Randomized Trial: Combined Vs Monotherapy in Male Hypogonadotropic Hypogonadism
Khalaf RI*, Amr NH, Hassan MM and Elsedfy H
Pediatric Endocrinology Clinic, Ain Shams University, Cairo, Egypt
Received Date: 30/09/2023; Published Date: 05/03/2024
*Corresponding author: Randa I Khalaf, Ph.D., Pediatric Endocrinology Clinic, Ain Shams University, Cairo, Egypt Postal address: 28 Mohamed Tawfik Diab, Nasr City, Cairo, Egypt 11371
Summary
In this randomized controlled trial, 22 males with hypogonadotropic hypogonadism were treated with gonadotropins for 1 year and were followed up using higher doses for another year. Puberty progressed significantly after the first year more than the second year questioning the need to increase doses of gonadotropins.
Abstract
Male HH is characterized by incomplete or absent puberty and infertility. Therapy aims to induce virilization and to gain fertility.
Aim of work: To compare two different treatment protocols given to HH male in terms of puberty induction and spermatogenesis.
Design: Randomized trial.
Setting: Pediatric endocrinology clinic, Ain Shams university, Cairo, Egypt.
Materials and methods: 22 HH males were randomly divided sequentially into 2 groups. Group 1 (9) received combined therapy with both FSH and hCG from the start of the study for 12 months. Group 2 (11) received FSH for 3 months, then combined treatment for further 9 months. Then, all patients were shifted to combined therapy with higher doses. Tanner staging was done every 6 months and trial of semen retrieval.
Main outcomes: puberty induction and spermatogenesis
Results: both groups showed significant increase in testicular sizes, tanner stage and combined testicular volumes by ultrasound (p value = 0.011, 0.007, 0.007 and 0.008 respectively for group 1 and 0.005, 0.005, 0.008 and 0.03 respectively for group 2). For sperm retrieval: In group 1, successful in 1 patient after first year of therapy compared to 3 patients after the second year of therapy. In group 2: 4 patients after the first year of therapy compared to only 1 patient after the second year.
Conclusion: the increase in testicular sizes and tanner stage in both groups was significant after first year of treatment when compared to second year questioning the need to increase gonadotropins dose in treatment of HH male.
This study has been approved by the Research Ethics Committee REC with Registration number FWA 000017585 and Registration date: 2/2/2020
Keywords: Hypogonadotropic hypogonadism; Spermatogenesis; Puberty
Introduction
Puberty in males is dependent on gonadotropin releasing hormone (GnRH) pulsatile secretion from hypothalamic neurons starting at week 7 fetal life under effect of placental human Chorionic Gonadotropins (hCG). This is followed by a mini puberty stage in infancy due to transient activation of hypothalamic pituitary gonadal axis (HPA) from third trimester to 6th month postnatally. A long quiescent stage follows till attaining full puberty at early adolescence after the reactivation of the pulsatile GnRH secretion. GnRH stimulates the pituitary gonadotropins; Follicle Stimulating Hormone (FSH) and Luteinizing Hormone (LH) release. LH is required to stimulate the Leydig cells in the testis to produce testosterone which acts on the Sertoli cells to stimulate sperm production. FSH is required for spermatid maturation during the initiation, and for maintenance of quantitatively normal spermatogenesis at puberty and thereafter [1].
Hypogonadotropic Hypogonadism (HH) is the clinical syndrome resulting from gonadal failure due to deficient GnRH or pituitary gonadotropin secretion. HH is considered treatable in some individuals. HH can be due to congenital CHH (rare) or acquired causes (pituitary tumors or drugs as in prolonged use of opioids) [1].
CHH has two types either anosmic (or hyposmic) as in Kallmann syndrome or normosmic [2]. Although it is congenital yet it usually presents during adolescence [3]. It is caused by the deficient secretion or action of GnRH and is clinically characterized by incomplete or absent puberty and infertility [4]. It can range from severe (e.g., complete absence of puberty with cryptorchidism and micropenis) to relatively milder forms with partial pubertal development. [5] Owing to the delayed presentation of CHH, it can be so difficult to distinguish from constitutional delay of growth and puberty (CDGP), an extreme variant of normal pubertal timing [6]. Low levels of inhibin B (secreted from Sertoli cells) (<16.3 pg/mL) are useful to diagnose severe GnRH deficiency [7]. Inhibin B levels less than 35 pg/ml [8] and FSH levels less than 1.2 IU/L [9] were 100 % predictive of central HH.
In cases of delayed or arrested puberty after the age of 14 years, pubertal induction can be initiated by different methods. To date randomized controlled trials on hormonal treatment in HH are limited. Till now no agreed uniform treatment is used worldwide. Therapeutic goals for treatment of HH are to induce virilization, to reach target height, to acquire normal bone mass and body composition, to achieve normal psychosocial development, and to gain fertility, which may not be covered totally with each of the available treatment protocols.
Monthly IM Testosterone (T) injections stimulate linear growth, virilization, masculinization and psycho-sexual maturation, with no effect on testicular size and consequently fertility due to the absence of intragonadal T production needed to stimulate spermatogenesis [10]. Another used therapy is the administration of gonadotropins that successfully initiates testicular growth and spermatogenesis improving the psychological burden and patients’ self-esteem yet not proven as the best due to the limited published data regarding their effect on final height and attainment of fertility [11].
hCG has LH-like action [12] in stimulating T production by Leydig cells while FSH is required for spermatogenesis initiation and maintenance [13]. Both hCG as a source of LH and urinary human menopausal gonadotropin (hMG) as a substitute for FSH have been used since a very long time. Highly purified urinary FSH has been available since 1997/1998, and recombinant FSH (rFSH) since 1995 [14]. Therapy starts with either hCG alone or in combination with FSH but due to the relatively few numbers of published studies, the outcome cannot be properly evaluated [15].
Some centers start by priming with FSH before the combination to increase Sertoli cell [16]. It was found that CHH patients and absent puberty with/without micropenis and cryptorchidism suffer from suboptimal Sertoli cell count owing to the lack of minipuberty, as evidenced by low serum inhibin B levels, and could thus benefit from FSH priming. Additionally, rFSH priming in patients with gonadotropins deficiency showed significant rise in inhibin B and testicular volumes without increase in intragonadal T production suggesting Sertoli cell proliferation [17]. Despite the seen advantages of this combined protocol, it requires many injections and close monitoring through frequent visits that might not be convenient to all patients.
Aim of the Study
To compare two different treatment protocols given to male patients with hypogonadotropic hypogonadism in terms of puberty induction and spermatogenesis.
Materials and Methods
This randomized controlled trial included 22 males with clinical and laboratory criteria fitting the diagnosis of HH. All clinical and laboratory parameters were evaluated at the Pediatrics Endocrinology Clinic, Children’s hospital, Ain Shams University. Study duration was 2 years.
Inclusion criteria: Males >14 years with HH diagnosed clinically with delayed pubertal maturation, in addition to laboratory evidence in the form of low basal and LHRHa (triptorelin) stimulated levels of FSH, LH and total testosterone [18]. The causes of HH were idiopathic, congenital, or acquired. Idiopathic HH was defined as absence of secondary sexual characters after the age of 14 years with normal Magnetic Resonance Imaging (MRI) results of pituitary gland. Congenital HH was diagnosed when there was a history of micropenis +/ cryptorchidism at birth or congenital brain defects evidenced by MRI of pituitary gland. Acquired HH was diagnosed in the presence of a condition that led to failure of start or arrest of puberty such as brain tumors.
Exclusion criteria: Patients with primary hypogonadism, with HH secondary to chronic diseases or HH receiving treatment at the time of the study.
All patients and their guardians signed an informed consent upon recruitment in the study, after full explanation of the study protocol. Approval of the research ethics committee of Ain Shams University was obtained (FWA 000017585). Birth history was obtained specifically for birth weight, history of neonatal hypoglycemia, NICU admissions, intracranial insults as well as the presence of micropenis +/ cryptorchidism at birth. History of brain tumor, brain surgeries, cranial radiation, and prior hormone therapy particularly T and hCG. Family history of similar condition was noted. Weight and height were measured. Body mass index (BMI) and subsequent standard deviation score (SDS) were calculated. Pubertal staging was performed according to norms of Tanner [19].
Patients who took hCG or T therapy previously were subjected to a washout period for at least 3 months before enrollment in the study.
The following laboratory tests were done to all patients: triptorelin test; FSH, LH and total testosterone levels were measured at baseline and after S.C injection with triptorelin (Decapeptyl® 0.1 mg, Ferring Pharmaceuticals). Blood samples were drawn 4 hours after injection for FSH and LH and after 24 hours for total T levels. LH response <5 IU/L occurs in prepubertal children and in HH and/or hypothalamic disease. (20) Inhibin B was measured at baseline, and again after 6- and 12-months during treatment. All samples for inhibin B were collected at the same time of the day at 10- 11 a.m and centrifuged within 2 hours and stored at -80℃. Samples were analyzed using inhibin B kit based on sandwich enzyme-linked assay technology (MyBioSource, Inc. P.O. Box 153308 San Diego, CA92195-3308 USA).
Patients were randomly divided sequentially into 2 groups by the principal investigator:
Group 1: Included 11 patients who received combined therapy with both FSH and hCG from the start of the study for 12 months. 2 patients refused to continue the study after initial enrollment leaving group 1 with only 9 patients.
Group 2: Included 11 patients who received FSH for 3 months, and then combined treatment (FSH + hCG) for further 9 months. The dose of FSH was 75 units twice weekly while that of hCG was 5000 units once weekly. After the first year, all patients were shifted to combined therapy with higher doses as follows: FSH 3 times weekly (150 units) in addition to hCG once weekly (5000 units). Tanner staging was done every 6 months and trial of semen retrieval once the patient achieved tanner stage 3 was done.
Scrotal ultrasound was repeated to all patients after completing the first year of treatment. Patients refused to repeat the ultrasound after the second year of treatment so we were unable to include the data.
Statistical Methods
IBM SPSS statistics (V. 26.0, IBM Corp., USA) was used for data analysis. Data were expressed as mean and SD for parametric values and additional median and percentiles were used for quantitative non-parametric measures. Where normality of distribution was determined, t-tests for independent samples were conducted; otherwise, the Mann–Whitney U-test was used. Wilcoxon Rank test was used to compare between two groups for non-parametric data. Significance was defined as p value < 0.05. Only statistically significant data were further analyzed by the effect size to compare the standardized difference between baseline and year1 or year 1 and year 2 in both groups. The effect size is calculated using Cohen’s d formula as follows: d = (M1 – M2) / SDpooled. Small effect size is 0.1 to 0.3, medium 0.3 to 0.5 and large is 0.5 or greater. For non-statistically significant data, the effect size was not applicable.
Results
A total of twenty male HH patients aged 15–27 years were enrolled and divided into group 1 (n=9) with mean (SD) age of 20.06 (3.3) years and group 2 (n=11) with mean (SD) age of 18.85 (3.5) years. Tables 1 & 2 describe the history, clinical, laboratory and radiological data of both groups at baseline, year 1 and year 2.
Three patients in group 2 were lost to follow up during the second year of treatment (2 refused to continue and 1 died in car accident).
End-points
- Puberty induction after first year:
Group 1: Mean (SD) right testicular size significantly increased from 2.6 (1.6) ml to 6.5 (4.2) ml (p value = 0.011). Mean (SD) left testicular size significantly increased from 2.4 (1.42) ml to 6.8 (4) ml (p value = 0.007). Mean (SD) Tanner stage significantly increased from 1.2 (0.4) to 2.2 (0.833) (p value = 0.007). Mean (SD) combined testicular volumes measured by ultrasound significantly increased from 2.4 (2) cm3 to 5.5 (4) cm3 (p value = 0.008) (Table 1).
Group 2: Mean (SD) right testicular size significantly increased from 2.1 (1.4) ml to 6.7 (4.6) ml (p value = 0.005). Mean (SD) left testicular size significantly increased from 2.5 (1.0) ml to 6.3 (3.9) ml (p value = 0.005). Mean (SD) Tanner stage significantly increased from 1.09 (0.3) to 2.27 (1.009) (p value = 0.008) Mean (SD) combined testicular volumes measured by ultrasound significantly increased from 2.8 (2.2) cm3 to 6 (5.2) cm3 (p value = 0.03).
Despite the evident statistically significant increase in right, left testicular sizes as well as Tanner stage in both groups, yet there was no statistically significant difference in their effect size (Table 2 & 3).
- Puberty progression after second year:
Group 1: Mean (SD) right testicular size significantly increased to 9.38 (4.3) ml (p value = 0.007) without statistically significant difference in its effect size. Mean (SD) left testicular size increased non-significantly to 7.1 (6.6) ml. Mean (SD) Tanner stage increased non-significantly to 8.44 (4.5).
Group 2: Mean (SD) right testicular size increased non-significantly to 9.63 (6.8) ml. Mean (SD) left testicular size increased non-significantly to 7.8 (6.9) ml. Mean (SD) Tanner stage increased non-significantly to 9.88 (4.97). (Table 2 & 3 and Figures 1 & 2).
- Spermatogenesis:
Group 1: Sperm retrieval was successful in 1 patient (azoospermia) after first year of therapy compared to 3 patients after the second year of therapy (1 azoospermia, 1 oligospermia &1 normal sperm count).
Group 2: Sperm retrieval was successful in 4 patients (azoospermia) after the first year of therapy compared to only 1 patient with normal sperm count after the second year of therapy (Table 4).
- Inhibin B:
There was a statistically significant difference on comparing results of inhibin B levels at baseline, 6 months and 1 year in group 2 only (p value = 0.008) (Table 5).
Serum inhibin B levels correlated positively with sperm concentration (p value = 0.0001), and negatively with FSH levels (p value = 0.01) and LH levels (p value = 0.05). hCG therapy altered inhibin B levels.
Possibly FSH initially increased sperm cell progenitors and then with prolonged treatment they failed to mature to sperm cells.

Figures 1 & 2: Right and left testicular volumes at baseline, 1 year and 2 years.
Table 1: History of patients in both groups.

Table 2: Clinical, laboratory and radiological data at baseline, year 1 and year 2.
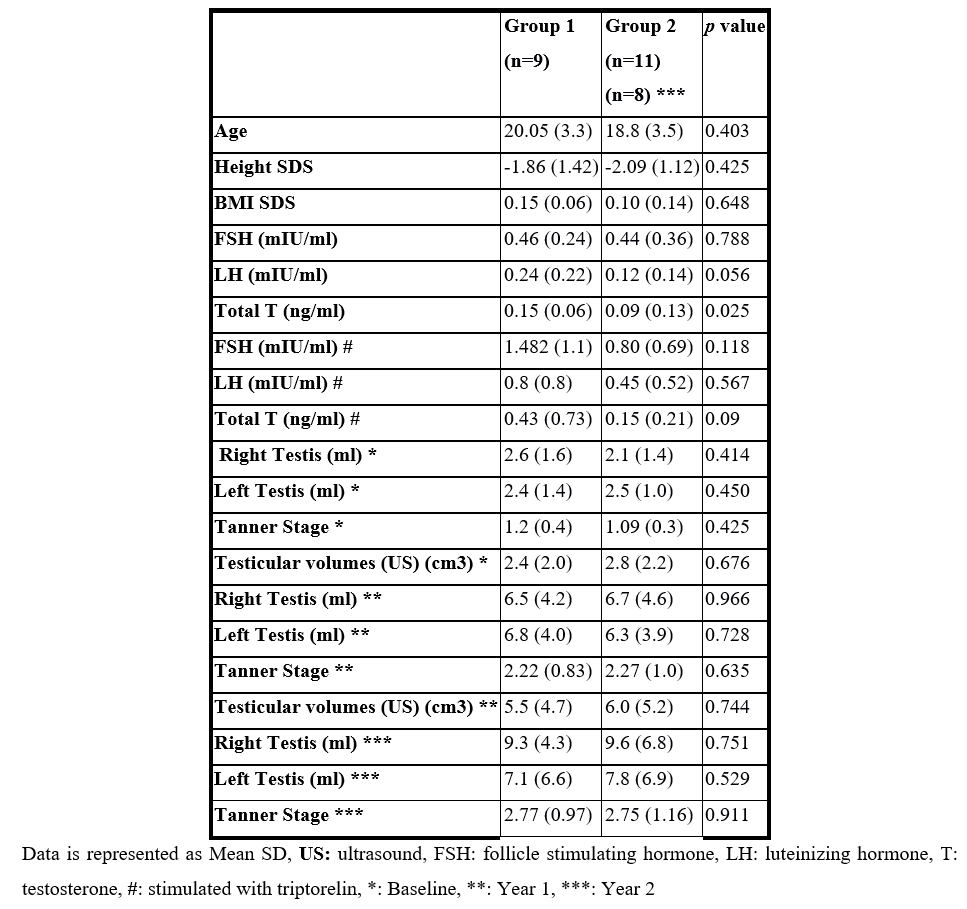
Table 3: Clinical response in group 1 and group 2.
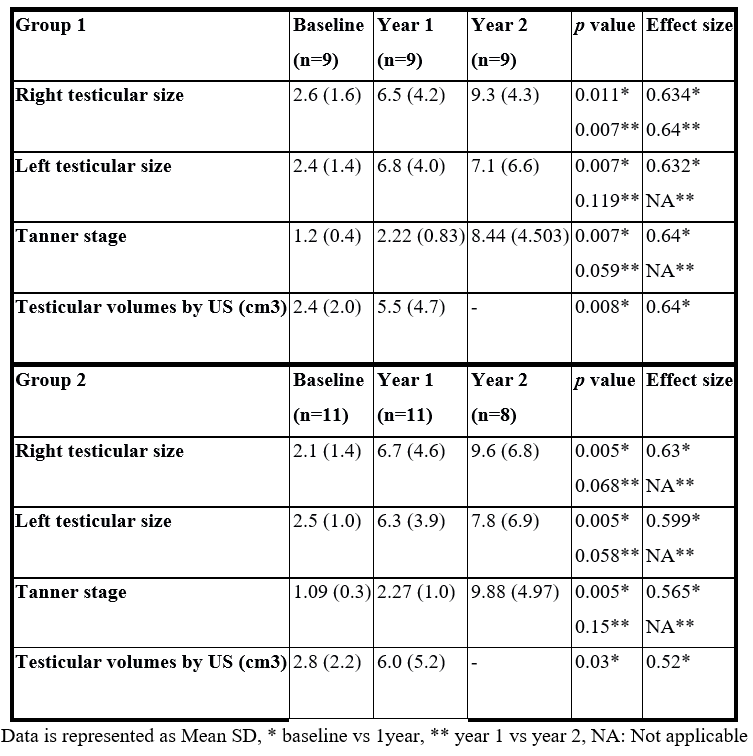
Table 4: Spermatogenesis.
Table 5: Inhibin B.

Table 6: Examples of different treatment protocols for HH and their outcomes.

Discussion
This study was done on 20 patients with HH over a period of 2 years. We were studying different treatment protocols given to patients with HH after dividing them into two groups; group 1 received combined FSH and hCG for 12 months. Group 2 received FSH for 3 months then combined therapy with FSH and hCG for further 9 months. During the second year of the study, both groups were given combined therapy of FSH and hCG at a higher dose. The primary outcome was the puberty induction in terms of increase in testicular sizes and progression of Tanner stage while the secondary outcome was spermatogenesis. Our results showed no statistical difference between both groups in all clinical and radiological data at baseline, 1 year and 2 years. Regarding the laboratory data, baseline testosterone was significantly different between the two groups which may be explained by the higher number of those who received testosterone and hCG prior to the study although a wash out period of 3 months was done. On comparing the change in testicular sizes measured both manually and by ultrasound, there was a statistically significant difference in both groups during the first year of treatment only. Increasing the doses of FSH and hCG during the second year increased testicular sizes and tanner stages further yet non- significantly. Inhibin B levels at baseline, 6 months and 1 year were higher in group 2 denoting the possible effect of FSH priming on testes in agreement with other studies [17,21,22].
Previous studies were done on HH patients receiving different treatment protocols as illustrated in table (10). Bouloux et al., (23), Zacharin et al., [16], Sanyal & Chatterjee [24] and Prior et al., [25] compared the effect of monotherapy with hCG vs combined therapy with both FSH and hCG. Other studies used combined therapy with both drugs with either higher dose of FSH as Liu et al., [26] and Rohayem et al., [15] or for longer duration as Lui et al., [27] and Kohva et al., [20] as shown in Table 6.
Primary outcome: The right and left testicular sizes as well as Tanner staging increased significantly during the first year of treatment and non-significantly during the second year suggesting that increasing doses of FSH and hCG may not be needed in treatment plans of male patients with HH. A study done by Zacharin et al. [16] concluded that after adding FSH to hCG for only 9 months, the testicular sizes and spermatogenesis were hastened. Kohva et al., [20] concluded that r-hFSH doubled testicular volumes after using higher doses of FSH reaching 112.5 IU three times weekly for longer duration reaching 34 months.
Rohayem et al., [15] achieved adult testicular volumes (≥24 ml) after an average of 23-25 months of combined treatment with hCG (250-500 IU twice weekly with dose titration according to serum testosterone level up to a maximum of 2500 IU 3 times weekly) and FSH (75-150 IU three times weekly).
Secondary outcome: spermatogenesis: After the first year of therapy 1 patient in group 1 and four patients in group 2 were able to produce azoospermic semen. While after the second year of therapy 3 patients (1 azoo, 1 oligo, 1 normal) produced sperms in addition to only 1 patient in group 2 (normal sperm count). This was in contrast to other studies that achieved successful spermatogenesis in all their studied patients after using either higher dose of FSH as Liu et al., [26] and Sanyal & Chatterjee [24] or longer duration of therapy as Liu et al., [27] and Dwyer et al., [28].
HH patients were treated by the same researchers in two different studies by Liu et al., [26,27], the first study was in 2009 and used 75-150 IU FSH three times weekly in combination with hCG in HH patients of diverse etiologies seeking fertility and achieved successful spermatogenesis after a median duration of 7 months in 90 % of the patients. The second study was performed in 2016 on male patients with CHH and used 75-150 IU of FSH twice weekly in combination with hCG and reached successful spermatogenesis after a longer duration of time reaching 42 months in some patients. So, continuation of our work using higher doses of FSH for a longer duration might be recommended.
All patients included in the study done by Zacharin et al., [16] were able to achieve spermatogenesis with a sperm count ranging from (0.2 - 15 x 10 ^6/ml) after receiving combined hCG (500–1,500 IU twice weekly) and FSH (150–300 IU three times per week) for only 9 months. Spermatogenesis was not achieved in all of our patients, probably owing to the lower doses of FSH used during the first year of treatment with subsequent negative impact on Sertoli cell proliferation.
In conclusion, this study demonstrated that the increase in testicular sizes as well as tanner stage in both groups was statistically significant after the first year of treatment when compared to the second year. This conclusion could possibly suggest that there is no need to increase the doses of FSH and hCG in treatment of male patients with hypogonadotropic hypogonadism. However further studies with bigger number of patients and with longer duration of therapy with FSH and hCG are needed.
Acknowledgment: The authors are grateful to Sarah El Qusi for her help in collection of clinical data. This is a continued work from our previous publication doi: 10.12816/0052914
Funding: None
Conflict of interest: none
References
- Kamel RM. Management of the infertile couple: an evidence-based protocol. Reproductive Biology and Endocrinology, 2010; 10: 21-25.
- Boehm U, Bouloux PM, Dattani MT, de Roux N, Dode C, Dunkel L, et al. Expert consensus document: European Consensus Statement on congenital hypogonadotropic hypogonadism pathogenesis, diagnosis and treatment. Nature Reviews. Endocrinology 2015; 11: 547–564.
- Pitteloud N, Hayes FJ, Boepple PA, DeCruz S, Seminara SB, MacLaughlin DT, et al. The role of prior pubertal development, biochemical markers of testicular maturation, and genetics in elucidating the phenotypic heterogeneity of idiopathic hypogonadotropic hypogonadism. Journal of Clinical Endocrinology and Metabolism, 2002; 87: 152–160.
- Gerner Lawaetz J, Hagen CP, Grunnet Mieritz M, Blomberg Jensen M, Holm Petersen J & Juul. Evaluation of 451 Danish boys with delayed puberty: diagnostic use of a new puberty nomogram and effects of oral testosterone therapy. Journal of Clinical Endocrinology and Metabolism, 2015; 100: 1376–1385.
- Harrington J, Palmert MR. Clinical review: distinguishing constitutional delay of growth and puberty from isolated hypogonadotropic hypogonadism: critical appraisal of available diagnostic tests. J Clin Endocrinol Metab, 2012; 97(9): 3056–3067.
- Varimo T, Miettinen PJ, Ka ̈ns ̈akoski J, Raivio T, Hero M. Congenital hypogonadotropic hypogonadism, functional hypogonadotropism or constitutional delay of growth and puberty? An analysis of a large patient series from a single tertiary center. Hum Reprod, 2017; 32(1): 147–153.
- Fang Q, George AS, Brinkmeier ML. Genetics of combined pituitary hormone deficiency: roadmap into the genome era. Endocr Rev, 2016; 37: 636–675.
- Coutant, Estelle Biette-Demeneix, Claire Bouvattier, Natacha Bouhours-Nouet, Frédérique Gatelais, Sylvie Dufresne, et al. Baseline inhibin B and anti-Mullerian hormone measurements for diagnosis of hypogonadotropic hypogonadism (HH) in boys with delayed puberty J Clin Endocrinol Metab, 2010; 95(12): 5225-5232.
- Grinspon, María Gabriela Ropelato, Silvia Gottlieb, Ana Keselman, Alicia Martínez, María Gabriela Ballerini, et al. Basal follicle-stimulating hormone and peak gonadotropin levels after gonadotropin-releasing hormone infusion show high diagnostic accuracy in boys with suspicion of hypogonadotropic hypogonadism J Clin Endocrinol Metab, 2010; 95(6): 2811-2818.
- Kiess W, Conway G, Ritzen M, Rosenfield R, Bernasconi S, Juul A, et al. Induction of puberty in the hypogonadal girl—practices and attitudes of pediatric endocrinologists in Europe. Horm Res, 2002; 57(1–2): 66–71.
- Bouloux P, Warne DW, Loumaye E. Efficacy and safety of recombinant human follicle-stimulating hormone in men with isolated hypogonadotropic hypogonadism. Fertility and Sterility, 2002; 77: 270–273.
- Siris ES, Nisula BC, Catt KJ. New evidence for intrinsic follicle-stimulating hormone-like activity in human chorionic gonadotropin and luteinizing hormone. Endocrinology, 1978; 102: 1356–1361.
- Tapanainen JS, Aittomaki K, Min J. Men homozygous for an inactivating mutation of the follicle-stimulating hormone (FSH) receptor gene present variable suppression of spermatogenesis and fertility. Nature Genetics, 1997; 15: 205–206.
- European Metrodin HP Study Group EMHSG Efficacy and safety of highly purified urinary follicle-stimulating hormone with human chorionic gonadotropin for treating men with isolated hypogonadotropic hypogonadism. European Metrodin HP Study Group. Fertility and Sterility, 1998; 70: 256–262.
- Rohayem J, Hauffa BP, Zacharin M, Kliesch S, Zitzmann M. “German Adolescent Hypogonadotropic Hypogonadism Study Group”. Testicular growth and spermatogenesis: new goals for pubertal hormone replacement in boys with hypo- gonadotropic hypogonadism? —a multicentre prospective study of hCG/rFSH treatment out- comes during adolescence. Clin Endocrinol, 2017; 86(1): 75–87.
- Zacharin M, Sabin MA, Nair VV, et al. Addition of recombinant follicle-stimulating hormone to human chorionic gonadotropin treatment in adolescents and young adults with hypogonadotropic hypogonadism promotes normal testicular growth and may promote early spermatogenesis. Fertility and Sterility, 2012; 98: 836–842.
- Raivio T, Wikstro ̈m AM, Dunkel L. Treatment of gonadotropin-deficient boys with recombinant human FSH: long-term observation and outcome. Eur J Endocrinol, 2007; 156(1): 105–111.
- Fraietta R, Zylberstejn DS, Esteves SC. Hypogonadotropic hypogonadism revisited. Clinics, 2013; 68: 81-88.
- Tanner J, Whitehouse R, Takaishi MJ. Standards from birth to maturity for height, weight, height velocity, and weight velocity: British children II, 1965; 41(220): 613.
- Kohva E, Huopio H, Hero M, Miettinen PJ, Vaaralahti K, Sidoroff V, et al. Recombinant Human Follicle Stimulating Hormone Treatment Outcomes in Five Boys with Severe Congenital Hypogonadotropic Hypogonadism. J Endocr Soc, 2018; 2(12): 1345–1356.
- Layman LC. Hypogonadotropic hypogonadism. Endocrinology and metabolism clinics of North America, 2007; 36(2): 283-296.
- Bougnères P, François M, Pantalone L, Rodrigue D, Bouvattier C, Demesteere E, et al. Effects of an early postnatal treatment of hypogonadotropic hypogonadism with a continuous subcutaneous infusion of recombinant follicle-stimulating hormone and luteinizing hormone. The Journal of Clinical Endocrinology & Metabolism, 2008; 93(6): 2202-2205.
- Bouloux PMG, Nieschlag E, Burger HG, Skakkebaek NE, Wu FC, Handelsman DJ, et al. Induction of spermatogenesis by recombinant follicle‐stimulating hormone (puregon) in hypogonadotropic azoospermic men who failed to respond to human chorionic gonadotropin alone. Journal of andrology, 2003; 24(4): 604-611.
- Sanyal D, Chatterjee S. Treatment preferences and outcome in male hypogonadotropic hypogonadism: an Indian perspective. Andrologia, 2016; 48(5): 601-602.
- Prior M, Stewart J, McEleny K, Dwyer AA, Quinton R. Fertility induction in hypogonadotropic hypogonadal men. Clinical endocrinology. Clinical Endocrinology, 2018; 89: 712–718.
- Liu PY, Baker HG, Jayadev V, Zacharin M, Conway AJ, Handelsman DJ. Induction of spermatogenesis and fertility during gonadotropin treatment of gonadotropin-deficient infertile men: predictors of fertility outcome. The Journal of Clinical Endocrinology & Metabolism, 2009; 94(3): 801-808.
- Liu Z, Mao J, Wu X, Xu H, Wang X, Huang B, et al. Efficacy and outcome predictors of gonadotropin treatment for male congenital hypogonadotropic hypogonadism: a retrospective study of 223 patients. Medicine, 2016; 95(9):
- Dwyer AA, Raivio T, Pitteloud N. Gonadotrophin replacement for induction of fertility in hypogonadal men. Best Practice & Research Clinical Endocrinology & Metabolism, 2015; 29(1): 91-103.

