Characterization of Prostatic Adenocarcinomas in Cancer Patients
Rimsha Farooq1 , TaherehKashkoulinejad-Kouhi2, Zehra Agha3, Marjan Assefi4 and Nadeem Kizilbash5,*
1School of Public Health, Sechenov University, Moscow, Russia
2Centre for Cooperative Research in Biomaterials (CIC biomaGUNE), San Sebastian, Spain
3Department of Biosciences, COMSATS University, Pakistan
4Joint School of Nanoscience and Nanoengineering, University of North Carolina, Greensboro, USA
5Department of Chemistry, Quaid-i-Azam University, Pakistan
Received Date: 01/05/2023; Published Date: 16/08/2023
*Corresponding author: Nadeem Kizilbash, Department of Chemistry, Quaid-i-Azam University, Islamabad-45320, Pakistan
Abstract
Gleason score is the most common way of grading the Adenocarcinomas of the Prostate Gland. An aggressive tumor is indicated only by a high Gleason score. This study employed the2005International Society of Urological Pathology (ISUP) Consensus Gleason Gradingfor characterization of Prostate Cancer. The study examined the samples from 38 cancer patients exhibiting Prostatic Adenocarcinomas. Amodified-combined Gleason score of 7 was decided for twenty of the cases which indicated that there is an upgradation of the Gleason scores to higher values and an increase inthe frequency of occurrence of the score of 7 in more than 45% of cases.
Keywords: Adenocarcinoma; Prostate cancer; Conventional Gleason grading; 2005 ISUP Modified Gleason System
Introduction
The leading cause of cancer in the Western world is prostate cancer. It seems to be more prevalent in men as compared to women. Almost 10%-20% of cases exhibit bone metastasis despite increasing efforts for early detection [1]. Table 1 summarizes the distribution and number of prostate cancer cases reported in Saudi Arabia. The data is based on Prostate Specific Antigen (PSA) measurements and Digital Rectal Examination (DRE) [2]. It indicates that Saudi Arabia has low incidence rates for Prostate Cancer (PCa) as compared to Western countries [3].
A series of conditions are involved in the development of Prostate Cancer. The Proliferative Inflammatory Atrophy (PIA) occurrs initially due to prostatitis as a result of certain environmental factors and diet [4]. This condition may lead to the loss of epithelial cells and cause inflammation [5,6]. In essence, due to defective proliferation control pathways, Prostate Cancer (PCa) arises when apoptosis is decreased. This may increase the number of cells and eventually leads continuous growth of tumors [7]. The Prostatic Intraepithelial Neoplasis (PIN) is the immediate precursor of PCa.It arises initially from PIA to Low-grade form (LGPIN) towards the High-grade (HGPIN) leading to PCa [8]. There is an association found between PIN causing a rise in apoptosis [9]. This coincides with the rise in proliferation linked to PIA and accelerates a high turnover of cells which increases the chances of genetic mutations [7]. A correlation is found between the volume of PIN and tumor volume, the PIN is found in 82% of PCa tissue, but it decreases to 43% in benign tissue [10,11,26].
Table 1: A summary of the distribution of Prostate Cancer cases reported in Saudi Arabia in the time period of 1975-1996.

Figure 1: Development of Prostatitis.
Adenocarcinomas may also be referred to as invasive ductal carcinoma [12]. They have different characteristics and certain generalizations as well but adenocarcinoma is the most commonly diagnosed cancer among men [13]. They are most common in AfricanAmericans [14] and can rise in several parts of the body and can arise in several parts of the body [2] (Figure 2). Almost 95% of prostate cancers are Adenocarcinomas. The tumor is considered as multifocal as well as heterogeneous having small glands that infiltrate to larger but benign glands. Nuclear anaplasia is exhibited by the cells and there is perineural and vascular lymphatic invasion [15]. The remaining 5% accounts for signet-ring carcinoma, squamous cell carcinoma, neuroendocrine carcinoma, and sarcoma. The PCa can spread locally by an invasion of seminal vesicles directly to the urinary bladder or surrounding tissues and distantly by origination from an initial lymphatic spread or hematogenous spread mainly to bones [16].
The diagnostic confirmation for an Adenocarcinoma is by histopathological examination of the tissue samples obtainedvia needle biopsies [17]. Commonly only 6-8 needle biopsies are taken for Trans-Rectal Ultrasound (TRUS) [18] but additionally taken only if some suspicious areas are identified by Digital Rectal Examination (DRE) or Trans-Rectal Ultrasound (TRUS). Only 10%-30% of repeat biopsies with one previous negative set can be found positive because the sensitivity of the first round of biopsies is not perfect for prostate cancer [18,19]. The WHO grades and Gleason score were employed for the description of the degree of differentiation of tumors [20,21].
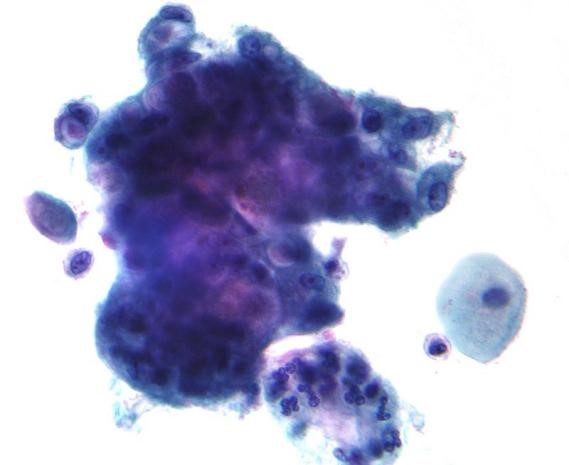
Figure 2: Micrograph showing adenocarcinoma found by Pap test. Normal intermediate (squamous) cells are seen on the right side of the image. The malignant cells have prominent mucin-filled intra-cytoplasmic vacuoles.
Different studies are pointing out the deficiencies in the diagnosis of PCa by PSA and TRUS. The Gleason score describes the appearance of cancer cells after viewing them under the microscope (Gleason, 1992) (Figure 3(a)). The degree of glandular architectural differentiation and the growth pattern of the tumor is utilized for the grading system. The predominant pattern is assigned a score of 1-5 after grading, and the second most prevalent, if it is present, is also assigned a score of 1-5 after grading. The combined Gleason score is obtained by adding both values [22] (Figure 3(b)). A Gleason score of 6 or lower is assigned to well-differentiated tumors, and patients with this score are often candidates for a program referred to as "watchful waiting therapy". The moderately or intermediately differentiated tumors receive a Gleason score of 7 and a score of 8- 10 or more is assigned to poorly differentiated tumors and a patient can need radiation therapy or adjuvant therapy. The study demonstrated that the conventional and 2005 ISUP-modified Gleason system is well-suited for the characterization of Adenocarcinomas of the Prostate Gland in Saudia Arabia.
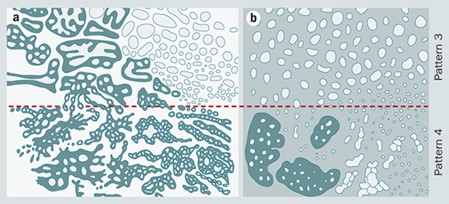
Figure 3: (a) Gleason's original grading system. (b) ISUP 2005 modified system. The general result of the 2005 changes was to narrow the definition of pattern 3 carcinoma and widen the definition of pattern 4 carcinomas.
Material and Methods
Thirty-eight samples of Prostatic Adenocarcinomas were collected by the Department of Pathology, King Abdul Aziz University Hospital (Jeddah, Saudi Arabia), for 24 months. Detailed examinations were performed followed by laboratory tests for liver and renal functions. The Prostate Specific Antigen (PSA) level was also investigated. The samples were kept in 10% formaldehyde solution and sent for the preparation of formalin-fixed paraffin-embedded blocks and tissue sections having a thickness of 3m. The Hematoxylin and Eosin (H&E) stains were used for processing and staining of the samples. All the samples were graded as per the Gleason grading system for further evaluation (Epstein et al., 2005).
Results and Discussion
Prostate Cancer depends on sexual history. Various sexually transmitted diseases have been suggested as causative factors. Sexually transmitted infection by the Human Papillomavirus (HPV) contribute to the development of Prostate Cancer (PCa). However, other factors like education, meat consumption, marriage status, smoking, and vasectomy are not found to impact prostate cancer [23].
The low specificity of PSA combined with the subjectivity of DRE offers a real possibility for the development of more stringent diagnostic criteria. Using prostatic tissue from TRUS‐guided biopsy specimens, the glandular architecture can be assessed to calculate the Gleason score (Amin et al., 2003). The twenty‐year survival rates for PCa treated conservatively show Gleason score to correlate with PCa‐associated mortality rates, as might be intuitively expected [24,25]. However, 7% of those with Gleason scores of 2–4 still die from PCa and only 66% of those having the highest Gleason scores at biopsy will die from their disease. Thus, even Gleason score is an imperfect prognostic indicator.
In 2005, the International Society of Urological Pathology modified the Gleason system by altering the criteria. It has been previously shown that this "modified Gleason score" outperforms the original one. It is currently used as the standard in urological pathology. This study characterized samples taken from 38 PCa patients and characterized them by the 2005 ISUP-modified Gleason system and conventional Gleason System. The histopathological examination of these samples revealed that all 38 cases could be characterized as Adenocarcinomas (Figures 4-6).
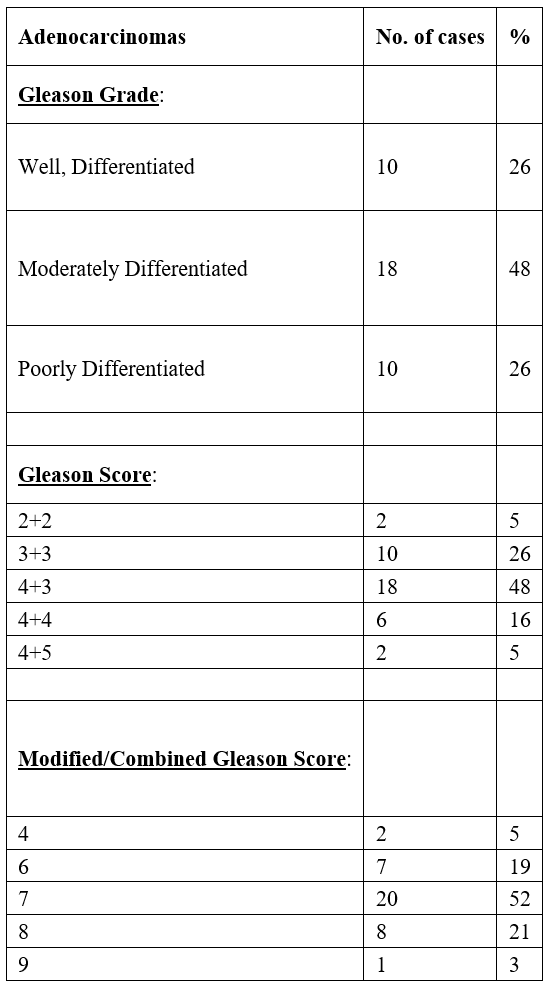
Gleason's grading of Prostatic Adenocarcinoma showed that two of the cases had a Gleason score of 02+02 and eventually, a combined score of 04. Eight cases were identified as having a Gleason score of 03+03 which led to a combined score of 06. The Gleason combined scores of 02 and 06 were found to be lower than Gleason scores for well-differentiated tumors. A total of eighteen cases were reported to be moderately differentiated as the Gleason score assigned for them was 04+03, with a combined score of 07. Ten cases were found to have poorly differentiated Prostatic Adenocarcinoma;six cases among them had a Gleason score of 04+04 and a combined Gleason score of 08, whereas four cases were given a Gleason score of 04+05 and a combined Gleason score of 09 (Table 2).
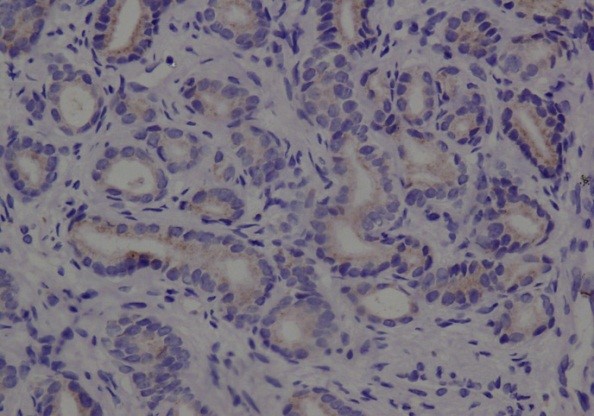
Figure 4: Well-differentiated Adenocarcinoma Gleason score (2+2), combined score 4 (DAB 200×).
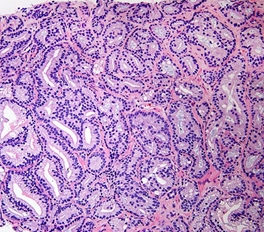
Figure 5: Gleason score 7 (3+4) with a minor component of Cribriform Glands.
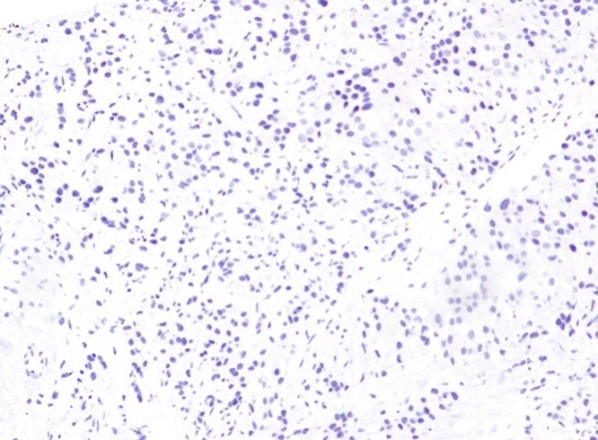
Figure 6: Poorly differentiated Adenocarcinoma with a Gleason score (5+4), combined score 9 (DAB 100×).
Table 2: Histopathological findings in cases of Adenocarcinomas.
Conclusion
Prostate Cancer cases in the Jeddah region of Saudi Arabia exhibited the same pattern and demographics as reported in the international literature for the world population as well as for the various regions of the Middle East and Saudi Arabia. This study also showed that an Adenocarcinoma of the prostate can be better characterized by using the 2005 ISUP modified Gleason system. There is an upgradation of Gleason scores to a higher level and an increase in the score of 07 in 45% of cases. The 2005 ISUP Modified Gleason System has been found to be more efficient for the diagnosis of Adenocarcinomas.
Acknowledgement: We are grateful for the help provided by the Department of Pathology, King Abdul Aziz University Hospital (Jeddah, Saudi Arabia).
References
- Subhamoy Dasgupta SJKV. Carcinogenesis 2012. New Delhi, India, International conference, , 2012; 19-21.
- Henderson BE, Ross RK, Pike MC, Casagrande JT. Endogenous Hormones as a Major Factor in Human Cancer1. Cancer Research, [online], 1982; 42(8): pp.3232–3239.
- Mosli HA, Abdel-Meguid TA, Jaudah Al-Maghrabi, Wisam Saad Kamal, Saadah HA, Hasan MA Farsi. The clinicopathologic patterns of prostatic diseases and prostate cancer in Saudi patients. Saudi Medical Journal, 2009; 30(11); pp.1439–1443.
- Pol Servián Vives. Clinical significance of prostatic proliferative inflammatory atrophy. TDX (Tesis Doctorals en Xarxa) 2016; (6).
- De Marzo AM, Putzi MJ, Nelson WG. New concepts in the pathology of prostatic epithelial carcinogenesis. Urology, 2001; 57(4): pp.103–114. doi: https://doi.org/10.1016/s0090-4295(00)00952-3.
- De Marzo AM, Meeker AK, Zha S, Luo J, Nakayama M, Platz EA, et al. Human prostate cancer precursors and pathobiology. Urology, 2003; 62(5): pp.55–62. doi: https://doi.org/10.1016/j.urology.2003.09.053.
- Ohe H, Watanabe H. Kinetic analysis of prostatic volume in treating prostatic cancer and its predictability for prognosis. Cancer, 1988; 62(11): pp.2325–2329. doi: https://doi.org/10.1002/1097-0142(19881201)62:11%3C2325::aid-cncr2820621112%3E3.0.co;2-s.
- Bostwick DG. High-grade prostatic intraepithelial neoplasia. The most likely precursor of prostate cancer. Cancer, 1995; 75(S7): pp.1823–1836. doi: https://doi.org/10.1002/1097-0142(19950401)75:7+%3C1823::aid-cncr2820751612%3E3.0.co;2-7.
- Sakr W, Partin AW. Histological markers of risk and the role of high-grade prostatic intraepithelial neoplasia. Urology, 2001; 57(4): pp.115–120. doi:https://doi.org/10.1016/s0090-4295(00)00953-5.
- Qian J, Wollan P, Bostwick DG. The extent and multicentricity of high-grade prostatic intraepithelial neoplasia in clinically localized prostatic adenocarcinoma. Human Pathology, 1997; 28(2): pp.143–148. doi:https://doi.org/10.1016/s0046-8177(97)90097-6.
- Montironi R, Mazzucchelli R, Lopez-Beltran A, Cheng L, Scarpelli M. Mechanisms of Disease: high-grade prostatic intraepithelial neoplasia and other proposed preneoplastic lesions in the prostate. Nature Clinical Practice Urology, 2007; 4(6): pp.321–332. doi:https://doi.org/10.1038/ncpuro0815.
- Malumbres M, Barbacid M. Correction: RAS oncogenes: The first 30 years. Nature Reviews Cancer, 2003; 3(9): pp.708–708. doi:https://doi.org/10.1038/nrc1193.
- Denis L. Prostate Cancer 2000. Springer, 2011.
- Landis SH, Murray T, Bolden S, Wingo PA. Cancer statistics. CA: A Cancer Journal for Clinicians, 1999; 49(1): pp.8–31. doi: https://doi.org/10.3322/canjclin.49.1.8.
- DeMarzo AM, Nelson WG, Isaacs WB, Epstein JI. Pathological and molecular aspects of prostate cancer. The Lancet, 2003; 361(9361): pp.955–964. doi:https://doi.org/10.1016/s0140-6736(03)12779-1.
- Mayadas TN, Rosenkranz AR, Cotran RS. Glomerular inflammation: use of genetically deficient mice to elucidate the roles of leukocyte adhesion molecules and Fc-gamma receptors in vivo. Current Opinion in Nephrology and Hypertension, 1999; 8(3): pp.293–298. doi:https://doi.org/10.1097/00041552-199905000-00004.
- Varadhachary GR, Raber MN, Matamoros A, Abbruzzese JL. Carcinoma of unknown primary with a colon-cancer profile—changing paradigm and emerging definitions. The Lancet Oncology, 2008; 9(6): pp.596–599. doi: https://doi.org/10.1016/s1470-2045(08)70151-7.
- Fleshner NE, O’Sullivan M, Fair WR. Prevalence and Predictors of a Positive Repeat Transrectal Ultrasound Guided Needle Biopsy of the Prostate. The Journal of Urology, 1997; pp.505–508. doi:https://doi.org/10.1097/00005392-199708000-00050.
- Crook J, Robertson S, Collin G, Zaleski V, Esche B. Clinical relevance of trans-rectal ultrasound, biopsy, and serum prostate-specific antigen following external beam radiotherapy for carcinoma of the prostate. International Journal of Radiation Oncology*Biology*Physics, 1993; 27(1): pp.31–37. doi:https://doi.org/10.1016/0360-3016(93)90418-u.
- Gleason DF. Histologic grading of prostate cancer: A perspective. Human Pathology, 1992; 23(3): pp.273–279. doi:https://doi.org/10.1016/0046-8177(92)90108-f.
- Baydar DE, Epstein JI. Gleason grading system, modifications, and additions to the original scheme. Turkish Journal of Pathology, 2009; 25(3): p.59. doi: https://doi.org/10.5146/tjpath.2009.00975.
- Epstein JI, Allsbrook WC, Amin MB, Egevad LL. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. The American Journal of Surgical Pathology, 2005; 29(9): pp.1228–1242. doi: https://doi.org/10.1097/01.pas.0000173646.99337.b1.
- Partin AW, Kattan MW, Subong EN, Walsh PC, Wojno KJ, Oesterling JE, et al. Combination of prostate-specific antigen, clinical stage, and Gleason score to predict pathological stage of localized prostate cancer. A multi-institutional update. JAMA, 1997; 277(18): pp.1445–1451.
- Albertsen PC, Hanley JA, Fine J. 20‐year outcomes following conservative management of clinically localized prostate cancer. JAMA, 2005; 293(17): pp.2095‐2101.
- Albertsen PC, Hanley JA, Gleason DF, Barry MJ. Competing risk analysis of men aged 55 to 74 years at diagnosis managed conservatively for clinically localized prostate cancer. JAMA, 1998; 280(11): pp.975‐980.
- Hisham AM Mosli “Prostate Cancer in Saudi Arabia: A Review of the Literature” (1975-1996) Annals of Saudi Medicine, 1997; 17(5): 510-514.

