Cutting the Gordian Knot of Diuretic Resistance in Heart Failure; Combination of Continuous Ultrafiltration with Diuretic Therapy
Yannis Dimitroglou, Emmanouil Mantzouranis, Christina Chrysohoou*, Styliani Brili and Constantinos Tsioufis
First Department of Cardiology, Hippokration General Hospital, National and Kapodistrian University, Athens, Greece
Received Date: 15/05/2023; Published Date: 06/09/2023
*Corresponding author: Christina Chrysohoou, First Department of Cardiology, Hippokration General Hospital, National and Kapodistrian University, 114 Vasilissis Sophias avenue, Athens, Greece
Abstract
Background: Continuous ultrafiltration has emerged as a decongestion method for patients with refractory decompensated heart failure with diuretic resistance as it enables the energetic withdrawal of isotonic fluid under controlled rate according to the patient’s vital signs, offering decongestion without exceeding plasma refill rate.
Methods: A 62-year-old male with history of HOLT-ORAM syndrome with Eisemenger physiology presented with worsening dyspnea. Patient initial clinical and laboratory examination, renal vein ultrasound and echocardiogram were consistent with significant congestion. A combined strategy of intravenous furosemide with early initiation of continuous ultrafiltration at an adjustable rate for four days was finally selected.
Results: Patient remained hemodynamically stable during the total treatment time and exhibited significant clinical and laboratory improvement. Consecutive renal vein ultrasounds and echocardiograms demonstrated a continuous and steady recession of congestion. During the 4 days of ultrafiltration total fluid loss was estimated at 42 liters. Patient remained asymptomatic without signs of worsened congestion at 1, 3 and 5-months follow-up.
Conclusion: Our case depicts that continuous ultrafiltration without exceeding plasma refill rate allows an impaired right ventricle to maintain significant preload. This suggests that it might be considered for patients in whom a session of short classic ultrafiltration might have detrimental results regarding cardiac output.
Keywords: Continuous ultrafiltration; Heart failure; Congestion; Cardiorenal
Introduction
Congestion has a central role in patients with decompensated Heart Failure (HF) as it has been related to increased morbidity and higher readmission rates, while on the other hand leads to the vicious cycle of diuretic resistance [1]. Ultrafiltration, the energetic withdrawal of isotonic fluid from the venous system, presents as an alternative method when the intravenous diuretics and combination of diuretics strategies fail [2]. However, in preload dependent situations this method might exert detrimental hemodynamic consequences. In such cases, continuous ultrafiltration has emerged as a decongestion method for patients with refractory decompensated heart failure. This method enables the energetic withdrawal of isotonic fluid from the venous system under a controlled rate according to the patient’s vital signs and thus offering decongestion without exceeding plasma refill rate [3]. Here we present our experience of the application of continuous ultrafiltration in a patient with known history of Holt-Oram syndrome and Eisenmenger physiology presenting with decompensated heart failure.
Materials and Methods
Present complaint and history
A 62-year-old male with known history of frequent hospitalizations due to HF decompensation, presented at the emergency department with worsening dyspnea at mild exertion, orthopnea and peripheral oedema; while he had gained 20 kg during last two months. Patient had a prior medical history of HOLT-ORAM syndrome, type 2 diabetes mellitus, atrial flutter and chronic obstructive pulmonary disease under oxygen therapy. HOLT-ORAM was diagnosed at the age of 58 when patient sought medical attention because of worsening dyspnea and fatigue at mild exertion. Clinical suspicion was raised due to malformation of extremities, low height and the presence of an Atrial Septal Defect (ASD) at the echocardiogram. At that time, he had already developed Eisenmenger physiology, right heart failure and severe pulmonary hypertension. Regarding his medication he reported taking daily oral furosemide 80mg, eplerenone 50mg, dapagliflozin 10mg, dabigatran 300mg, valsartan 160mg, metformin 1000mg, macitentan 10mg and digoxin 0.25mg three times per week. He did not report use of tobacco, alcohol or any illicit drug.
Initial assessment and workup
Initial physical assessment showed blood pressure 110/75 mmHg, heart rate of 80 bpm and oxygen saturation of 75% on ambient air. Patient clinical examination revealed prominent signs of right heart failure such as dilated jugular veins, ascites and oedema anasarca. Heart auscultation revealed a split S2 and a systolic ejection murmur whereas lung auscultation revealed diffuse wheezing and basal coarse crackles.
Chest radiograph showed an enlarged cardiac silhouette with signs of congestion and pulmonary hypertension. 12-lead electrocardiogram showed atrial flutter with satisfactory rate control and RBBB. Initial renal vein ultrasound demonstrated a monophasic pattern of renal vein flow with a single flow phase in diastole. Baseline echocardiogram showed left ventricle with normal dimensions and wall thickness and preserved ejection fraction, with systolic and diastolic D-shape movement of intraventricular septum; whereas right cavities were notably enlarged with a systolic tissue doppler velocity of right ventricle equal of 5cm/s. Doppler study revealed Atrial Septal Defect (ASD) with bidirectional flow. Inferior vena cava (IVC) was dilated without respiratory variation and systolic pulmonary pressure (PASP) was estimated at 95 to 100 mmHg (Figure 1A, C, E).
Decision making
Due to the prominent congestion, as well as the decreased urine volume and urine sodium with intravenous furosemide, a combined strategy of maintaining intravenous furosemide with a parallel initiation of continuous ultrafiltration was selected. Intravenous furosemide daily dosage remained at 160mg during the total treatment time. Initial ultrafiltration rate was at 200ml/hr with a gradual increase up to 250ml/hr for the first 48-hour ultrafiltration session. Ultrafiltration rate at the second session gradually decreased from 250ml/hr to 180ml/hr because of increase in hemoglobin. Patient remained hemodynamically stable during the total treatment time and exhibited significant clinical improvement regarding NYHA class, oedema, respiratory function and oxygen saturation. Renal function, after the initial expected deterioration, showed a steady improvement, whereas patient, surprisingly, did not exhibit any electrolyte abnormalities despite the continuous usage of furosemide and the high daily urine output.

Figure 1: Baseline echocardiography depicting bidirectional shunt through the ASD due to increased diastolic pressure in the right atrium (A). The shunt was left to right after the decongestion therapy leading to an increase of the arterial blood oxygen saturation (B). IVC diameter and respiratory variation was improved due to the decrease of right atrial pressure (C, D). Renal venous pressure decrease was documented with Doppler ultrasound as the pattern was improved from monophasic before the ultrafiltration therapy, to biphasic after the first ultrafiltration session (E) and finally continuous before discharge (F).
Materials and Methods
Outcome
Consecutive x-rays, renal vein ultrasounds and echocardiograms demonstrated a continuous and steady recession of congestion (Figure 1). His mean daily urine output was 3 liters, whereas during the 4 days of ultrafiltration the total ultrafiltrate was 15 liters. Total fluid loss was estimated at 42 liters (27 liters of urine and 15 liters of ultrafiltrate). Treatment results were reflected in total body weight considering that patient presented with 110kg and after treatment weighted 86kg.
He was dismissed asymptomatic, with minor ankle edema, blood pressure of 120/80 mmHg, oxygen saturation of 90% on ambient air. His main lab and hemodynamic parameters at admission and exit are depicted at table 1 whereas figure 2 highlights the progression of specific clinical and laboratory parameters of interest.
A follow up echocardiogram at day 7 showed mainly left to right flow and less right to left compared to the baseline study. Inferior vena cava remained dilated without respiratory variation. Pulmonary valve flow showed premature acceleration time. Tricuspid valve exhibited medium to severe regurgitation and PASP was estimated at 95 to 100 mmHg. Renal vein ultrasound revealed a biphasic pattern with a tendency to the continuous form (Figure 1B, D, F).
Patient was dismissed with the following prescribed regime (daily dosage): pantoprazole 40mg, furosemide 125mg, torsemide 60mg, eplerenone 50mg, dapagliflozin 10mg, dabigatran 300mg, valsartan 160mg, allopurinol 100mg and digoxin 0.25mg three times a week.
Follow up
The patient remained asymptomatic during the 6 months of follow-up, showing substantial improvement in his functional capacity, leading to remarkable reduction in torsemide dose to 20 mg per day. His laboratory parameters at the follow up visits are presented in Figure 2.
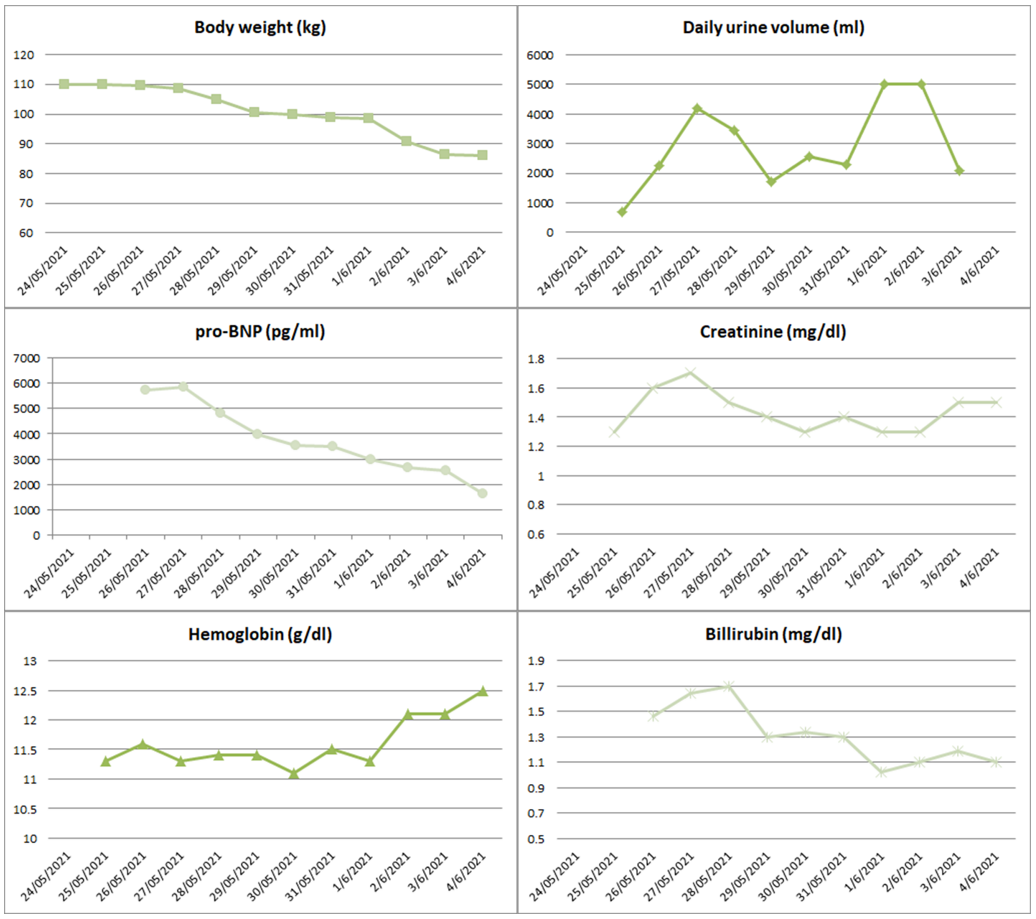
Figure 2: Graphs show the progression of various clinical and laboratory variables during the hospitalization.
Discussion
The main proposed mechanism of diuretic resistance in the context of decompensated HF is the increased renal vein pressure which decreases arteriovenous pressure gradient, with a parallel increase in the interstitial pressure within the kidney, thereby decreasing renal blood flow, ultrafiltration pressure and GFR [4]. Congestion also worsens diuretic resistance by decreasing intestinal absorption, and because of a stimulation of the SNS and RAAS systems. Moreover, worsened liver function in patients with right heart failure and congestion leads to hypoalbuminemia which can increase resistance to furosemide the most commonly used diuretic [5].
In patients without significant diuretic resistance, increased doses of diuretics may temporarily increase urine output succeeding in resolution of congestion. However, neurohormonal mechanisms involving continued activation of the SNS and RAS can maintain diuretic resistance at a high level so that a sustainable equilibrium cannot be achieved in out-of-hospital settings. Intravenous loop diuretics at high doses and combination of different diuretics often aid in overcoming the obstacle of resistance [5]. In case of low cardiac output and low blood pressure the use of inotropes and vasospastic drugs, often in combination, should be also considered. Ultrafiltration comes as the last resort but its use is limited due the potential further hemodynamic instability. When ultrafiltration rate significantly exceeds the plasma refill rate, there is intravascular volume depletion which in preload dependent HF patients can decrease cardiac output with detrimental effects for the patient. Continuous ultrafiltration, on the other hand, exerts a minimum impact on hemodynamic status and tissue perfusion as the low and continuous ultrafiltration rate contributes to the maintenance of the necessary preload and thus cardiac output. Furthermore, ultrafiltration prevents neurohormonal activation and could possibly reduce diuretic resistance [3].
In the RAPID-CHF trial 40 patients were evaluated and ultrafiltration resulted to higher weight loss than diuretic therapy with low complication risk. In the UNLOAD trial, 200 patients were randomized to either ultrafiltration or diuretic therapy. Ultrafiltration was associated with a significantly greater fluid removal, fewer hospitalizations for HF and lower readmission rate than diuretic therapy [6]. In the CARRESS-HF trial 188 patients with acute HF decompensation, congestion and worsened renal function were assigned to either pharmacologic treatment or ultrafiltration. The main findings were a similar weight loss with fewer complications in the pharmacologic treatment group than the ultrafiltration group [7]. However, in CARRESS-HF trial the average furosemide dose was relatively low at 120mg per day, while only 20% were on MRAs, indicating low diuretic resistance of the study population. Real world retrospective 10-year study which included 335 consecutive patients has shown superiority of the readmission rates compared to the data from the randomized trials, which could be attributed to the adjustable ultrafiltration rate and personalized ultrafiltration protocol [8].
According to the available data and experience, continuous ultrafiltration could be considered in patients with congestion refractory to intravenous diuretics and diuretic combinations, low urine output (<100ml), impaired renal function, compromised function of the right ventricle and frequent hospitalizations for decompensated HF. Termination of the procedure is indicated after clinical resolution of congestion and after either persistent elevation of creatinine by more than 1mg/dl compared to baseline or persistent hemodynamic instability [3].
This case demonstrated an impressive response to this method which most importantly was retained long term. Contrary to the main studies of UF in this case a combination of low dose of IV furosemide was sustained during the UF procedure, which resulted to great volume loss through UF and increased urine output resulting from decongestion of the kidney and the resulting decrease of diuretic resistance. The continuing application of the method supported loss of high volumes in short therapy duration, preserving cardiac output, as the degrees of hemoconcentration, venous saturation and arterial blood pressure were constantly monitored, and ultrafiltration rate and output were modified accordingly. However, it should be noted that despite the right ventricular systolic dysfunction and the prominent right heart failure patient had equal interventricular pressures, due to the Eisenmenger physiology as the right to left intra – atrial shunt, that contributed on the maintenance of cardiac output.
Conclusion
Our case depicts that continuous ultrafiltration may exert a minimum impact on hemodynamic status and tissue perfusion by allowing an impaired right ventricle to maintain a significant preload. This suggests that it might be considered for patients in whom a session of short classic ultrafiltration might have detrimental results regarding cardiac output. Combining continuous ultrafiltration with diuretic therapy may achieve significant decongestion rate with safety, by monitoring the intravascular volume and not exceeding plasma refill rate.
Conflicts of Interest: The authors declare no conflicts of interest related to the manuscript.
References
- Mullens W, Damman K, Harjola VP, Mebazaa A, Brunner-La Rocca HP, Martens P, et al. The use of diuretics in heart failure with congestion — a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail, 2019. DOI:10.1002/EJHF.1369
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American college of cardiology foundation/american heart association task force on practice guidelines. J Am Coll Cardiol, 2013. DOI: 10.1016/J.JACC.2013.05.019/SUPPL
- Costanzo MR. Ultrafiltration in Acute Heart Failure. Card Fail Rev, 2019. DOI:10.15420/CFR.2018.29.2
- Ikeda Y, Inomata T, Kida K, Shibagaki Y, Sato N, Izumi T, et al. Kanagawa Aquaresis Investigators. Different diuretic properties between tolvaptan and furosemide in congestive heart failure patients with diuretic resistance and renal impairment: a subanalysis of the K-STAR. Heart Vessels, 2019. DOI: 10.1007/s00380-018-1270-x
- Wilcox CS, Testani JM, Pitt B. Pathophysiology of diuretic resistance and its implications for the management of chronic heart failure. Hypertension, 2020. DOI: 10.1161/HYPERTENSIONAHA.120.15205
- Costanzo MR, Guglin ME, Saltzberg MT, Jessup ML, Bart BA, Teerlink JR, et al. UNLOAD Trial Investigators. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol, 2007. DOI: 10.1016/J.JACC.2006.07.073.

