Oxidative Status of Diabetic Rat Kidneys Administered Ethanol Extract of Cucumis sativus Whole Fruit
Abu OD*, Avenbuan SE and Osarhenomase EG
Department of Biochemistry, Faculty of Life Sciences, University of Benin, Nigeria
Received Date: 24/05/2023; Published Date: 15/09/2023
*Corresponding author: Osahon Abu, Department of Biochemistry, Faculty of Life Sciences, University of Benin, Benin City, Nigeria
Abstract
Diabetes Mellitus (DM) remains a serious health challenge, globally. Strategies currently employed for its treatment are targeted at ameliorating the different metabolic derangement associated with the disease. The aim of the present study was to evaluate oxidative status of diabetic rat kidneys administered ethanol extract of Cucumis sativus whole fruit. Male Wistar rats (n = 25, mean weight = 215 ± 15 g) were randomly assigned to five groups of 5 rats each: normal control, diabetic control, metformin, and 200 mg/kg body weight (bwt) and 300 mg/kg bwt extract groups. Diabetes mellitus was induced via intraperitoneal injection of Streptozotocin (STZ) at a dose of 50 mg/kg bwt. The diabetic rats were then treated with metformin (50 mg/kg bwt) or the extract (200 and 300 mg/kg bwt, respectively). Treatment lasted 21 days. Indices of oxidative stress were measured in rat kidney homogenate (20 %). The results showed that the activities of catalase, Superoxide Dismutase (SOD), Glutathione Peroxidase (GPx), and Glutathione Reductase (GR) as well as concentrations of Glutathione (GSH) and % GSH were significantly lower in diabetic control group, but they were increased by extract treatment (p < 0.05). However, the concentrations of Nitric Oxide (NO) and Malondialdehyde (MDA) elevated by STZ were greatly reduced after treatment with C. sativus fruit extract (p < 0.05). These results indicate that ethanol extract of the medicinal plant fruit has the capacity to potentiate the antioxidant defense system in rats exposed to the diabetogenic agent, STZ.
Keywords: Cucumis sativus; Catalase; Glutathione; Nitric oxide; Oxidative stress
Introduction
Diabetes mellitus is a heterogeneous group of syndromes characterized by an elevation of fasting blood glucose caused by a relative or absolute deficiency in insulin [1]. It is one of the oldest diseases of man, and the most common metabolic disorder that affects millions of people all over the world [2]. Nephropathy (damage to kidney leading to renal failure) is a microvascular complication of the disease [3].
Oxidative stress plays a role in insulin resistance. Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS) affect insulin signaling cascade [4]. Pancreatic β-cells are especially sensitive to ROS and RNS, because their natural enzymatic antioxidant defenses are lower compared to other tissues such as liver. Moreover, they lack the ability to adapt their low enzyme activity in response to stress such as high glucose or high oxygen [5]. Besides the provision of energy, glucose sensing in pancreatic β-cell is crucial for insulin secretion. It has been suggested that hyperglycemia results in chronic oxidative stress via glucose oxidation pathway, leading to an excess in mitochondrial superoxide production, which further activates uncoupling protein-2 (UCP-2) [6]. This protein lowers ATP/ADP through proton leak in β-cell, which reduces insulin secretion [7]. As in other cell types, NO in β-cells has physiological roles. It may regulate glucokinase activity via S-nitrosilation in β-cell, and possibly increase insulin secretion [8]. However, excess NO and concomitant NRS may cause apoptosis through caspase-3 activation and decrease in ATP levels [9]. The aim of the present study was to evaluate oxidative status of diabetic rat kidneys administered ethanol extract of C. sativus whole fruit.

Figure 1: Anatomy of the Kidney.
Materials and Methods
Chemicals
Chemicals, reagents and solvents used in this study were of analytical grade, and they were products of Sigma-Aldrich, Ltd. (USA).
Collection of Plant Material
Whole fruits of C. sativus were bought from a reputable supplier in Benin City, Nigeria. The plant was identified and authenticated at the University of Benin herbarium domiciled in the Department of Plant Biology and Biotechnology. The prepared plant specimen was deposited in the herbarium after obtaining the voucher number (No. UBHD330).
Plant Preparation and Extraction
The fruits were washed and shade-dried for 2 weeks at room temperature, and thereafter ground into powder using an electric blender. A portion (500 g) of powdered plant material was steeped in 5 L of absolute ethanol. The resultant extract was filtered through muslin cloth and freeze-dried with a lyophilizer [10].
Experimental Rats
Wistar rats (n = 25) weighing between 160 and 180 g were purchased from the Department of Anatomy, School of Basic Medical Sciences, University of Benin, Benin City, Nigeria. The rats were kept in metal cages under standard laboratory settings. They had unrestricted access to feed (pelletized mash) and potable drinking water. Seven days were used to acclimate the rats to laboratory conditions prior to the commencement of the study. The investigation followed a standard experimental protocol.
Experimental Design
The rats were randomly assigned to five groups of 5 rats each: normal control, diabetic control, metformin, and 200 mg/kg bwt and 300 mg/kg bwt extract groups. Diabetes mellitus was induced in the rats via intraperitoneal injection of STZ (50 mg/kg bwt). The diabetic rats were subsequently treated for 21 days with metformin (50 mg/kg bwt) or the extract (200 and 300 mg/kg bwt, respectively), leaving the diabetic control group untreated.
Tissue Sample Collection
At the end of the 21-day treatment, the rats were euthanized under mild anesthesia. Their kidneys were excised, washed in ice–cold saline, blotted dry and placed in plain containers. Weighted portions of the kidney were used to prepare 20 % tissue homogenate used for biochemical analyses.
Biochemical Analyses
The activities of catalase, SOD, GPx and GR were determined [11-14]. Concentrations of renal total protein, MDA and GSH were also measured [15-17]. Nitric oxide (NO) concentration was determined using a previously described method [18].
Statistical Analysis
Data are expressed as mean ± SEM (n = 5). Statistical analysis was performed using SPSS version 21. Statistical differences between means were compared using Duncan multiple range test. Values of p < 0.05 were considered statistically significant.
Results
Effect of Ethanol Extract of C. sativus Fruit on Kidney Weight
Induction of diabetes mellitus with STZ significantly reduced the weights of rat kidney (p < 0.05). However, treatment of the diabetic rats with extract of C. sativus markedly increased the weights of the organ as well as the corresponding kidney/body weight ratio (p < 0.05; Figure 2).

Figure 2: Comparison of the Weights of Rat Kidney Among the Groups.
Data are weights of rat kidneys and are expressed as mean ± SEM (n = 5).
N. Control = normal control; and D. Control = diabetic control
Effect of Ethanol Extract of C. sativus Fruit on Oxidative Status of Diabetic Rat Kidneys
The activities of all the antioxidant enzymes measured and concentrations of GSH were significantly lower in diabetic control group than in the normal control group, but they were increased by extract treatment (p < 0.05). However, the concentrations of NO and MDA elevated by STZ were greatly reduced after treatment with the medicinal plant extract (p < 0.05; Figures 2 to 5).
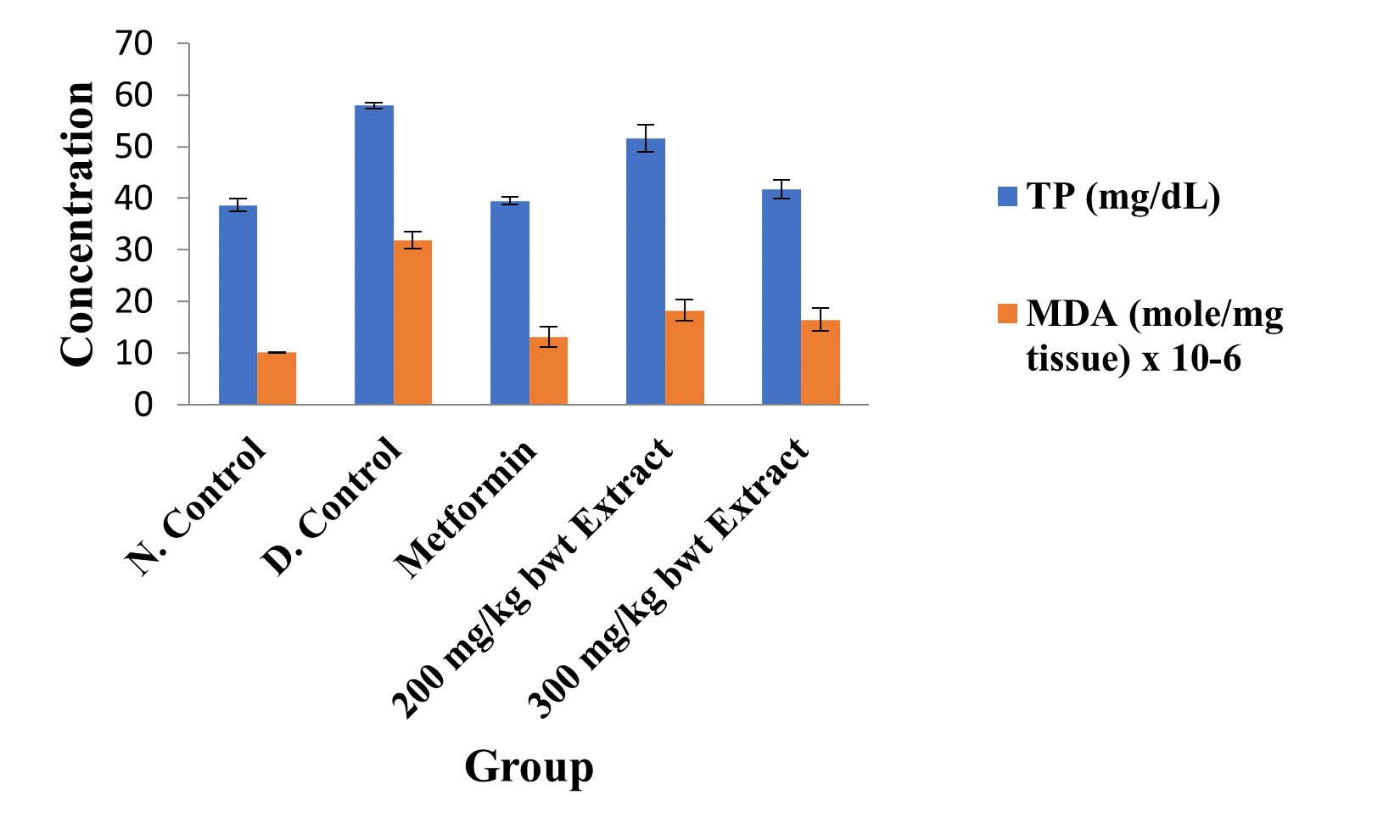
Figure 3: Concentrations of Kidney Total Protein and Lipid Peroxidation Index in the Different Groups.
Data are kidney total protein and MDA levels and are expressed as mean ± standard error of mean (SEM, n = 5).
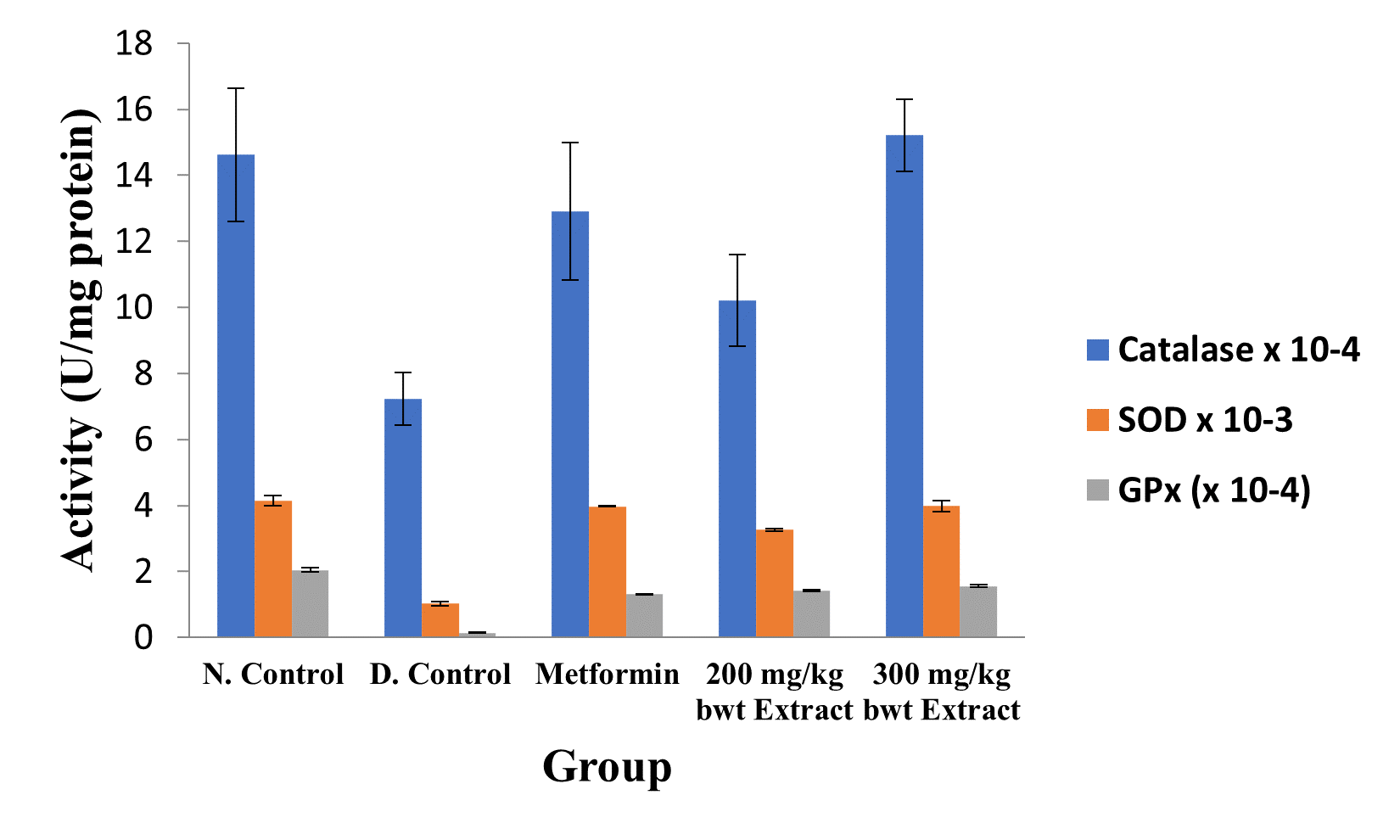
Figure 4: Activities of Antioxidant Enzymes in the Different Groups.
Data are markers of oxidative stress and are expressed as mean ± SEM (n = 5).
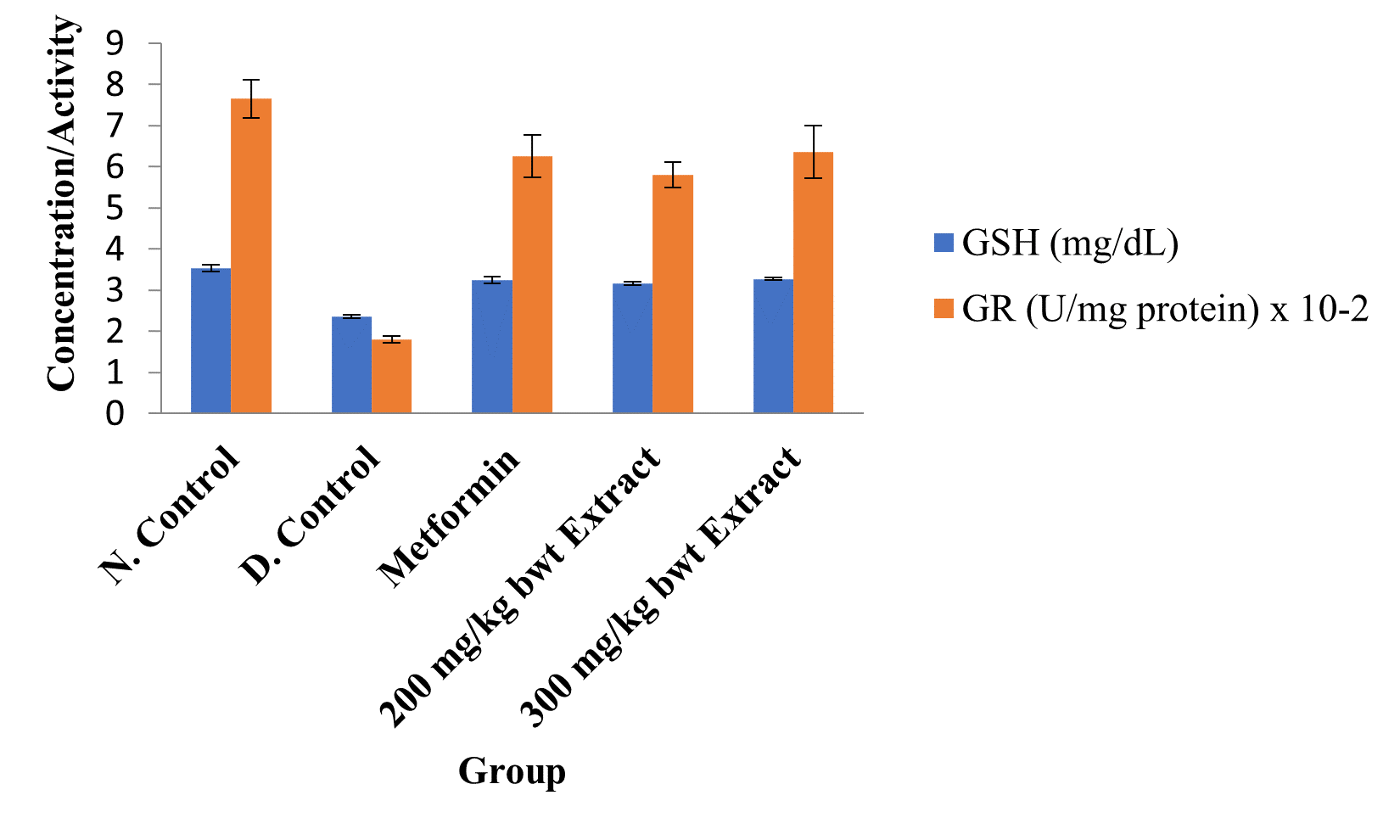
Figure 5: Activities of Glutathione Reductase and Concentrations of GSH in the Different Groups.
Data are markers of oxidative stress and are expressed as mean ± SEM (n = 5).
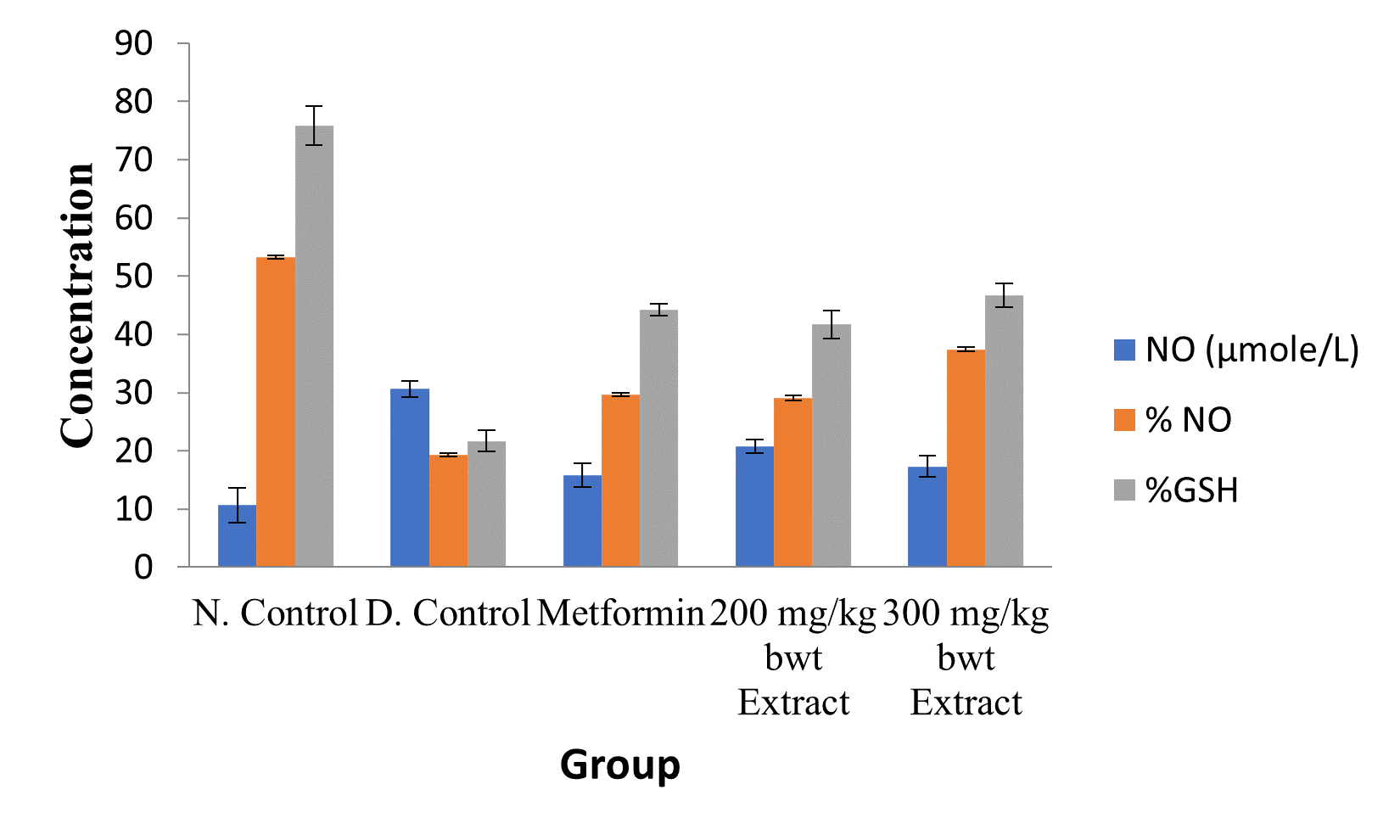
Figure 6: Effect of Ethanol Extract of C. sativus Fruit on Renal NO and GSH Level.
Data are markers of oxidative stress and are expressed as mean ± SEM (n = 5).
Discussion
Increasing evidence suggest that oxidative stress plays a role in the pathogenesis of diabetes mellitus and its complications [7]. One of the proposed mechanisms of STZ-induced toxicity is induction of oxidative stress. Hyperglycemia increases oxidative stress, which contributes to impairment of the main processes that fail during the disease (insulin action and secretion). In addition, antioxidant mechanisms are diminished in diabetic patients, which may further promote oxidative stress [4,19]. Hyperglycemia and free fatty acids are among the causes of oxidative stress [20].
Free radicals generated within an organism are readily removed by natural antioxidant defenses such as glutathione or catalase [21]. Natural antioxidants play a key role in health maintenance and prevention of chronic and degenerative diseases. Plants contain a wide variety of free radicals scavenging molecules such as phenols, flavonoids, vitamins and terpenoids [22]. Antioxidants protect cells against the damaging effects of Reactive Oxygen Species (ROS) such as singlet oxygen, superoxide anion (O2-), and peroxyl and hydroxyl radicals and peroxynitrite which results in oxidative stress leading to cellular damage [23]. Many plants, citrus fruits and leafy vegetables are the source of these antioxidant molecules.
Antioxidants are the agents that can interfere with oxidation process by various mechanisms, such as, reacting with free radicals, chelating free catalytic metals, and acting as oxygen scavengers [24]. Free radicals, with unpaired electrons, are produced in normal or pathological cell metabolism. Lipid peroxidation in cell membrane causes several types of biological damage. Interest in natural antioxidants, especially phytochemicals has greatly increased in recent years [25-27]. Many phytochemicals including phenolics, flavonoids, tannins, proanthocyanidins, and various plant extracts have been reported as antioxidants [28-30]. This study evaluated oxidative status of diabetic rat kidneys administered ethanol extract of C. sativus fruit. The results showed that the activities of catalase, SOD, GPx, and GR as well as concentrations of GSH and % GSH were significantly lower in diabetic control group, but they were increased by extract treatment. However, the concentrations of NO and MDA elevated by STZ were greatly reduced after treatment with C. sativus extract. These results are in agreement with reports of previous studies [27,31-36]. It has been demonstrated that different parts of C. sativus contain bioactive compounds responsible for particular pharmacological activity [37].
Conclusion
The results obtained in this study indicate that ethanol extract of the medicinal plant fruit has the capacity to potentiate the antioxidant defense system in rats exposed to the diabetogenic agent, STZ.
References
- Centers for Disease Control and Prevention. Diabetes Report Card. Atlanta, GA: Centers for Disease Control and Prevention, U.S Department of Health and Human Services, 2012.
- Onoagbe IO, Esekheigbe A. Studies on the anti-diabetic properties of Uvaria Chamae in streptozotocin-induced diabetic rabbits. Biokemistri, 1999; 9: 79-84.
- World Health Organisation. The World Health Report 2006: working together for health. Humans; Professional Competence, 2006.
- Rains JL,Jain SK. Oxidative stress, insulin signaling, and diabetes. Free Radic Biol Med, 2011; 50(5): 567-575.
- Tiedge M, Lortz S, Drinkgern J, Lenzen S. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes, 1997; 46(11): 1733–1742.
- Robertson RP, Harmon J, Tran PO, Tanaka Y, Takahashi H. Glucose toxicity in beta-cells: type 2 diabetes, good radicals gone bad, and the glutathione connection. Diabetes, 2003; 52(3): 581-587.
- Brownlee M. A radical explanation for glucose-induced beta cell dysfunction. J. Clin Invest, 2003; 112 (12): 1788-1790.
- Rizzo MA, Piston DW. Regulation of beta cell glucokinase by S-nitrosylation and association with nitric oxide synthase. J Cell Biol, 2003; 161(2): 243-248.
- Tejedo J, Bernabe JC, Ramirez R, Sobrino F, Bedoya FJ. NO induces a cGMP-independent release of cytochrome c from mitochondria which precedes caspase 3 activation in insulin producing RINm5F cells. FEBS Lett, 1999; 459(2): 238-243.
- Abu OD, Imafidon KE, Obayuwana HO, Okuofu ED. Phytochemical, proximate, and metal content analysis of citrullus lanatus (watermelon) seeds. FUDMA Journal of Sciences, 2017; 2(2): 153-156.
- Cohen G, Dembie CD, Marcus J. Measurement of catalase activity in tissue extracts. Analytic Biochemistry, 1970; 34: 30-38.
- Misra HR, Fridovich I. The role of superoxide anions in the auto oxidation of epinephrine and a single assay for superoxide dismutase. J Biol. Chem, 1972; 247: 3170-3175.
- Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hockstra WG. Selenium biochemical role as a component of glutathione peroxidase. Science, 1973; 179: 588-590.
- Abu OD, Ikponmwosa-Eweka O. Evaluation of the Potential of Total saponins and Tannins of Dialium guineense Stem Bark in the Amelioration of Carbon Tetrachloride-Induced Renal Oxidative Stress. SAU Science-Tech. Journal, 2022; 7(1): 42-50.
- Henry RJ, Sobel C, Beckman S. Determination of serum protein by the Biuret reaction. Anal. Chem, 1957; 92(149): 1-5.
- Ellman GL. Tissue sulphydryl groups. Archive of Biochemistry and Biophysics, 1959; 82(1): 70–77.
- Guttridge JMC, Wilkins C. Cancer dependent hydroxyl radical damage to ascorbic acid. Formation of thiobarbituric acid reactive product. FEBS Lett, 1982; 137: 327-340.
- Marcocci L, Packer L, Droy-Lefaix MT, Sekaki A, Gardes-Albert M. Antioxidant action of Ginkgo biloba extract EGb 761. Methods in Enzymology, 1994; 234: 462–475.
- Maritim AC, Sanders RA.Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol,2003; 17(1): 24-38.
- Evans JC, Huddler DP, Jiracek J, Castro C, Millian NS, Garrow TA, et al.Betaine-homocysteine methyltransferase: zinc in a distorted barrel. Structure, 2022; 10(9): 1159–1171.
- Hurst R, Bao Y, Jemth P, Mannervik B, Williamson G. Phospholipid hydroperoxide glutathione peroxidase activity of rat class Theta glutathione transferase T2-2. Biochem. Soc. Trans, 1997; 25: 559.
- Sun B, Ricardo-da-Silva JM, Spranger I. Critical factors of vanillin assay for catechins and proanthocyanidins. Journal of Agriculture and Food Chemistry, 1998; 46: 4267-4274.
- Jornot L, Petersen H, Junod AF. Hydrogen peroxide-induced DNA damage is independent of nuclear calcium but dependent on redox-active ions. Biochem. J, 1998; 335: 85-94.
- Mills EM, Takeda K, Yu ZX. Nerve growth factor treatment prevents the increase in superoxide Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Food and Cosmetics Toxicology, 1998; 71: 12-13.
- Abu OD, Okuo AV, Osemwota OF. Extracts of Dialium guineense Stem Bark Ameliorates CCl4-induced Oxidative Stress in Liver of Wistar Rats. Biomedical Journal of Scientific and Technical Research, 2022a; 46(2): 37297–37301.
- Abu OD, Iyare HE, Ogboi KU. Cardiac Oxidative Status in CCl4-Exposed Rats Treated with Extracts of Dialium guineense Stem Bark. Global Journal of Scientific Frontier Research, 2022b; 22(01): 1–6.
- Abu OD, Iyare HE, Ogboi KU. Antioxidant Property of Total Saponins and Tannins of Dialium guineense Stem Bark in Rats Hearts Exposed to CCl4. Journal of Clinical Epidemiology and Toxicology, 2022c; 3(3): 1–4.
- Abu OD, Onoagbe IO, Obahiagbon O. In Vitro Antioxidant Activities of Extracts of Dialium Guineense Stem Bark. American Journal of Sciences and Engineering Research, 2020a; 3(4): 68–75.
- Abu OD, Onoagbe IO, Obahiagbon O. Phenolic contents of extracts of Dialium guineense stem bark. American Journal of Sciences and Engineering Research, 2020b; 3(4): 92–96.
- Abu OD, Onoagbe IO, Obahiagbon O. Qualitative phytochemical screening and proximate analysis of Dialium guineense stem bark. IAR Journal of Agriculture Research and Life Sciences, 2020c; 1(4): 108–112.
- Abu OD, Ezike TV, Ajuwa OI. Cardioprotective property of extracts of Dialium guineense stem bark in rats exposed to CCl4. American Journal of Biomedical Science and Research, 2022d; 2022: 689–693.
- Abu OD, Umar A-B, Eiremiokhae CO. Investigation of the Cardioprotective Capacity of queous Extract of Icacina trichanta Leaves in Rats Exposed to CCl4. Journal of Genetics and Cell Biology, 2022e; 6(1): 322–328.
- Abu OD, Onoagbe IO, Ekugum E. Hepatotoxicity of Graded Doses of Ethanol Extract of Dialium guineense Stem Bark in Wistar Rats. Journal of Pharmaceutical and Bio-Medical Sciences, 2022f; 2(9): 347-352.
- Abu OD, Onoagbe IO, Ohikhuare F. Nephrotoxic Evaluation of Ethanol Stem Bark Extract of Dialium guineense in Normal Wistar Rats. International Journal of Forensic Medicine, 2022g; 4(2): 19–22.
- Abu OD, Umar A-B, Adekanle E. Cardiotoxic Effect of Aqueous Extract of Dialium guineense Stem Bark in Wistar Rats. East African Scholars Journal of Agriculture and Life Sciences, 2022h; 5(9): 167–172.
- Abu OD, Okuo AV, Ayele PE. Pancreatotoxic Effect of Aqueous Extract of Dialium guineense Stem Bark in Wistar Rats. International Journal of Novel Research in Life Sciences, 2022i; 9(5): 31–37.
- Patil MVK, Kandhare AD, Bhise SD. Effect of aqueous extract of Cucumis sativus Linn. fruit in ulcerative colitis in laboratory animals. Asian Pacific Journal of Tropical Biomedicine, 2012; 2(2): S962-S969.

