Intra-Abdominal Lymphatic Malformations
Jo Anamarie Cooke-Barber1,a, Joseph Gerard Brungardt1,a,*, Gregory M Tiao1, Adrienne M Hammill2, Kiersten W Ricci2, Manish N Patel3 and Roshni Dasgupta1
1Division of Pediatric General and Thoracic Surgery, Cancer and Blood Diseases Institute, Hemangiomas and Vascular Malformation Center, Cincinnati Children’s Hospital Medical Center, USA
2Division of Pediatric Hematology and Oncology, Cancer and Blood Diseases Institute, Hemangiomas and Vascular Malformation Center, Cincinnati Children’s Hospital Medical Center, USA
3Division of Radiology, Cancer and Blood Diseases Institute, Hemangiomas and Vascular Malformation Center, Cincinnati Children’s Hospital Medical Center, USA
aCo-first authors
Received Date: 14/09/2023; Published Date: 16/02/2024
*Corresponding author: Joseph Gerard Brungardt, MD, Division of Pediatric General and Thoracic Surgery, Cincinnati Children’s Hospital Medical Center, USA
Abstract
Background: Intra-abdominal lymphatic malformations are rare entities without a clear consensus on management or prognosis. These malformations are medically and surgically complex and often require a sophisticated, multi-disciplinary approach to treatment.
Methods: A single quaternary institutional, retrospective chart review between January 2000-December 2019 was performed on all patients with an initial diagnosis of a non-solid organ intra-abdominal lymphatic malformation. Demographics, presentation, treatment, and outcome were reviewed.
Results: Twenty-four patients were identified, 62.5% were male. Median age at time of diagnosis was 2.5 years old, 33% were present at birth, 50% presented before 2 years old, and 29% presented ≥10 years of age. Seventy-five percent were lymphatic (majority macrocystic), 20.8% were mixed lymphatic-venous malformations (LVM). Fifty-four percent had other associated lymphatic or vascular malformations including 4 patients with PIK3CA-Related Overgrowth Spectrum (PROS), 3 with Generalized Lymphatic Anomaly, and 2 with Capillary Venous Lymphatic malformation (CVLM). Patients most often presented with abdominal pain (50%). An intervention was performed in 95.8% and 87.5% were managed initially with sclerotherapy. Most interventions were performed for abdominal symptoms either before three years of age or during adolescence. Laparoscopic or open surgery was performed in 41.7% of patients. Surgery was performed as primary therapy in 8.3% of the group.
Conclusion: Intra-abdominal lymphatic malformations appear to have a bimodal distribution pattern of presentation; shortly after birth and again at puberty. Most patients present with abdominal symptoms and have other associated vascular anomalies. MRI appears to be the most accurate modality for treatment planning. Interventional procedures such as sclerotherapy and medical therapy with sirolimus are safe and effective and may be considered first line therapies. Surgical resection may be required when the diagnosis is in question or when more conservative therapies are no longer efficacious and is typically an option of last resort.
Keywords: Vascular malformations; Lymphatic malformations; Venous malformations; CLOVES; Intra-abdominal vascular malformations
Abbreviations Key: ILM- Intra-abdominal Lymphatic Malformations; CLOVES- Congenital Lipomatous Overgrowth, Vascular Malformations, Epidermal Nevi, and Scoliosis/Skeletal/Spinal Anomalies Syndrome; LM- Lymphatic Malformation; LVM- Lymphatic-Venous Malformation; CVLM- Capillary Venous Lymphatic Malformation; PROS- PIK3CA Related Overgrowth Syndrome; MRI- Magnetic Resonance Imaging; CT- Computed Tomography; GLA- Generalized Lymphatic Abnormality; ICD-10 - International Classification Of Diseases, Tenth Revision
Introduction
Intra-abdominal vascular malformations are rare entities, reported in the literature to have a 1% incidence, with the true incidence likely higher given small asymptomatic lesions [1–3]. There are three general types of vascular malformations which are seen in non-solid organs of the abdomen which include venous, lymphatic, arteriovenous, and combinations such as lymphatic-venous malformations, though the International Society for the Study of Vascular Anomalies (ISSVA) has classifications of lymphatic malformations in three groups: simple, complex, and lymphedema [4,5]. Lymphatic malformations (LM) occur during embryonic
development as a result of abnormally formed lymphatic vessels and represent the most common form of low-flow, non-solid organ, intra-abdominal vascular malformation [1,4].
Intraabdominal Lymphatic Malformations (ILM) are medically and surgically complex and require a sophisticated, multi-disciplinary approach to treatment. There are no consensus guidelines on the management or prognosis of these lesions. Recent studies have demonstrated that though LM are rare entities, both inpatient charges and resource utilization as well as mortality are on the rise for patients with lymphatic malformations [6]. Intraabdominal lymphatic malformations have a known association with other vascular malformations, particularly venous malformations. especially those involving the trunk. Patients with intraabdominal lymphatic malformations can present with abdominal pain, early satiety, obstruction, volvulus, constipation, and bladder outlet obstruction. Infection is rare though can present as septic shock.
Patients with CLOVES syndrome (Congenital lipomatous overgrowth, vascular malformations, epidermal nevi, and scoliosis/skeletal/spinal anomalies syndrome), a PIK3CA related overgrowth syndrome (PROS), frequently manifest with involvement/features on the flanks or abdomen [4,7]. Sirolimus therapy has been used to treat PROS and has demonstrated
safety and efficacy, though it is accepted that it does not cause shrinkage of the malformations, but rather stabilizes them and reduce symptoms including pain [4,8–11]. The purpose of this study was to investigate the natural history and clinical course of this disease process, and described required therapies and treatments in a lesion which can typically be observed.
Methods
A retrospective chart review was performed at a single quaternary institution after approval from the institutional review board. Patients with a diagnosis of intra-abdominal vascular malformation treated at our institution between January 2000 and December 2019 were included. Patients with an initial diagnosis of non-solid organ intra-abdominal lymphatic malformation were included. Isolated solid organ malformations such as those patients with pancreatic, splenic, liver, or bowel were excluded for the purposes of this study. Data was collected on demographics, presentation, treatment and outcomes, and follow-up.
Results
Demographics
Twenty-four patients with intra-abdominal lymphatic malformations were identified. Fifteen patients, 62.5%, were male, and the remaining 37.5% (n=9) were female. The median age at time of diagnosis was 2.5 (SD 6.19) years (range 0 – 17 years). Seven cases, 29%, were present at birth, 50% present (n=12) before 2 years old, and 29% (n=7) presented in patients older than 10 years. The ages at presentation created a peak incidence distribution pattern (Figure 1). Notably, the peak presentations appear before 2 years old and over 10 years old. The majority, 75% (n=18) were Caucasian, with 12.5% (n=3) African American and 8.3% (n=2) Hispanic. Most patients were privately insured (58.3%, n=14). See Table 1 for further demographic details of the population.
Malformation Characteristics
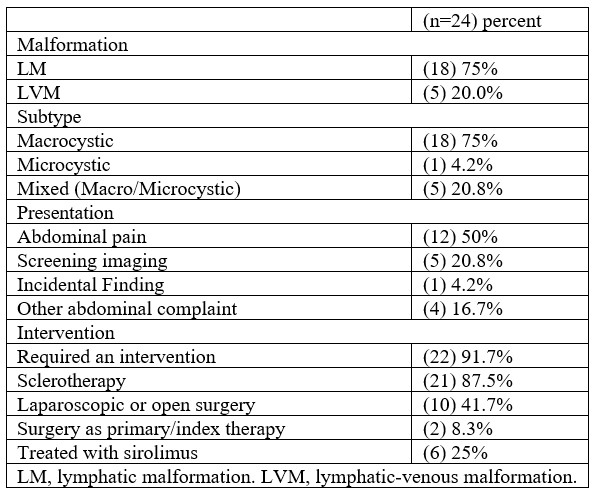
Primarily lymphatic malformations, and lymphatic-venous malformations were noted in this study group. Eighteen (75%) were Lymphatic Malformations (LM), 20.8% (n=5) were mixed lymphatic-venous (LVM). The majority of the LM in our patient population were primarily macrocystic (n=18, 75%) with 20.8% (n=5) being mixed macro-microcystic and one (4.2%) primarily microcystic (Table 2).
One patient with presumed LVM based on imaging was ultimately found to have extra-osseus Ewing’s Sarcoma of the abdomen. CT and MRI were consistent with LVM of the mesentery with multiple macrocysts as well as microcystic features. However, fluid from a sclerotherapy procedure was sent for cytology and noted to be consistent with Ewing’s sarcoma.
Clinical Features
Thirteen patients, 54.2%, had other lymphatic or vascular malformations including 4 patients with PROS, 3 with Generalized Lymphatic Anomaly (GLA), 2 with Capillary Venous Lymphatic malformation (CVLM), and 4 with other lymphatic malformations. Twelve patients, 50%, presented with abdominal pain, 20.8% were discovered incidentally on screening imaging,
16.7% presented with abdominal distension, rectal bleeding, or a palpable abdominal mass, and one was diagnosed incidentally during an inguinal hernia repair.
All patients in our study underwent some form of imaging to either confirm or diagnose ILM. Ultrasound and computed tomography scan were often performed, typically used in order to work up a patient with abdominal signs or symptoms or as part of a workup for another issue (i.e., trauma work up). Once an intra-abdominal malformation was identified, MRI was utilized in all patients as the primary modality of imaging for more complete evaluation of the ILM, surveillance, and treatment and intervention planning.
Management
Of the twenty-four patients, 23 (95.8%) required an intervention at some point during their clinical course. Twenty-one, 87.5%, were managed initially with sclerotherapy. Most interventions were performed for abdominal symptoms before 3 years old or during adolescence. Ten patients (41.7%) underwent laparoscopic or open surgery. Two patients (8.3%) underwent surgery as their primary therapy. Thirteen of 19 patients (68.4%) who underwent sclerotherapy did not require further invasive intervention. Four patients, 21%, received sclerotherapy and then underwent surgical resection for progressive disease. Six patients (25%) were treated with sirolimus at some point during their clinical course. Indications for surgery in all patients included abdominal pain, with some also having symptoms of distension or early satiety. Patients were followed for at least 1-2 years, with some lost to follow up but most having surveillance ongoing over 5 years.
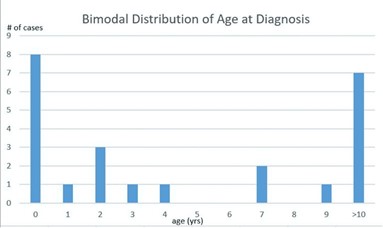
Figure 1: Peak incidence of age at diagnosis of intra-abdominal lymphatic malformations.

Figure 2: Magnetic resonance imaging of extensive intra-abdominal lymphatic malformation demonstrating both macrocystic and microcystic features.
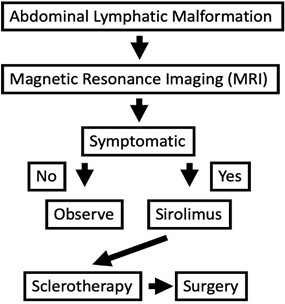
Figure 3: Pathway for management of abdominal lymphatic malformations.
Table 1: Demographics of patients with intra-abdominal lymphatic malformations.

Table 2: Malformation and therapy data of patients with intra-abdominal lymphatic malformations.
Discussion
Lymphatic malformations are a rare but costly disorder with a recent trend towards higher inpatient costs and resource utilization [6]. A recent study examining characteristics of inpatients with LM showed more admission in children under age 3 [12]. Our series of patients with ILM demonstrated a peak incidence curve as seen in Figure 1. This is consistent with hormonal sensitivity of these lesions as patients demonstrate hormonally driven growth in the first two years of life as well as hormone surges around the onset of puberty.
MRI was the most commonly used form of imaging for the diagnosis of ILM and has been shown to be sensitive and specific for the diagnosis of low-flow ILM [1,13]. With recent MRI advances, fetal diagnosis is possible [14]. Ultrasound provides an ideal modality for interval surveillance though MRI evaluates the anatomic extent and involvement of the LM respective to other structures (15). Specifically, MRI helps evaluate subtypes of lymphatic malformations, including macrocystic, microcystic, and mixed macro-microcystic (Figure 2).
This differentiation is necessary to direct approach to sclerotherapy. Macrocystic lesions may be approached directly, with a pigtail drain placed within the cavity for sclerosant access, while microcystic lesions are typically approached individually with small angiocatheters. Figure 3 describes a proposed pathway for management of abdominal lymphatic malformations.
Two of our patients had melena as a symptom on presentation. Venous enlargement adjacent to lymphatic malformations are frequent, though venous malformations associated with lymphatic malformations is rare. Thus, the bleeding may be from an associated engorged vein and not necessarily the malformation itself. Surgical resection may allow for decompression of the venous collaterals.
Most patients presented with mild to moderate symptoms, generally pain, mass, or distension [2]. 95.8% of our patients needed either sclerotherapy or surgery, with interventional treatment rates ranging from 60-100% similar to other reports [16]. Sclerotherapy was highly effective with only 4 of 16 sclerotherapy patients needing subsequent treatment with laparoscopic or open surgery. Sclerotherapy at our institution is typically image guided by ultrasound and fluoroscopy, the lesion aspirated, fluid collections drained, and doxycycline instilled as the sclerosing agent. Sclerotherapy for intraabdominal vascular malformations has been shown to be safe and effective in previous studies with demonstrated success rates of 96.7% and a low rate of complications [2]. Our follow up for most patients ranged from one to 5 years. Success with sclerotherapy on these lesions is characterized by both symptomatic relief and volumetric reduction in size of these lesions and not based on complete radiographic resolution as this is rarely achieved and should not be the goal of therapy [2]. Sclerotherapy is traditionally known to be more helpful in patients with macrocystic lesions, however, Chaudry, et al. demonstrates the utility of bleomycin as a sclerosing agent for the treatment of microcystic or mixed lesions [17]. One patient who developed a recurrent ILM years after treatment with sclerotherapy underscores the importance for long term management in a multi-disciplinary clinic and a high index of suspicion if recurrent symptoms develop.
Sirolimus has demonstrated both safety and efficacy in multiple studies on the use in patients with PROS and other lymphatic malformations [8–11]. This therapeutic benefit is believed to be related to sirolimus’ ability to inhibit lymphatic vessel invasion and regeneration through the mTor pathway [18,19]. Twenty-five percent of patients in our study were treated with sirolimus, which plays an important role in the multi-modal treatment of patients with PROS. It was difficult in this study to delineate with certainty if the patients treated with sirolimus required less interventions, however their treatment was aimed toward improvement in overall symptoms. Length of sirolimus prescription was not recorded. medications that also target the mTOR/PIK3CA pathway are on the horizon. BYL719 (alpelisib) has shown to have promise in both safety and efficacy and shown relatively dramatic improvement in the size of malformations, with recent approval [20].
Childbearing females may also note exacerbations or enlargement of their vascular malformations during pregnancy. One patient successfully brought two pregnancies to term and developed increase pain in her head and neck lymphatic malformation but did not report any significant increase in symptoms relating to her intraabdominal malformation. Female patients should be counseled to avoid taking exogenous estrogen containing medications as these can worsen symptoms from vascular malformations. Counseling becomes especially important as the patients near puberty and late adolescence, as many are lost to follow up.
One patient in our study was presumed to have mixed macro-microcystic ILM on preoperative MRI, however the surgical pathology returned Ewing’s Sarcoma. This underscores the importance of following the patient and considering surgical resection or at least biopsy should the course of the lesion not follow typical patterns. Rapid growth and changing symptoms should raise suspicion for malignancy [21].
Limitations of this study are its retrospective nature as well as the challenge of gathering data from a complex chart review process. Many of our patients are referred from outside facilities including across the country as well as from international centers which may affect our rates of procedures or interventions.
Intra-abdominal vascular malformations have a bimodal distribution pattern of presentation, shortly after birth and at puberty. Half of our patients presented with abdominal signs and symptoms such as abdominal pain, distension, constipation, and nausea which was consistent with presentations reported in existing literature [22]. Many of our patients had other associated vascular anomalies. Patients with PROS, GLA, and those who have other vascular malformations are at risk for the presence of intra-abdominal vascular malformations. There is a population of patients who have isolated intra-abdominal vascular malformations, therefore clinical suspicion and abdominal signs and symptoms should guide the diagnostic work up to rule out ILM. MRI appears to be the most accurate modality for treatment/intervention planning [1,13]. Interventional procedures such as sclerotherapy and medical therapy with sirolimus can be used with excellent efficacy and should be considered first line therapies. Surgery should not be considered as primary therapy unless the patient develops peritonitis or bowel obstruction. Despite the use of sirolimus, most patients will need an intervention at some point during childhood or adolescence for symptoms related to their ILM. Only one patient in our series was treated with sirolimus alone, the rest requiring some form of intervention. Surgical resection is often required when the diagnosis is in question or when more conservative therapies are no longerefficacious and in our experience in a last-line therapy.
Conflict of interest: The authors have no conflicts of interest. No funding received.
References
- Francavilla ML, White CL, Oliveri B, Lee EY, Restrepo R. Intraabdominal Lymphatic Malformations: Pearls and Pitfalls of Diagnosis and Differential Diagnoses in Pediatric Patients. AJR Am J Roentgenol [Internet]. 2016/12/23. 2017; 208(3): 637–649.
- Madsen HJ, Annam A, Harned R, Nakano TA, Larroque LO, Kulungowski AM. Symptom Resolution and Volumetric Reduction of Abdominal Lymphatic Malformations with Sclerotherapy. J Surg Res, 2019; 233: 256–261.
- Alqahtani A, Nguyen LT, Flageole H, Shaw K, Laberge JM. 25 years’ experience with lymphangiomas in children. J Pediatr Surg, 1999; 34(7): 1164–1168.
- Adams DM, Ricci KW. Vascular Anomalies: Diagnosis of Complicated Anomalies and New Medical Treatment Options. Hematol Oncol Clin North Am, 2019; 33(3): 455–470.
- ISSVA Classification of Vascular Anomalies ©2018 International Society for the Study of Vascular Anomalies, 2022.
- Cheng J, Liu B, Farjat AE, Routh J. The Public Health Burden of Lymphatic Malformations in Children: National Estimates in the United States, 2000-2009. Lymphat Res Biol, 2017; 15(3): 241–245.
- Martinez-Lopez A, Blasco-Morente G, Perez-Lopez I, Herrera-Garcia JD, Luque-Valenzuela M, Sanchez-Cano D, et al. CLOVES syndrome: review of a PIK3CA-related overgrowth spectrum (PROS). Clin Genet, 2017; 91(1): 14–21.
- Adams DM, Trenor 3rd CC, Hammill AM, Vinks AA, Patel MN, Chaudry G, et al. Efficacy and Safety of Sirolimus in the Treatment of Complicated Vascular Anomalies. Pediatrics, 2016; 137(2): e20153257.
- Hammill AM, Wentzel M, Gupta A, Nelson S, Lucky A, Elluru R, et al. Sirolimus for the treatment of complicated vascular anomalies in children. Pediatr Blood Cancer, 2011; 57(6): 1018–1024.
- Tian R, Liang Y, Zhang W, Wang J, Shan Y, Gao H, et al. Effectiveness of sirolimus in the treatment of complex lymphatic malformations: Single center report of 56 cases. J Pediatr Surg, 2020; 55(11): 2454–2458.
- Triana P, Dore M, Cerezo VN, Cervantes M, Sanchez A V, Ferrero MM, et al. Sirolimus in the Treatment of Vascular Anomalies. Eur J Pediatr Surg, 2017; 27(1): 86–90.
- Cheng J, Liu B, Farjat AE, Routh J. National Characteristics of Lymphatic Malformations in Children: Inpatient Estimates and Trends in the United States, 2000 to 2009. J Pediatr Hematol Oncol, 2018; 40(3): 221–223.
- Akita H, Yamada Y, Ito Y, Mikami S, Sugiura H, Okuda S, et al. Retroperitoneal low-flow vascular malformations: characteristic MRI findings correlated with histopathological findings. Abdom Imaging, 2015; 40(6): 1713–1720.
- Barrera CA, Victoria T, Escobar FA, Krishnamurthy G, Smith CL, Moldenhauer JS, et al. Imaging of fetal lymphangiectasias: prenatal and postnatal imaging findings. Pediatr Radiol, 2020; 50(13): 1872–1880.
- Liu YP, Huang YL, Tsai PS, Lin DC, Chen CP. Prenatal diagnosis of abdominal lymphatic malformations. Taiwan J Obs Gynecol, 2021; 60(1): 13–19.
- Elbaaly H, Piche N, Rypens F, Kleiber N, Lapierre C, Dubois J. Intra-abdominal lymphatic malformation management in light of the updated International Society for the Study of Vascular Anomalies classification. Pediatr Radiol, 2021; 51(5): 760–772.
- Chaudry G, Guevara CJ, Rialon KL, Kerr C, Mulliken JB, Greene AK, et al. Safety and efficacy of bleomycin sclerotherapy for microcystic lymphatic malformation. Cardiovasc Interv Radiol, 2014; 37(6): 1476–1481.
- Ricci KW. Advances in the Medical Management of Vascular Anomalies. Semin Interv Radiol, 2017; 34(3): 239–249.
- Ozeki M, Nozawa A, Yasue S, Endo S, Asada R, Hashimoto H, et al. The impact of sirolimus therapy on lesion size, clinical symptoms, and quality of life of patients with lymphatic anomalies. Orphanet J Rare Dis, 2019; 14(1): 141.
- Venot Q, Blanc T, Rabia SH, Berteloot L, Ladraa S, Duong JP, et al. Targeted therapy in patients with PIK3CA-related overgrowth syndrome. Nature, 2018; 558(7711): 540–546.
- Scorletti F, Patel MN, Hammill AM, Ricci KW, Myer CM th, Dasgupta R. Sclerotherapy for intramuscular vascular malformations: A single-center experience. J Pediatr Surg, 2018; 53(5): 1056–1059.
- Kosir MA, Sonnino RE, Gauderer MW. Pediatric abdominal lymphangiomas: a plea for early recognition. J Pediatr Surg, 1991; 26(11): 1309–1313.

