower among Bupivacaine group than Meloxicam and placebo groups. Also, VAS score was significantly lower in Bupivacaine and Meloxicam groups as compared placebo group at 8 hours. These results were in accordance with the study done by Atashkhoii et al. (2006) who found that Pain scores were significantly higher in the placebo group than in the bupivacaine group on awakening, and at 6h after surgery.
Another study by [10] there was no significant difference in VAS for pain at 1-hour post operatively and pethidine consumption concluded that local skin infiltration using Bupivacaine 0.5% was not successful in controlling post-cesarean section somatic pain. The results of their study contradict our study. This contradiction may be due to the difference between the number of patients in both studies and due to different concepts in infiltration of the wound (pre-incision skin infiltration was done). [11] showed that the VAS was significantly lower in the levobupivacaine group compared to the other two groups. This comes parallel to study by [12] reviewed data from 20 studies that together involved 1.150 women who gave birth by CS. They found that women treated with local anaesthetic didn't require as much morphine or other opioid drugs for pain relief after their operation which agree with the results of our study. Likewise, [13] showed that it was a safe and simple technique that provides effective analgesia and reduces Morphine requirements after C.S.
This is in accordance with another study conducted by [14] concluded that pain scores were significantly reduced at 12 h but not 6 h after surgery in the LA group compared with placebo group. At 24 h and 48 h after surgery, the pain sore was lower, but the difference did not meet the common level of significance. A lower rate of post-operative nausea was observed in the LA group. Similarly, Sedek and Kassab, (2015) found that infiltration of the wound with bupivacaine reduced post-operative pain and analgesic requirements which agree with the results of our study. There was a statistical highly significant difference in visual analogue scale values after 30 min, 2h, 4h as comparing group C (control) to group A (Lidocaine) and group B (Bupivacaine). There was no statistically significant difference in visual analogue scale after 6h, 12 h and 24 h comparing group C to group A and group B.
In the current study, Bupivacaine and Meloxicam groups had significantly higher satisfaction than placebo group. These results were corresponding to [15] who found that Mean satisfaction score of patient, surgeons and anesthesiologist were significantly higher in the Group BF and Group RF; as compared to group S. Mean Satisfaction score of patients, surgeon and anesthesiologist in Group BF was also significantly higher than in Group RF. Thus, satisfaction score was Group BF > Group RF > Group S. This finding is also consistent with [16] found that nine patients in the L group (levobupivacaine) and 10 patients in the B group (bupivacaine) experienced excellent satisfaction. Six patients in each group experienced good satisfaction and five patients in each group experienced moderate satisfaction. Finally, two patients in the L group and one patient in the B group reported poor satisfaction. While, in the study conducted by [17] found that the levobupivacaine group significantly showed the highest patient satisfaction rate among the three study groups (Levobupivacaine, Meloxicam, Placebo) with 90.4% (47 patients out of 52).
The present study revealed that there were no statistically significant differences between the studied groups regarding complications and drug side effects. There was decrease in the incidence of local itching, Nausea and vomiting in the three groups. Our results were intimately in accordance with the study of [16] who found no significant complication was observed among Levobupivacaine and bupivacaine groups. [18] found no perioperative complications directly related to the injection of a combination of bupivacaine, epinephrine, and morphine. Another recent study by [17] showed that no significant post-operative complications occurred with any of the three drugs (Levobupivacaine, Meloxicam, Placebo). While Atashkhoii et al. (2006) found that there were significant differences regarding postoperative complications such as nausea, vomiting, pruritus, and respiratory depression and patients who asked for antiemetic between the two groups at the first 24h after surgery. This agrees with the well-known safety profile of bupivacaine that was proved before by a lot of studies as well as that of meloxicam.
No adverse effects are detected from the dose of bupivacaine used in previous studies. This observation is consistent with pharmacokinetic studies in which no ad-verse clinical effects were reported from intraperitoneal bupivacaine. In our study bupivacaine was administered in doses like that of these studies and peak plasma concentrations were much smaller than the generally accepted toxic value of 3 μg/mL [19,20]. The dose of bupivacaine used was 150 mg in 45 mL bupivacaine 0.375%, which is lower than the maximum dose (175 mg) of drug for infiltration anesthesia [21].
Conclusion
Local infiltration with either bupivacaine or meloxicam decreases pain score at 6, 12, and 24 hours and decreases need of postoperative diclofenac requirements. Bupivacaine is well tolerated and effective local anesthetic that can be used to reduce pain scores and postoperative analgesic use if infiltrated in surgical wound site in women after cesarean section deliveries and is more effective than meloxicam local infiltration in the same way. Further studies are suggested on adding NSAID to LA in wound infiltration solution for post-operative pain management to increase the chance of effectiveness by different modes of action.
References
- Arroyo-Novoa CM, Figueroa-Ramos MI, Miaskowski C, Padilla G, Paul SM, Rodríguez-Ortiz P, et al. Efficacy of small doses of ketamine with morphine to decrease procedural pain responses during open wound care. The Clinical journal of pain, 2011; 27(7): 561-566.
- Sng BL, Sia AT, Quek K, Woo D, Lim Y. Incidence and risk factors for chronic pain after caesarean section under spinal anaesthesia. Anaesthesia and intensive care, 2009; 37(5): 748-752. https://doi.org/10.1177/0310057X0903700513.
- Kainu JP, Sarvela J, Tiippana E, Halmesmäki E, Korttila KT. Persistent pain after caesarean section and vaginal birth: a cohort study. International journal of obstetric anesthesia, 2010; 19(1): 4-9. https://doi.org/10.1016/j.ijoa.2009.03.013
- Aksoy H, Ak M, Gökahmetoğlu G, Aksoy Ü. The comparison of intraincisional bupivacaine infiltration and intravenous paracetamol administration for pain alleviation after cesarean section: a double-blind randomized placebo controlled clinical trial. European Review for Medical & Pharmacological Sciences, 2023; 27(8).
- Eisenach JC, Pan PH, Smiley R, Lavand’homme P, Landau R, Houle TT. Severity of acute pain after childbirth, but not type of delivery, predicts persistent pain and postpartum depression. Pain, 2008; 140(1): 87-94.
- Nguyen NK, Landais A, Barbaryan A, M'barek MA, Benbaghdad Y, McGee K, et al. Analgesic efficacy of pfannenstiel incision infiltration with ropivacaine 7.5 mg/mL for caesarean section. Anesthesiology research and practice, 2010; 2010. https://doi.org/10.1155/2010/542375
- Bamigboye AA, Hofmeyr GJ. Local anaesthetic wound infiltration and abdominal nerves block during caesarean section for postoperative pain relief. Cochrane Database of Systematic Reviews, 2009; 3.
- Ahmad RK. Validation and Determination of Meloxicam and Pantoprazole in a Combined Formulation (Doctoral dissertation, University of Petra (Jordan)), 2021.
- Bekker A, Kloepping C, Collingwood S. Meloxicam in the management of post-operative pain: Narrative review. Journal of anaesthesiology, clinical pharmacology, 2018; 34(4): 450. https://doi.org/10.4103%2Fjoacp.JOACP_133_18.
- Tverskoy M, Cozacov C, Ayache M, et al. Postoperative pain after inguinal herniorrhaphy with different types of anesthesia. Anesthesia and Analgesia, 2012; 70: 29–35.
- Mohamed SA, Abdel-Ghaffar HS, Kamal SM, et al. Effect of topical morphine on acute and chronic postmastectomy pain: what is the optimum dose? Regional Anesthesia & Pain Medicine, 2015; 41(6): 704-710.
- Nadhima HH, Zahra’s MS. The effect of local anaesthetic infiltration on postoperative pain after caesarean section. Journal of surgery Pakistan, 2010; 15: 131-134.
- Fredman B, Zohar E, Tarabykin A, et al. Bupivacaine wound instillation via an electronic patient-controlled analgesia device, and a double catheter system does not decrease postoperative pain or opioid requirements after major abdominal surgery. Anesth Analg, 2001; 92: 189-189.
- Li X, Zhou M, Shi X, et al. Local anaesthetic wound infiltration used for caesarean section pain relief: a meta-analysis. International journal of clinical and experimental medicine, 2015; 8(6): 10213.
- Naithani U, Kumari I, Roat R, et al. Efficacy of wound infiltration using bupivacaine versus ropivacaine along with fentanyl for postoperative analgesia following abdominal hysterectomy under spinal anesthesia. Journal of Evolution of Medical and Dental Sciences, 2013; 2(34): 6478-6490.
- Fathi HM, Ezz GF. Periarticular infiltration of bupivacaine versus levobupivacaine in postoperative analgesia in patients undergoing total knee arthroplasty. Research and Opinion in Anesthesia & Intensive Care, 2017; 4(2): 70-76.
- Mohie Edine Sh, Aly MS. The Comparison of Intraincisional Injection of Levobupivacaine versus Meloxicam on Post cesarean Section Pain relief thesis for MSC Ain shams faculty of medicine, 2016.
- Lombardi AV, Berend KR, Mallory TH, Dodds KL, Adams JB. Soft-tissue and intra-articular injection of bupivacaine, epinephrine, and morphine has a beneficial effect after total knee arthroplasty. Clin Orthop, 2004; 428: 125–130.
- Ng A, Swami A, Davidson AC, Ememolu J. The analgesic effects of intraperitoneal and incisional bupivacaine with epinephrine after total abdominal hysterectomy. Anesth Analg, 2002; 95(1): 158-162.
- Ong CKS, Lirk P, Seymour RA, Jenkina BJ. The efficacy of pre-emptive analgesia for acute postoperative pain management: A meta-analysis. Anesth Analg, 2005; 100(3): 757-773.
- Miller RD. Miller's Anesthesia, 6th ed, Philadelphia: El-sevier, 2005; 586: 2731-2732.

