The Association Between Selenium and Epithelial Ovarian Cancer (EOC) Among Women; A Protocol of a Systematic Review and Meta-Analysis
Perera KCM1,*, Perera WND2 and Abeysena HTCS3
¹Acting Consultant Community Physician, Visiting Research Fellow, The School of Public Health, The University of Queensland, Post Graduate Institute of Medicine University of Colombo, Ministry of Health Sri Lanka, Sri Lanka
²Acting Consultant Community Physician, Visiting Research Fellow, The School of Public Health, The University of Queensland, Post Graduate Institute of Medicine University of Colombo, Ministry of Health Sri Lanka, Sri Lanka
³Professor in Community Medicine, University of Kelaniya, Sri Lanka
Received Date: 13/06/2023; Published Date: 03/11/2023
*Corresponding author: Dr. KCM Perera, Acting Consultant Community Physician, Visiting Research Fellow, The School of Public Health, University of Queensland, Australia Post Graduate Institute of Medicine University of Colombo, Ministry of Health Sri Lanka, Sri Lanka
Abstract
Introduction: Selenium (Se) may have a protective effect against some selected cancers. Ovarian cancer is ranked as one of the major killers of all gynecological malignancies worldwide. The objective of this study is to find the relationship between selenium intake and Epithelial Ovarian Cancer risk in women who have not had an oophorectomy.
Methods A comprehensive electronic search was carried out according to the prepared strategy from the starting date of the PubMed/Medline, EMBASE, Scopus, Proquest, and Web of Science databases up to 30th of September 2022 without limitations related to language and publication status. Studies were screened by COVIDENCE. Cohort studies, case-control studies, cross-sectional analytical studies, ecological studies, and randomized control studies were included, and descriptive studies were excluded from the systematic review. The exposure of interest is high selenium intake from either food sources or supplements and also high measures of selenium in blood, toenails, or other biological samples, and high measures of serum selenoproteins. Data extraction will be done. New Castle Ottawa Scale will be used to assess the bias of observational studies. The findings will be synthesized first via a narrative description. If data permits results will be displayed via forest plots. All analyses will be conducted using STATA-17.
Discussion: Ovarian cancer is the most fatal gynecological malignancy among women. Due to the lack of recommended screening tools, the identification of modifiable effective risk factors and preventive tools are essential to reduce ovarian cancer burden. Selenium is a powerful antioxidant; therefore, it prevents cell damage. It was proven in some studies that selenium protects against the development of some selected cancers. Therefore, it is envisaged to find whether there is an inverse relationship between selenium and ovarian cancer for future preventive strategies.
Systematic review registration: Registered in the International Prospective Register of Systematic Reviews (PROSPERO)- CRD42022356472
Keywords: Cancer, ovarian cancer, epithelial ovarian cancer, association, selenium, systematic reviews, meta-analysis
Abbreviations: Se: Selenium; ASR: Age- Standardized Rate EMBASE: Excerpta Medica data BASE EOC: Epithelial Ovarian Cancer; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-analysis PROSPERO: International Prospective Register of Systematic Reviews; RCT: Randomized Controlled Trials; STATA: Statistics and Data (USPSTF): United States Preventive Service Task Force
Background
Antioxidants are compounds in foods that prevent cell damage caused by free radicals. Free radicals are normal byproducts of processes like metabolism that are formed in the body daily. A balance between free radicals and antioxidants is necessary for proper physiological function [1]. However, things like smoking, alcohol use, and any type of change that causes physical, emotional, or psychological strain can cause excess free radicals. If free radicals overwhelm the body's ability to regulate them, a condition known as oxidative stress ensues, which damages healthy cells [2].
Selenium (Se) is a micronutrient and powerful antioxidant that fights against oxidative stress, therefore helps to prevent cell damage [3]. Selenium is an essential dietary component for animals including humans and is regarded as a protective agent against some selected cancers. A higher intake of selenium may reduce the risk of breast cancer, lung cancer, esophageal cancer, gastric cancer, and prostate cancer, but it was not associated with colorectal cancer, bladder cancer, and skin cancer [4].
According to the age-standardized incidence rate (ASR), ovarian cancer is ranked as the seventh most commonly diagnosed cancer among women in the world (6.1 per 100,000 women), and it is the sixth most common cause of cancer deaths in women [5]. More than 90% of ovarian cancers occurring in women aged over 40 years are epithelial cancers. Mainly there are 5 different histotypes of ovarian cancers; high-grade serous, low-grade serous, endometrioid, clear cell, and mucinous carcinoma [6]. Despite being classified as ovarian, a high proportion of high-grade serous cancers are now thought to originate from the fallopian tube [7]. During the process of ovulation and luteinization, reactive oxidants are produced in excess and surrounding cells are exposed to excessive reactive oxidants. Therefore, limiting oxidative stress to the ovarian tissues could be considered a first-line defense against ovarian cancer [2].
Several observational studies have assessed the relationship between Se and ovarian cancer risk. For epithelial ovarian cancer, an inverse association was observed for the highest compared to the lowest tertile of Se intake from food sources [1,2]. In addition, women with the highest intakes of supplemental Se (>20 mg/d) had a 30% lower risk of ovarian cancer than those with no supplemental intake [8]. In contrast, some other studies have concluded that there is no association between serum Se concentration and the incidence of ovarian cancer [9].
Upon searching the literature, there was no published systematic review and meta-analysis assessing the association between Se and EOC risk. Epidemiological studies have yielded inconsistent results on the association between Se and EOC risk. Therefore, we aimed to conduct a systematic review and meta-analysis to assess whether there is an inverse relationship between Se and EOC. Such a relationship would have implications for future preventive measures.
Review question:
What is the relationship between Se (food sources or supplements, measures of Se in blood, toenails, or other biological samples, and measures of serum selenoproteins) and EOC risk among women who have not had an oophorectomy?
Methodology
Systematic Review and Meta-analysis
Inclusion and Exclusion criteria:
- Inclusion criteria:
- Cohort studies that compared women with the lowest Se intake (food or supplements), the lowest level of serum, toenail, or other biological samples Se, and the lowest level of serum selenoprotein with the highest Se intake(food or supplements), the highest level of serum, toenail, or other biological samples Se, and the highest level of serum selenoprotein, and provided adjusted risk estimates (i.e. hazard ratios, or risk ratios with 95% confidence intervals [CIs]) or provide data allowing the calculation of the risk estimates and 95% CIs for the association between Se and
- Case-control studies that defined the control group as women without ovarian cancer and compared the Se intake (food or supplements), level of serum, toenail, or other biological samples Se, and level of serum selenoprotein of women with ovarian cancers, and provided adjusted risk estimates (i.e., odds ratio [OR] with 95%CIs) or provided data allowing the calculation of the OR and 95%CIs of the association between selenium and
- Randomized Control studies (RCTs).
- Ecological studies will be considered for inclusion in the systematic
- Cross-sectional analytical
- Studies published across all dates, times, and
- Studies published in other languages (translated to English by Google translator).
- Exclusion criteria:
i. Descriptive studies (i.e., case reports, case series, editorials, and opinion pieces).
2. Participants/population:
Women from the general population, are at risk of developing ovarian cancer.
3. Exposure:
The exposure of interest is high Se intake from either food sources or supplements. We will also consider high measures of Se in blood, toenails, or other biological samples and high measures of serum selenoproteins.
4. Control:
Women with a low Se intake from either food sources or supplements. We will also consider low measures of Se in blood, toenails, or other biological samples and low measures of serum selenoproteins.
5. Main outcome:
In this review, the primary outcome will be EOC. Where possible associations will also be explored by EOC histotype.
6. Measure:
Relative risk measures (relative risks, rate ratios, risk ratios, hazard ratios, odds ratios, and their 95%CIs) of the association between Se and ovarian cancer will be extracted from publications.
7. Additional outcome:
Not applicable.
8. Databases to be Searched and proposed search strategy
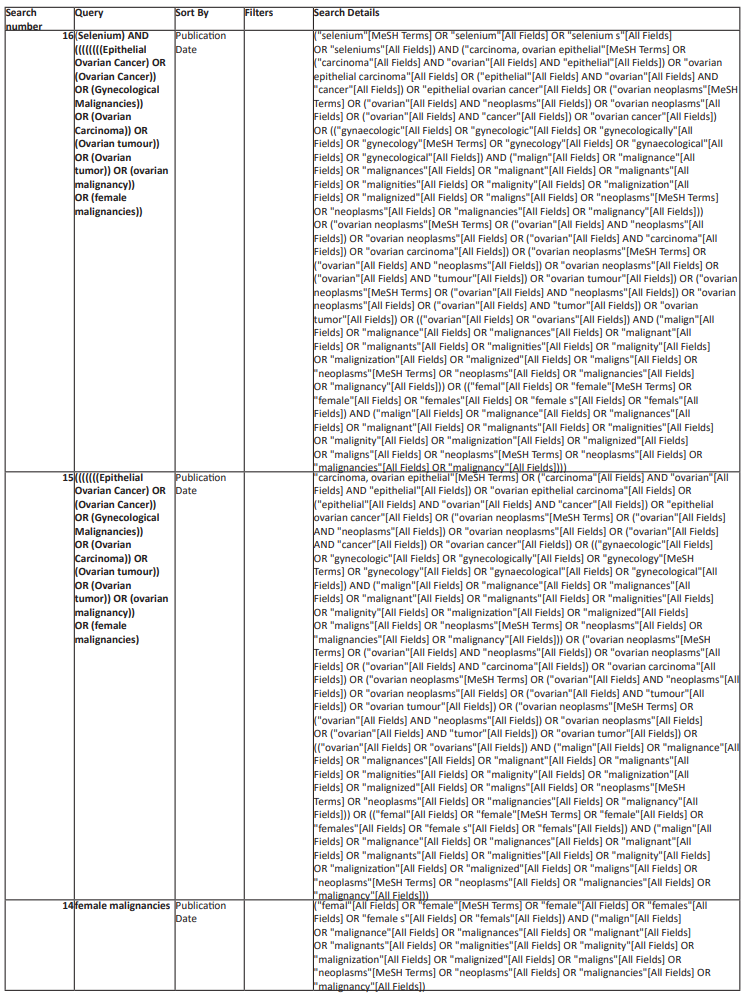
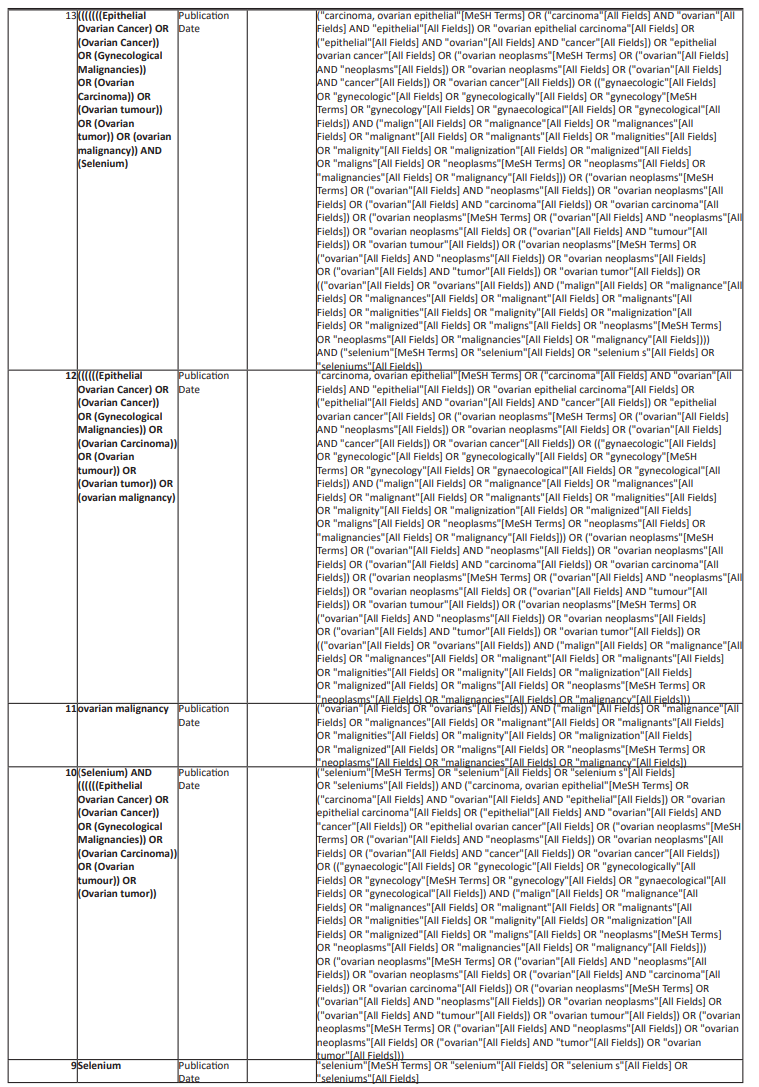
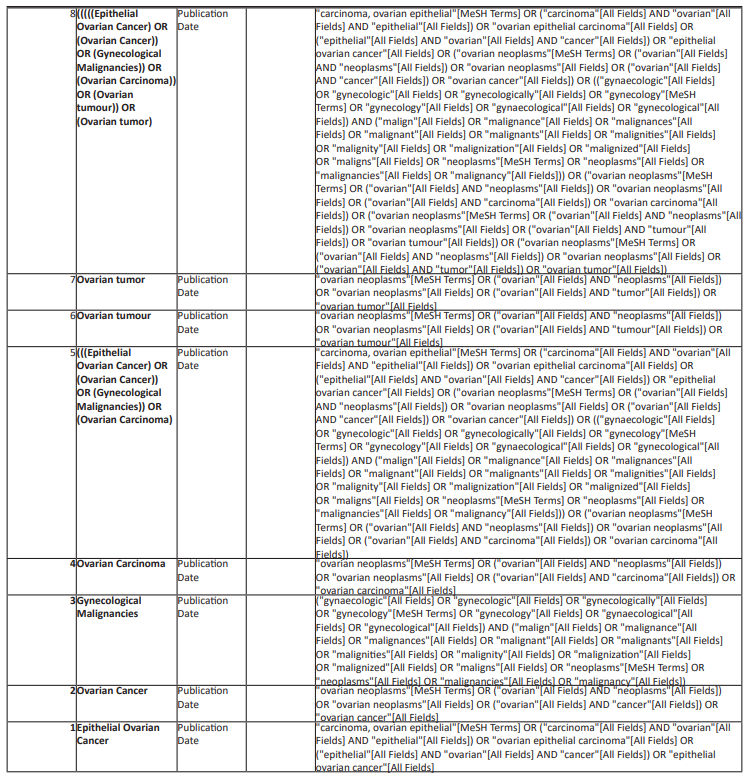
A comprehensive electronic search in Pubmed/Medline, EMBASE, Scopus, Proquest, and Web of Science from the starting date of the databases up to September 30th, 2022 will be undertaken without limitations related to the language and publication status. To prepare the complete search strategy a librarian was involved. Studies will be retrieved from the mentioned databases using the following search strategy and the complete search strategy was mentioned below in Table 1:
(Selenium) AND ((((((((Epithelial Ovarian Cancer) OR (Ovarian Cancer)) OR (Gynecological Malignancies)) OR (Ovarian Carcinoma)) OR (Ovarian tumour)) OR (Ovarian tumor)) OR (ovarian malignancy)) OR (female malignancies)) Sort by: Publication Date
Table 1: Complete search strategy for electronic data bases.
In addition, references to review articles, systematic reviews, meta-analyses, commentaries, editorials, meeting abstracts, and references of the included studies will be screened for relevant articles. Further, books related to gynecological malignancies and hand searches of journals will be done. And also search for grey literature such as conference abstracts/proceedings, published lists of thesis and dissertations, and other literature outside of the main journal literature, where possible will be done. Further, one needs to identify and include unpublished outcomes and studies by searching informal sources, including meeting abstracts and PhD theses and contacting authors of included studies.
9. Data Extraction
Two review authors (K. C. M. Perera and W.N.D. Perera) will independently screen studies using COVIDENCE software for systematic reviews under the University of Queensland multiple systematic review license according to the following procedure;
- Retrieve studies to the COVIDENCE software and remove duplicates
- Assess the title and abstract of all studies and remove irrelevant
- Assess the full text of all studies identified as possibly relevant
- Select studies for systematic review
Any disagreement between two review authors over the eligibility of particular studies will be resolved through discussion with a third reviewer. As per the PRISMA-P expanded checklist 2020, We will cite studies that might appear to meet the inclusion, but which were excluded, and explain why they were excluded.

Figure 1: Exporting and Interpreting PRISMA Flowchart.
Figure 1 shows Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) will be used to construct the flow chart (Figure 1) of the studies.
Two review authors (K. C. M. Perera and W.N.D. Perera) will independently retrieve the following general study information, where available, from all included studies: author, publication year, study design, study setting; study population, participant demographics, and baseline characteristics, details of the intervention and control conditions, recruitment and study completion rates, outcomes with risk estimates and 95%CIs, adjusted/matched factors for individual studies and times of outcome measurement, and information for the assessment of the risk of bias. Discrepancies will be identified and resolved through discussion (with a third author where necessary). Missing data will be requested from the study authors. PRISMA-P expanded checklist 2020 will be used in writing once the systematic review is completed.
10. Risk of bias (quality assessment)
Two review authors (K. C. M. Perera and W.N.D. Perera) will independently use the New Castle Ottawa scale of quality assessment for observational studies (i.e. selection bias, measurement bias, and confounding bias for each study). If there are any Random Control Trials (RCTs) bias will be assessed by the Cochrane Collaboration tool. Disagreements between the two review authors over the risk of bias in particular studies will be resolved by discussion, with the involvement of a third review author where necessary.
11. Synthesis of the findings
One review author (Dr. K. C. M. Perera) will abstract data into standard evidence tables, and the second review author (Dr. W. N. D. Perera) will check them for accuracy. The findings will be first synthesized via a narrative description. If possible, a quantitative synthesis will be undertaken using random effect meta-analysis for pooling data assuming that all of the studies are estimating the same underlying effect and variation between their results is due to chance. The results will be displayed via forest plots. All analyses will be conducted using STATA-17.
Heterogeneity between the studies in effect measures will be assessed using the I2 statistic. An I2 value greater than 50% is indicative of substantial heterogeneity and sensitivity analyses will be undertaken to assess the cause of heterogenicity. A subgroup analysis will be performed to examine any sources of significant heterogeneity according to the different types of studies (cohort, case-control, and cross-sectional analytical), study quality, and exposure (highest Se intake (food sources and supplements)) and controls (lowest selenium intake (food sources and supplements)). To assess the possibility of publication, bias a funnel plot will be drawn and an associated statistical test will be used.
12. Dissemination plan
The systematic review and meta-analysis will be written up for publication in a peer-reviewed journal.
Results
The total number of 652 studies from all 5 databases (Pubmed/Medline n=47, Embase n=222, Scopus n=72, Proquest n=4, and Web of Science n=307) were uploaded in the title and abstract screening to the COVIDENCE software and 146 total duplicates were removed. Four hundred and five studies were irrelevant in the title and abstract screening and selected 102 studies for the full-text screening. Seventy-three studies were excluded from the full-text screening and 29 studies were selected for the data extraction and bias assessment from the full-text screening by the COVIDENCE software.
Discussion
Ovarian cancer is associated with the highest mortality among all gynecologic cancers [2]. It has poor survival, due to late diagnosis at advanced stages, and treatment is unlikely to be curative. The symptoms of ovarian cancer are often non-specific and delays in diagnosis may, in part, explain why the disease has so often been diagnosed with metastasis [12].
Transvaginal ultrasound and serum CA-125 testing can be used effectively at present to evaluate symptomatic women for ovarian cancer but the United States Preventive Service Task Force (USPSTF) does not recommend routine screening for ovarian cancer using any method [13]. Due to the lack of recommended screening tools, the identification of modifiable risk factors and preventive tools is essential to reduce ovarian cancer mortality and morbidity [8].
Accumulating epidemiological and translational studies suggest that selenium protects against the development of various cancers including prostate, colon, esophagus, lung, and stomach [4]. Blood and serum levels of Se [14,15] and toenail levels of Se [16] are proxy measures of Se intake from food sources and supplements.
Epidemiological studies have yielded inconsistent results on the association between Se and EOC risk. Some studies have given the inverse relationship between Se from food sources and EOC [1,2], while, some others have given no association [9,17]. Some studies found an inverse relationship between Se from supplements [17,18] and EOC. In contrast some other studies were given no association [19,20]. There is no systematic review conducted so far on the association between Se and EOC risk. Therefore, it is versatile to assess the real association between
Se and EOC, which would give implications for ovarian cancer prevention.
Strengths and Limitations
The search strategy was created with the assistance of an expert librarian at the University of Queensland. To minimize the bias two independent reviewers will screen the title and abstracts of uploaded articles, will screen the full text of selected articles, will do the data extraction, will assess the risk of bias of included studies, and data synthesis and conflicts are resolved by the third review author are strengths of this study. No limitations related to the language and publication status.
Conclusion
It is envisaged to conduct a systematic review and meta-analysis to assess the real relationship between Se and EOC. If there is a true inverse relationship between Se and EOC, it would have implications for future preventive strategies for ovarian cancer.
Patient and public involvement: It was not appropriate or possible to involve patients or the public in the design, or conduct, or reporting, or dissemination plans of our research.
Ethical approval and consent to participate: The need for Ethical approval and consent was waived as the Systematic Review and Meta- analysis are based on ethically approved and conducted studies only.
Consent for publication: Not applicable
Availability of data and materials: The datasets used to analyze this systematic review and meta-analysis are available at the corresponding author upon reasonable request.
Competing interests: The authors declared that they have no known competing interests.
Funding: Not applicable.
Authors contributions: KCMP drafted the research protocol manuscript. KCMP and WNDP were assigned to screen studies and extract data. HTCSA was assigned as a third review author. HTCSA helped to draft the manuscript. All three authors read and approved the final protocol manuscript.
Acknowledgement: The guidance to prepare the search strategy for systematic review and meta-analysis was provided by Marcos Riba, a librarian at the University of Queensland. We are grateful to the School of Public Health at the University of Queensland, the Post Graduate Institute of Medicine, the University of Colombo and the Ministry of Health, Sri Lanka.
References
- Gifkins DM. Antioxidants and Cancer of The Endometrium and Ovary. A Dissertation submitted to the Graduate School-New Brunswick Rutgers, The State University of New Jersey,
- Gifkins D, Olson SH, Paddock L, King M, Demissie K, Shou-En Lu, et al. Total and individual antioxidant intake and risk of epithelial ovarian cancer. BMC Cancer,
- Guo Y, Lu Y, Jin H. Appraising the role of circulating concentrations of micro-nutrients in epithelial ovarian cancer risk: A Mendelian randomization analysis. Sci Rep, 2020; 10(1): 7356.
- Cai X, Wang C, Yu W, Fan W, Wang S, Shen N, et al. Selenium Exposure and Cancer Risk: an Updated Meta-analysis and Meta-regression. Sci Rep, 2016; 6: 19213.
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer, 2015; 136(5): E359-386.
- Peres LC, Cushing-Haugen KL, Anglesio M, Wicklund K, Bentley R, Berchuck A, et al. Histotype classification of ovarian carcinoma: A comparison of approaches. Gynecol Oncol, 2018; 151(1): 53-60.
- Jordan SJ, Nelson AE, Zorbas HM, Luxford KA, Webb PM, Group ACSOCaAOCS. Pathways to the diagnosis of epithelial ovarian cancer in Australia,
- Webb PM, Jordan SJ. Epidemiology of epithelial ovarian cancer. Best Pract Res Clin Obstet Gynaecol, 2017; 41: 3-14.
- Terry PD, Qin B, Camacho F, Moorman PG, Anthony JA, Barnholtz-Sloan JS, et al. Supplemental Selenium May Decrease Ovarian Cancer Risk in African-American Women. The Journal of Nutrition Nutritional Epidemiology,
- Fleischauer AT, Olson SH, Mignone L, Simonsen N, Caputo TA, Harlap S. Dietary antioxidants, supplements, and risk of epithelial ovarian cancer. Nutr Cancer, 2001; 40(2): 92-98.
- Ko-Hui Tung LRW, Anna H Wu, Katharine McDuffie, Jean H, Abraham MY, Nomura LNK, et al. Association of Dietary Vitamin A, Carotenoids, and Other Antioxidants with the Risk of Ovarian Cancer. Cancer Epidemiol Biomarkers Prev, 2005; 14.
- Knekt P, Aromaa A, Alfthan G, Maatela J, Hakama M, Hakulinen T, et al. Prospective Study of Serum Micronutrients and Ovarian Cancer. Journal of the National Cancer Institute,
- Jori S Carter LSDJ. Ovarian Cancer Tests and Treatment,
- Helzlsouer KJ AA, Norkus EP, Morris JS, Hoffman SC, Comstock GW. Prospective Study of Serum Micronutrients and Ovarian Cancer. Journal of the National Cancer Institute,
- Lubinski L, Marciniac W, Derkacz R, Baszuk P, Muszyńska M, Kuświk M, et al. Read-Gene SA -DP. Increased Risk of Cancers Among BRCA1 Carriers with Low Blood Selenium Levels,
- Garland M, Morris JS, Stampfer MJ CG, Spate VL, Baskett CK, Rosner B, et al. Prospective Study of Toenail Selenium Levels and Cancer Among Women,
- Terry PD, Qin B, Camacho F, Moorman PG, Alberg AJ, Barnholtz-Sloan JS, et al. Supplemental Selenium May Decrease Ovarian Cancer Risk in African-American Women. The Journal of Nutrition, 2017; 147(4): 621-627.
- Huzarski T, TB, JG, EbK, SaZc, BG, et al. A Lowering of Breast and Ovarian Cancer Risk in Women with a BRCA1 Mutation by Selenium Supplementation of Diet Hereditary Cancer in Clinical Practice, 2006.
- Fleischauer AT, Olson SH, Mignone L, Simonsen N, Caputo TA, Harlap S. Dietary antioxidants, supplements, and risk of epithelial ovarian cancer. Nutr Cancer, 2001; 40(2): 92- 9
- Thomson CA, Neuhouser ML, Shikany JM, Caan BJ, Monk BJ, Mossavar-Rahmani Y, et al. The role of antioxidants and vitamin A in ovarian cancer: results from the Women's Health Initiative. Nutr Cancer, 2008; 60(6): 710-719.

