Genetics, Epigenetics and Ecogenetics of Cancer
Alain L Fymat*
International Institute of Medicine & Science, Rancho Mirage, USA
Received Date: 27/04/2023; Published Date: 14/08/2023
*Corresponding author: Dr. Alain L Fymat, Professor, International Institute of Medicine & Science, Rancho Mirage, California, USA
Abstract
With deeper understanding of cell biology and genetics, it now appears that cancer is less an organ disease and more a disease of molecular mechanisms caused by mutations of specific genes. It is fundamentally a disease of tissue growth regulation failure when the genes that regulate cell growth and differentiation are altered. Most cancers have multiple possible concurring causes, and it is not possible to prevent all such causes. However, only a small minority of cancers (5-10%) are due to inherited genetic mutations whereas the vast majority (90-95%) are non-hereditary epigenetic and ecogenetic mutations that are caused by various agents (environmental factors, physical factors, and hormones). Epigenetics is the study of cellular and physiological traits inherited by daughter cells, but not caused by changes in the DNA sequence. Important examples of epigenetic and ecogenetic mechanisms and agents are discussed. Analyzing the epigenome and ecogenome of cancer, as well another genomic information included in the transcriptome and reverse transcriptome, actionable treatment indicators are identified and recommended for personalizing the cancer treatment.
Keywords: Actionable treatment indicators; Cancer theories; Cancer treatments; Clonal cancer; Chromatin remodeling; DNA Methylation; Drug resistance; Environmental exposure; Epimutations; Epitome; Genomic imprinting; Histone modification; Immunotherapy; Oncoecogenomics; Oncoepigenomics; Oncogenomics; Reverse transcriptome;.Transcriptome; Transgenerational inheritance
Abbreviations: AD: Alzheimer's disease; AIDS: Acquired ImmunoDeficiency Syndrome; ALL: Acute Lymphoblastic Lymphoma; ALS: Amytrophic Lateral Sclerosis (or Lou Gehrig disease); AML: Acute Myeloid Leukemia; ATI: Actionable Treatment Indicators; BBB: Blood-Brain Barrier; BRCA: Breast Cancer; BVP (Bleomycin, Vinblastine, Cisplatin abbreviated P for platinum),CAR: Chimeric Antigen Receptor; ChiP: Chromatin immuno Precipitation; CVD: Cardiovascular Diseases; CNV: Copy Number Variants; DNA: DeoxyriboNucleic Acid; DNAMT: DNA Methyl Transferase; EBV: Epstein-Barr Virus; ecoATI: ecogenetic ATI; ECT: Electrochemotherapy; EGP: Environmental Genome Project; epiATI: epigenetic ATI; GBM: GlioBlastoma Multiform; GE: Gene-Environment (interactions); GF: Growth Factors; GGE: Gene-Gene-Environment (interactions); GH: Growth Hormones; GIS: Growth Inhibitory Signals; HAT: Histone Acetyl Transferase; HCGP: Human Cancer Genome Project; HDAC: Histone DeACetylase; HDAT: Histone DeACetylase Transferease; HDI: Human Development Index; HGP: Human Genome Project; HIV: Human Immundefiency Virus; HLMT: Histone Lysine Methyl Transferase; HRT: Hormone Replacement Therapy; IARC: International Agency for Research on Cancer; IGF: Insulin Growth Factor; mi-RNA: Micro RNA; MLL: Mixed Lineage Leukemia; MRSA: Methycillin Resistant Staphylococcus Aureus; NCT: Nano Chemo Therapy; ND: Nano Devices; NDD: NeuroDegenerative Diseases; NIEHS: (U.S.) National Institute of Environmental Health Sciences; NM: Nano Medicine; NP: Nano Particles; NT: Nanotechnology; OS: Osteosarcoma; PAMT: Protein Arginine Methyl Transferase; PCR: Polymerase Chain Reaction; PD: Parkinson's Diseae; PD: Programmed Death; PTX: Paclitaxel; qPCR: quantitative PCR: RNA: RiboNucleic Acid; RP: Repressor Proteins; RSV: Rous Sarcoma Virus; SM: Somatic Mutations; SR: Silencer Regions;T2D: Type 2 Diabetes; TF:Transcription Factors; TPG: Tumor Promoter Genes; TS: Tumor Suppressor; TSG: Tumor Suppressor Genes; WHO: World Health Organization
Introduction
Africa is witnessing significant improvements in population health as witnessed by the declining infant mortality rates, plummeting HIV/AIDS fatality rates, rising life spans, and falling burden of communicable diseases. At the same time, morbidity and mortality from non-infectious diseases have been rising. Thus, cancer incidence has increased from 715,000 in 2008 to 1.1 million in 2020, and cancer mortality has also increased from 542,000 in 2008 to 711,000 in 2020 – the numbers varying with gender and distributing roughly two-thirds for males and one-third for females. As a matter of fact, cancer has now become the fifth leading cause of death in Africa.
Previous spatial epidemiological studies of cancer in Africa had focused on sub-Saharan countries. Recently, Sharma et al. (2022) have provided a more comprehensive epidemiology of different cancer groups in all African countries and have also estimated the relationship between the Human Development Index (HDI) and the cancer burden. Using the 2020 GLOBOCAN data recently released by the International Agency for Research on Cancer (IARC), a member of the World Health Organization (WHO), these authors projected that by 2040 all neoplasms will increase to 2.1 million new cases and 1.4 million deaths. These findings prompted them to advocate a holistic approach toward cancer control and management to naturally also include enhancing disease awareness, adopting primary and secondary prevention, mitigating risk factors, and improving cancer infrastructure and timely treatment. The GLOBOCAN data are considered the best available in each country worldwide. However, caution must be exercised when interpreting it because of the current limitations in the quality and coverage of cancer data, particularly in some low- and middle-income countries.
As a sobering observation, it is sad to note that cancer has not been cured, not in developed countries and even less in Africa despite more than a four-decade “war” against the disease, the expenditure of hundreds of billions of dollars, the conduct of hundreds of clinical trials, and the development of numerous drugs. Why? In short, it is essentially because of our lack of understanding of the basic underlying molecular mechanisms. Such mechanisms have not been elucidated by chemotherapy, as evidenced by the fact that this approach does not work universally or permanently. Fortunately, with the more recent deeper understanding of cell biology, genetics, epigenetics, and ecogenetics, it now appears that cancer is less an organ disease and more a disease of molecular mechanisms caused by mutations of specific genes.
In this Chapter, as a preamble, I will provide a brief primer on cancer (including its causes, prevention, development, malignant progression, transformative processes from a normal cell into cancer, and how cancer cells become resistant to treatment). This will be followed by another brief primer on epigenetics (including its mechanisms, changes, classification, agents, evidence in humans and inheritance). These primers will serve as a background for the study of the genetics, epigenetics, and ecogenetics of cancer (including the cause of genetic instability characteristic of cancer; DNA repair genetics and epigenetic carcinogens; environmental agents with known ecogenetic variation, the role of enzymes in processing toxic substances, exogenous and endogenous DNA damage, and gene-environment and gene-gene-environment interactions). This is followed by a detailed comparison between genetics, epigenetics, and ecogenetics in terms of their respective types of study, characteristics, processes, mechanisms, controls, agents, changes, inheritance, and applications and linkages. I will then detail the various cancer theories propounded so far, pointing the road to the personalized treatment of cancer (blood suppuration, somatic mutation, viral propagation, retroviral propagation, anti-vitamins, mono- and combo-chemotherapy, proto-oncogene, two-hit, and metastatic mechanism). A section will be dedicated to a discussion of the major recent developments in cancer treatment (including nano chemotherapy, innate and synthetic immunotherapy, DNA origami/Trojan, mnk-2 conversion to overcome drug resistance, antiangiogenesis, self-eradication during meiosis, inflammation, and electrochemotherapy. In a final section, oncoepigenomcs and oncoecogenomics will be presented as sequencing the epigenome and the ecogenome of the patient in order to evidence epigenetic and ecogenetic actionable treatment indicators.
A Brief Primer on Cancer
In earlier publications (see section References), I have reviewed the long quest for cancer cures along with developments in anti-cancer therapy, including the new strategy of immunotherapy. This latter strategy and its synthetic version utilizing Chimeric Antigen Receptors (CAR) aim to harness the body's immune system to fight cancer. Similarly, recent developments in Nanomedicine (NM) research and more specifically the application of Nanotechnology (NT) in cancer research and treatment have also been reviewed. Let me begin with a discussion of our present understanding of the causes of cancer.
Causes of Cancer
A small minority of cancers (some 5-10%) are due to inherited genetics, being caused by an inherited genetic defect (e.g., inherited mutations in the genes such as BRCA1 and BRCA2 in the case of breast cancer). The vast majority of cancers (some 90-95% of cases) are non-hereditary and due to various agents (environmental factors, physical factors, and hormones).
Environmental factors include lifestyle (diet and obesity; excessive tobacco smoking; alcohol over-consumption; excessive stress; lack of physical activity), physical factors (pollutants; viral, bacterial and parasitic infections; ionizing and non-ionizing electromagnetic radiations), and economic and behavioral factors. It is nearly impossible to prove what truly caused a cancer in any individual because most cancers have multiple possible concurring causes. Also, excepting the rare transmissions that occur with pregnancies and only a marginal few organs donor, cancer is generally not a transmissible disease. Some substances cause cancer primarily through their physical rather than chemical effects on cells (asbestos; synthetic asbestos-like fibers; non-fibrous particulate materials). It is controversial whether chronic inflammation can directly cause mutations. It is recognized, however, that inflammation can contribute to the angiogenesis, survival, proliferation, and migration of cancer cells by influencing the microenvironment around tumors. Furthermore, oncogenes are known to build up an inflammatory pro-tumorigenic microenvironment.
Some hormones play a role in the development of cancer by promoting cell proliferation. Likewise, Insulin-like Growth Factors (IGF) and their binding proteins play a key role in cancer cell proliferation, differentiation, and apoptosis, suggesting possible involvement in carcinogenesis. Hormones are also important agents in cancers of the sex organs (breasts, endometrium, ovaries, prostate, and testes), and also of thyroid and bone cancer. Other factors are also relevant: obese people have higher levels of some hormones associated with cancer and a higher rate of those cancers. Women who take Hormone Replacement Therapy (HRT) have a higher risk of developing cancers associated with those hormones. On the other hand, people who exercise far more than average have lower levels of these hormones, and a lower risk of cancer. Growth Hormones (GH) may promote Osteosarcoma (OS) and, by artificially reducing hormone levels, hormone-sensitive cancers may be discouraged.
Cancer Development
The process of cancer development in the body is a combination of events. Individuals differ in their inherited tendency to develop cancer. Mutations occasionally occur within cells in the body as they divide. Unless they occur in germ cells, these mutations will not be inherited by any offspring although they can affect the behavior of cells, sometimes causing them to grow and divide more frequently. There are biological mechanisms that attempt to stop this process: signals are given to inappropriately dividing cells that should trigger cell death (apoptosis), but sometimes additional mutations occur that cause cells to ignore these messages. An internal process of natural selection occurs within the body and eventually mutations accumulate within cells to promote their own growth, creating a cancerous tumor that grows and invades various tissues of the body.
Normally, a cell divides in response to signals called Growth Factors (GF) and stops growing once in contact with surrounding cells in response to Growth Inhibitory Signals (GIS). It usually then divides a limited number of times and dies, staying within the epithelium where it is unable to migrate to other organs. To become cancerous, a cell has to accumulate mutations in a number of genes that allow it to bypass this regulation and then no longer needs GFs to divide. It continues growing when making contact with neighboring cells, ignoring inhibitory signals, and growing indefinitely to become immortal. It will then escape from the epithelium and ultimately from the primary tumor, cross the endothelium of a blood vessel, be transported by the blood stream to colonize a new organ, forming metastases. Although there are some genetic predispositions in a small fraction of cancers, the major fraction is due to a set of new genetic mutations. These new Somatic Mutations (SM) originally appear and accumulate in one or a small number of cells that will divide to form the tumor but are not transmitted by the progeny. The most frequent mutations are a loss of function of the p53 protein, a Tumor Suppressor (TS), or in the p5 pathway, and gain of function mutations in the protein or in other oncogenes.
Malignant Progression of Cancer
Douglas Hanahan and Robert Weinberg proposed the following steps, which they dubbed as the “Hallmarks of Cancer”:
- “Evasion of apoptosis (or programmed cell death);
- “Self-sufficiency in growth signaling;
- “Insensitivity to anti-growth signals;
- “Induction and sustainment of angiogenesis;
- “Enabling of a limitless replicative potential;
- “Activation of metastasis and invasion of tissue;
- “Reprogramming of energy metabolism; and
- “Evasion of immune destruction”.
The above multi-step progression from normal cells to cells that can form a discernible mass to outright cancer is known as “malignant progression”. When cancer begins, it invariably produces no symptoms. Signs and symptoms only appear as the mass continues to grow. Symptoms can be local or systemic, the resultant finding depending on the type and location of the cancer.
Metastasis is the spread of cancer from its original site to distant sites by one or more pathways: local spread, lymphatic spread to regional lymph nodes, and by blood. In the latter instance, cancer spreads all over the body. In the so-called “soil and seed hypothesis” of cancer metastasis, cancer 'seeds' grow in certain selected site(s) only ('soil'). The symptoms of metastatic cancers depend on the location of the tumor, and can include enlarged lymph nodes, enlarged liver, enlarged spleen, and neurological symptoms.
Cancer Prevention
While hereditary genetic disorders may cause cancer, as stated earlier, the vast majority of cancer cases are due to environmental risk factors. Many, but not all, of these environmental factors are controllable lifestyle choices. Thus, cancer is considered a largely preventable disease. Greater than 30% of cancer deaths could have been prevented by avoiding certain risk factors including: tobacco use (smoking and chewing), alcohol consumption, overweight, obesity, poor diet, physical inactivity, transmitted infections, and air pollution. However, not all environmental causes are controllable such as, for example, naturally occurring background electromagnetic radiation. Thus, it is not possible to prevent all causes of cancer.
Transformative Processes of a Normal Cell into Cancer
Some environments make errors more likely to arise and propagate. Such environments can include the presence of disruptive substances called carcinogens, repeated physical injury, heat, ionizing radiation, or hypoxia. The errors that cause cancer are self-amplifying and compounding, for example:
- A mutation in the error-correcting machinery of a cell might cause that cell and its daughters to accumulate errors more rapidly;
- A further mutation in an oncogene might cause the cell to reproduce more rapidly and more frequently than its normal counterparts;
- Another mutation may cause loss of a TSG, disrupting the apoptosis signaling pathway and result in the cell becoming immortal;
- Yet another mutation in the signaling machinery of the cell might send error-causing signals to nearby cells; and
- The transformation of normal cell into cancer is akin to a chain reaction caused by initial errors, which compound into more severe errors, each progressively allowing the cell to escape the controls that limit normal tissue growth. This rebellion-like scenario becomes an undesirable survival of the fittest where the driving forces of evolution work against the body's design and enforcement of order. Once cancer has begun to develop, this ongoing process (termed “clonal evolution”) drives progression towards more invasive stages. Clonal evolution leads to intra-tumor heterogeneity that complicates designing effective treatment strategies.
How Cancer Cells Become Resistant to Treatment
While many types of chemotherapy have been developed against cancer, oncologists do not know before starting treatment whether a patient might benefit from a particular drug. So being able to identify through a laboratory test whether a patient’s tumor is either resistant or sensitive to a specific drug is crucial to enabling the rapidly developing field of “personalized medicine”.
In a breakthrough discovery, Prof. Karni and his team of researchers at the Hebrew University Medical Center, Jerusalem, Israel, have identified a process by which cancer cells become resistant to certain drugs. This process will lead to a reliable prediction as to which patients will be helped by chemotherapy and recover, and which patients will not be helped by these drugs. This finding could enable the reversal of the process and inhibit metastasis of malignant tumor cells. The researchers found that breast, lung, and colon cancer cells change the structure of an enzyme called mnk-2, which is involved in the transmission of information from the environment/body into the cell. They also showed that the enzyme has two forms, a “normal” one that inhibits cancer and another form that promotes cancer development. They further showed that cancer cells change the structure of the mnk-2, so they eliminate the form that inhibits cancer and enhance the form that induces it, thus allowing the cancer cells to survive and grow faster. Still further, they found that the anti-cancer form of the enzyme activates a program of apoptosis (suicide) in normal cells under stress conditions.
To fight the resistance process, the Karni team developed molecules that can convert the cancerous form of the mnk-2 enzyme back into its normal form so they become sensitive to stress and to absorbing chemotherapeutic drugs. More importantly, the molecules that change the cancerous form of mnk-2 into the normal form will make it possible to overcome the drug resistance of cancer cells, making them instead sensitive and responsive to various anti-cancer treatments. Further laboratory work on this aspect is continuing.
In summary, the mechanism discovered by Prof. Karni et al explains how cancer cells eliminate the anti-cancer form of mnk-2 without changing their DNA and how they become resistant to anti-cancer treatments, a problem that exists for almost every cancer treatment today. The new molecules they developed to change the structure of the mnk-2 enzyme back to its normal form will enable re-sensitizing cancer cells into anti-cancer therapies. This research could lead to the development of a new biomarker for testing the sensitivity of a patient to specific drugs. The possibility of examining whether a patient will benefit from a specific drug treatment before the treatment starts is of primary medical interest. The Israeli scientists are now developing a diagnostic test for the marker they found.
A Brief Primer on Epigenetics
Defining Epigenetics
The generally accepted definition of “epigenetics” (epi-from the Greek word επι meaning over, outside of, on top off, around + genetics) is the “study of cellular and physiological traits inherited by daughter cells, but not caused by changes in the DNA sequence”. It is the study of stable, long-term alterations in the heritable transcriptional potential of a cell. Thus, unlike genetics, which is based on changes to the DNA sequence (the genotype), in epigenetics, the changes in gene expression (or cellular phenotype) have other causes.
The term epigenetics also refers to the changes themselves, the relevant changes to the genome that do not involve a change in the nucleotide sequence. Gene expression can be controlled through the action of repressor proteins (RP) that attach to silencer regions (SR) of the DNA. These epigenetic changes may last through cell divisions for the duration of the cell's life, and may also last for multiple generations even though they do not involve changes in the underlying DNA sequence of the organism; instead, non-genetic factors cause the organism's genes to behave (or "express themselves") differently.
Epigenetic Mechanisms and Health Endpoints
Several types of epigenetic inheritance systems may play a role in what has become known as “cell memory”. (Note, however, that not all of these are universally accepted as examples of epigenetics.) Important examples of epigenetic mechanisms are DNA methylation, histone modification and chromatin remodeling, each of which alters how genes are expressed without altering the underlying DNA sequence.
Epigenetic mechanisms are affected by several factors and processes including development in utero and in childhood, environmental chemicals, drugs and pharmaceuticals, aging, and diet. DNA methylation is what occurs when methyl groups, an epigenetic factor found in some dietary sources, can tag DNA and activate or repress genes. Histones are proteins around which DNA can wind for compaction and gene regulation. Histone modification occurs when the binding of epigenetic factors to histone “tails” alters the extent to which DNA is wrapped around histones and the availability of genes in the DNA to be activated. All of these factors and processes can have an effect on people’s health and influence their health possibly resulting in cancer, autoimmune disease, mental disorders, or diabetes among other illnesses (Figure 1).

Figure 1: Epigenetic Mechanisms and Health Endpoint.
Source: U.S. National Institutes of Health http://commonfund.nih.gov/epigenomics/figure.aspx
DNA Methylation
Much is known about the mechanism of heritability of DNA methylation state during cell division and differentiation. Heritability of methylation state depends on certain enzymes, such as DNA methyltransferase (DNAMT), that have a higher affinity for 5-methylcytosine than for cytosine. If this enzyme reaches a "hemimethylated" portion of DNA (where 5-methylcytosine is in only one of the two DNA strands) the enzyme will methylate the other half.
DNA methylation patterns are known to be established and modified in response to environmental factors by a complex interplay of at least three independent DNA methyltransferases (DNAMT1, DNAMT3A, and DNAMT3B). DNAMT1 is often referred to as the maintenance methyltransferase. It is essential for proper embryonic development, imprinting, and X-inactivation. To emphasize the difference of this molecular mechanism of inheritance from the canonical Watson-Crick base-pairing mechanism of transmission of genetic information, the term epigenetic templating was introduced. Furthermore, in addition to the maintenance and transmission of methylated DNA states, the same principle could work in the maintenance and transmission of histone modifications, as further discussed in a later section of this Chapter.
DNA methylation is an important regulator of gene transcription and a large body of evidence has demonstrated that aberrant DNA methylation is associated with unscheduled gene silencing, and the genes with high levels of 5-methylcytosine in their promoter region are transcriptionally silent. DNA methylation is essential during embryonic development and, in somatic cells, patterns of DNA methylation are in general transmitted to daughter cells with high fidelity. Aberrant DNA methylation patterns have been associated with a large number of human malignancies and found in two distinct forms: hypermethylation and hypomethylation compared to normal tissue. Hypermethylation is one of the major epigenetic modifications that repress transcription via promoter region of tumor suppressor genes. Hypermethylation typically occurs at CpG islands in the promoter region and is associated with gene inactivation. Global hypomethylation has also been implicated in the development and progression of cancer through different mechanisms.
Histone Modifications
Mechanisms of heritability of histone state are not well understood. Although histone modifications occur throughout the entire sequence, the unstructured N-termini of histones (called histone tails) are particularly highly modified. These modifications include:
- Acetylation: This is the formation of an acetyl derivative (acetyl is the atom grouping CH3CO, an acetic acid molecule from which the hydroxyl group has been removed). It is the most highly studied. It has a tendency to be associated with “active” transcription and is biophysical in nature. Because it normally has a positively charged nitrogen at its end, lysine can bind the negatively charged phosphates of the DNA backbone. The acetylation event converts the positively charged amine group on the side chain into a neutral amide linkage. This removes the positive charge, thus loosening the DNA from the histone. When this occurs, complexes and other transcriptional factors can bind to the DNA and allow transcription to occur. This is the "cis" model of epigenetic function. In other words, changes to the histone tails have a direct effect on the DNA itself. Another model of epigenetic function is the "trans" model in which changes to the histone tails act indirectly on the DNA. Further, acetylation at one position is likely to function differently from acetylation at another position. The idea that multiple dynamic modifications regulate gene transcription in a systematic and reproducible way is called the histone code.
- Methylation: This is the addition of methyl groups (methyl is the radical - CH3). It bears the idea that modifications act as docking modules for related factors. It is a chemical endogenous damage to DNA and an important regulator of gene transcription.
- Phosphorylation: This is the addition of phosphate to an organic compound such as glucose to produce glucose monophosphate through the action of a phosphotransferase (phosphorylase) or kinase.
- Ribosylation: This is the chemical transformation into a ribosyl (a radical formed by loss of the hemiacetal OH group from either of two cyclic forms of ribose) yielding ribofuranosyl andrybopyranosyl compounds by combination with an H of -nH- or -CH group.
Other modifications include Citrillination, Sumoylation, and Ubiquitylation that will not be detailed here.
Chromatin Remodeling
Because chromatin remodeling (and other mechanisms such as DNA methylation) play such a central role in many types of epigenetic inheritance, the word "epigenetics" is sometimes, albeit misleadingly, used as a synonym for these processes. Chromatin remodeling is not always inherited and not all epigenetic inheritance involves chromatin remodeling. Chromatin remodeling is accomplished through two main mechanisms:
- Post-translational modification of the amino acids that make up histone proteins: Histone proteins are made up of long chains of amino acids. If the amino acids that are in the chain are changed, the shape of the histone might be modified. DNA is not completely unwound during replication. It is possible, then, that the modified histones may be carried into each new copy of the DNA. Once there, these histones may act as templates, initiating the surrounding new histones to be shaped in the new manner. By altering the shape of the histones around them, these modified histones would ensure that a lineage-specific transcription program is maintained after cell division.
- Addition of methyl groups to the DNA, mostly at CpG sites, to convert cytosyne to 5-methylcytosine: 5-Methylcytosine performs much like a regular cytosine, pairing with a guanine in double-stranded DNA. However, some areas of the genome are methylated more heavily than others, and highly methylated areas tend to be less transcriptionally active, through a mechanism not fully understood. Methylation of cytosines can also persist from the germ line of one of the parents into the zygote, marking the chromosome as being inherited from one parent or the other (genetic imprinting).
Epigenetic Changes
As we know, epigenetic changes can modify the activation of certain genes, but not the sequence of DNA. Additionally, the chromatin proteins associated with DNA may be activated or silenced. This is why the differentiated cells in a multi-cellular organism express only the genes that are necessary for their own activity.
Epigenetic changes are preserved when cells divide. Most epigenetic changes only occur within the course of one individual organism's lifetime but, if gene inactivation occurs in a sperm or egg cell that results in fertilization, then some epigenetic changes can be transferred to the next generation. This raises the question of whether or not epigenetic changes in an organism can alter the basic structure of its DNA (in contradistinction with the very definition of epigenetics)
Specific epigenetic processes are multiple and varied:
- Bookmarking;
- Carcinogenesis progress;
- Cloning technical limitations;
- Gene silencing;
- Heterochromatin;
- Histone modification, regulation, imprinting, maternal effects;
- Paramutation;
- Pathogenesis technical limitations;
- Position effect;
- Reprogramming;
- Teratogens' effects;
- Transvection; and
- X-chromosome inactivation.
DNA damages can also cause epigenetic changes. They are very frequent, occurring on average about 10,000 times a day per cell of the human body. These damages are largely repaired, but at the site of a DNA repair, epigenetic changes can remain. DNA-damaging chemicals, such as benzene, hydroquinone, styrene, carbon tetrachloride, and trichloroethylene cause considerable hypomethylation of DNA.
Foods are known to alter the epigenetics of rats on different diets. Some food components epigenetically increase the levels of DNA repair enzymes while others can reduce DNA damage, such as soy isoflavones and bilberry anthocyanins. The effects on humans have been lees studied.
Epigenetic Classification
Epigenetics can be divided into predetermined and probabilistic epigenesis. Predetermined epigenesis is a unidirectional movement from structural development in DNA to the functional maturation of the protein. Predetermined here means that development is scripted and predictable. Probabilistic epigenesis, on the other hand, is a bidirectional structure-function development with experiences and external molding development.
Epigenetic Agents
Epigenetic agents are prions, RNA, and micro-RNA:
Prions
In general, proteins fold into discrete units that perform distinct cellular functions, but some proteins are also capable of forming an infectious conformational state known as a prion (protein+infection). Although often viewed in the context of infectious diseases, prions are more loosely defined by their ability to catalytically convert other native state versions of the same protein to an infectious conformational state. It is in this latter sense that they can be viewed as epigenetic agents capable of inducing a phenotypic change without a modification of the genome.
Fungal prions are considered by some to be epigenetic because the infectious phenotype caused by the prion can be inherited without modification of the genome.
RNA and micro-RNA
Sometimes a gene, after being turned on, transcribes a product that (directly or indirectly) maintains the activity of that gene. RNA signaling includes differential recruitment of a hierarchy of generic chromatin-modifying complexes and DNAMTs to specific loci by RNAs during differentiation and development. Other epigenetic changes are mediated by the production of different splice forms of RNA, or by formation of double-stranded RNA. Descendants of the cell in which the gene was turned on will inherit this activity, even if the original stimulus for gene-activation is no longer present. These genes are often turned on or off by signal transduction, although in some systems RNA may spread directly to other cells or nuclei by diffusion. A large amount of RNA and protein is contributed to the zygote by the mother during oogenesis or via nurse cells resulting in maternal effects' phenotypes. A smaller quantity of sperm RNA is transmitted from the father, but there is recent evidence that this epigenetic information can lead to visible changes in several generations of offspring.
Micro-RNAs (mi-RNAs) are members of non-coding RNAs that range in size from 17 to 25 nucleotides. They regulate a large variety of biological functions. About 2000 mi-RNAs have so far been discovered in humans. It appears that about 60% of human protein coding genes are regulated by mi-RNAs. Many mi-RNAs are epigenetically regulated. About 50% of mi-RNA genes are associated with CpG islands that may be repressed by epigenetic methylation. Other mi-RNAs are epigenetically regulated by either histone modifications or by combined DNA methylation and histone modification.
Evidence in Humans
There are at least three types of epigenetic evidence in humans:
Environmental Exposure
Epigenetic changes have been observed to occur in response to environmental exposure. In the case of humans with different environmental exposures, monozygotic (identical) twins were epigenetically indistinguishable during their early years, while older twins had remarkable differences in the overall content and genomic distribution of 5-methylcytosine DNA and histone acetylation. The twin pairs who had spent less of their lifetime together and/or had greater differences in their medical histories were those who showed the largest such differences.
Recent studies involving both dizygotic (not identical) and monozygotic twins have produced some evidence of epigenetic influence in humans. Direct comparisons between identical twins constitute the ideal experimental model for testing environmental epigenetics because DNA sequence differences that would be abundant in a singleton-based study do not interfere with the analysis. Research has shown that a difference in the environment can produce long-term epigenetic effects, and different developmental monozygotic twin subtypes may be different with respect to their susceptibility to be discordant from an epigenetic point of view.
One of the first high-throughput studies of epigenetic differences between monozygotic twins focused on comparing global and locus-specific changes in DNA methylation and histone modifications in a sample of 40 monozygotic twin pairs. In this case, only healthy twin pairs were studied, but a wide range of ages was represented, between 3 and 74 years. One of the major conclusions from this study was that there is an age-dependent accumulation of epigenetic differences between the two siblings of twin pairs. This accumulation suggests the existence of epigenetic drift. A more recent study, where 114 monozygotic twins and 80 dizygotic twins were analyzed for the DNA methylation status of around 6,000 unique genomic regions, concluded that epigenetic similarity at the time of blastocyst splitting may also contribute to phenotypic similarities in monozygotic co-twins. This supports the notion that the microenvironment at early stages of embryonic development can be quite important for the establishment of epigenetic marks.
Genomic Imprinting
Some human disorders are associated with genomic imprinting, a phenomenon in mammals where the father and mother contribute different epigenetic patterns for specific genomic loci in their germ cells. The best-known cases of imprinting in human disorders are those in Angelman, Prader-Willi, and Beckwith-Wiedemann syndromes. The former two syndromes can be produced by the same genetic mutation (chromosome 15q partial deletion), and the particular syndrome that will develop depends on whether the mutation is inherited from the child's mother or father. This is due to the presence of genomic imprinting in the region. The latter syndrome is often caused by abnormalities in maternal genomic imprinting of a region on chromosome 11.
Transgenerational Inheritance
In the Overkalix study, Marcus Pembrey and colleagues observed that the paternal (but not maternal) grandsons of Swedish men who were exposed during preadolescence to famine in the 19th century were less likely to die of cardiovascular disease. If food was plentiful, then diabetes mortality in the grandchildren increased, suggesting that this was a transgenerational epigenetic inheritance. The opposite effect was observed for females—the paternal (but not maternal) granddaughters of women who experienced famine while in the womb (and therefore while their eggs were being formed) lived shorter lives on average. Similar transgenerational effects were observed during the 1944 Dutch Famine. Such transgenerational inheritance traits were noted and studied in the case of these two famines because, especially in the cae of the Dutch Famine, accurate records were kept over long periods of time for both male and female descendants, some continuing even to this day. No doubt such observations could have been made in the previous numerous famines except for the lack of the required meticulous records.
Epigenetic Inheritance
Somatic epigenetic inheritance through epigenetic modifications, particularly through DNA methylation and chromatin remodeling, is very important in the development of multicellular eukaryotic organisms. The genome sequence is static (with some notable exceptions), but cells differentiate into many different types, which perform different functions, and respond differently to the environment and intercellular signaling. Thus, as individuals develop, morphogens activate or silence genes in an epigenetically heritable fashion, giving cells a "memory".
In mammals, most cells terminally differentiate, with only stem cells retaining the ability to differentiate into several cell types ("totipotency" and "multipotency"). Some stem cells continue producing new differentiated cells throughout life, such as in neurogenesis, but mammals are not able to respond to loss of some tissues, for example, the inability to regenerate limbs, of which some other animals are capable. Unlike animals, plant cells do not terminally differentiate, remaining totipotent with the ability to give rise to a new individual plant. While plants do utilize many of the same epigenetic mechanisms as animals, such as chromatin remodeling, it has been hypothesized that some kinds of plant cells do not use or require "cellular memories", resetting their gene expression patterns using positional information from the environment and surrounding cells to determine their fate.
The Genetics of Cancer
Cancer is fundamentally a disease of tissue growth regulation failure, as already stated. In order for a normal cell to transform into a cancer cell, the genes that regulate cell growth and differentiation must be altered. The affected genes are divided into two broad categories: Tumor Promoter Genes (TPGs) or oncogenes that promote cell growth and reproduction, and Tumor Suppressor Genes (TSGs). that inhibit cell division and survival. Malignant transformation can occur through the formation of new oncogenes, the inappropriate over-expression of normal oncogenes, or else by the under-expression or disabling of TSGs. Typically, changes in many genes are required to transform a normal cell into a cancer cell.
Genetic changes can occur at different levels and by different mechanisms. The gain or loss of an entire chromosome can occur through errors in mitosis. More common are mutations, which are changes in the nucleotide sequence of genomic DNA. Large-scale mutations involve the deletion or gain of a portion of a chromosome. The following processes are noted:
- Genomic amplification: It occurs when a cell gains many copies (often 20 or more) of a small chromosomal locus, usually containing one or more oncogenes and adjacent genetic material;
- Genomic translocation: It occurs when two separate chromosomal regions become abnormally fused, often at a characteristic location;
- Small-scale mutations including point mutations, deletions, and insertions: They may occur in the promoter region of a gene and affect its expression, or may occur in the gene's coding and alter the function or stability of its protein product; and
- Disruption of a single gene: It may result from integration of genomic material from a DNA virus or retrovirus, leading to the expression of viral oncogenes in the affected cell and its descendants.
Replication of the enormous amount of data contained within the DNA of living cells will probabilistically result in some errors (mutations). Complex error correction and prevention are built into the process to safeguard the cell against cancer. However, if a significant error occurs, the damaged cell can "self-destruct" through programmed cell death (apoptosis). If the error control processes fail, then the mutations will survive and be passed along to daughter cells.
The Epigenetics of Cancer
Defining Cancer Epigenetics
The accepted definition of cancer epigenetics is “...the study of epigenetic modifications to the genome of cancer cells that do not involve a change in the nucleotide sequence”. Epigenetic alterations are as important as genetic mutations in the transformative processes of a normal cell into cancer. As will further be elaborated, such alterations include the silencing of tumor suppressor genes (TSGs) and the activation of tumor promoter genes (TPGs, or oncogenes) by altered CpG island methylation patterns, histone modifications, and dysregulation of DNA binding proteins.
Classically, cancer has been viewed as a set of diseases that are driven by progressive genetic abnormalities including mutations in oncogenes, TSGs, and chromosomal abnormalities. However, it has become apparent that cancer is also driven by epigenetic alterations, which refer to functionally-relevant modifications to the genome that do not involve a change in the nucleotide sequence. Examples of such modifications were given already (changes in DNA methylation: hyper- and hypo-methylation; histone modifications; and changes in chromosomal architecture caused by inappropriate expression of proteins).
Each of these epigenetic alterations serves to regulate gene expression without altering the underlying DNA sequence. These changes may remain through cell divisions, last for multiple generations, and can be considered to be “epimutations” (equivalent to mutations). Epigenetic alterations occur frequently in cancers. However, while large numbers of epigenetic alterations are found in cancers, the epigenetic alterations in DNA repair genes, causing reduced expression of DNA repair proteins, may be of particular importance. Such alterations are thought to occur early in progression to cancer and to be a likely cause of the genetic instability characteristic of cancers. Reduced expression of DNA repair genes causes deficient DNA repair.
In summary, as pointed out above, under genetic alterations, cancer is caused by failure to regulate tissue growth, when the genes that regulate cell growth and differentiation are altered. It has become clear that these alterations are caused by both DNA sequence mutation in oncogenes and TSGs as well as by epigenetic alterations. The epigenetic deficiencies in the expression of DNA repair genes, in particular, likely cause an increased frequency of mutations, some of which then occur in oncogenes and TSGs.
Epigenetics has the potential to explain mechanisms of aging, human development, and the origins of cancer, heart disease, and mental illness as well as several other conditions. Some investigators even think that epigenetics may ultimately turn out to have a greater role in disease than genetics.
A variety of compounds are considered as epigenetic carcinogens, They result in an increased incidence of tumors, but they do not show mutagen activity. Examples include: diethylstilbestrol, arsenite, hexachlorobenzene, and nickel compounds.
Recent studies have shown that the mixed lineage leukemia (MLL) gene causes leukemia by rearranging and fusing with other genes in different chromosomes, which is a process under epigenetic control. Other investigators have concluded that alterations in histone acetylation and DNA methylation occur in various genes influencing prostate cancer. Gene expression in the prostate can be modulated by nutrition and lifestyle changes.
Epimutations as the Cause of Genetic Instability Characteristic of Cancer
Cancer epigenetics is again the study of epigenetic modifications to the genome of cancer cells that do not involve a change in the nucleotide sequence. Epigenetic alterations are as important as genetic mutations in the transformative processes of a normal cell into cancer. Such alterations include the silencing of Tumor Suppressor Genes (TSGs) and the activation of Tumor Promoter Genes (TPGs, or oncogenes) by altered CpG island methylation patterns, histone modifications, chromatin remodeling, and dysregulation of DNA binding proteins. Whereas cancer has been viewed as a set of diseases that are driven by progressive genetic abnormalities (mutations in oncogenes, TSGs, and chromosomal abnormalities), it is also driven by epigenetic alterations (changes in DNA methylation: hyper- and hypo-methylation; histone modifications; and changes in chromosomal architecture caused by inappropriate expression of proteins). These changes may remain through cell divisions, last for multiple generations, and can be considered to be “epimutations” (equivalent to mutations). They occur early in the progression to cancer and likely cause the genetic instability characteristic of cancers. The epigenetic deficiencies in expression of DNA repair genes, in particular, likely cause an increased frequency of mutations, some of which then occur in oncogenes and TSGs.
Epigenetics of DNA Repair
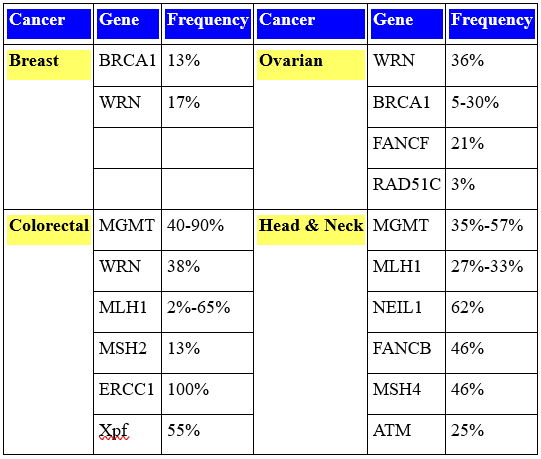
Epigenetic reductions in the expression of DNA repair genes are very frequent in sporadic (non-germ line) cancers, as shown among the representative cancers illustrated in Table 1, while mutations in DNA repair genes in sporadic cancer are very rare.
Deficiencies in expression of DNA repair genes cause increased mutation rates and genome instability, which is likely the main underlying cause of the genetic alterations leading to cancer. In fact, the first event in many sporadic neoplasias is a heritable alteration that affects genetic instability; also, epigenetic defects in DNA repair are somatically heritable.
Table 1: Frequency of Epigenetic Changes (CpG Island Methylation) in DNA Repair Genes in Sporadic Cancers.
Epigenetic Carcinogens
A variety of toxic compounds or pathogens (diethylstilbestrol, arsenite, hexachlorobenzene, nickel) are considered as epigenetic carcinogens in that they increase the incidence of tumors. However, they do not show mutagen activity. By epigenetic mechanisms, many teratogens (such as diethylstilbestrol) exert specific effects on the fetus and throughout the life of an affected child. However, the possibility of birth defects resulting from the child's parents and their parents' exposures have not been observed although a range of male-mediated abnormalities have been demonstrated.
Recent studies have shown that the mixed lineage leukemia (MLL) gene causes leukemia by rearranging and fusing with other genes in different chromosomes, which is a process under epigenetic control. Other investigations have concluded that alterations in histone acetylation and DNA methylation occur in various genes influencing prostate cancer. Gene expression in the prostate can be modulated by nutrition and lifestyle changes. Figure 2 illustrates epigenetic patterns in normanl and cancer cells. The Figure is separated into two parts for a normal cell: an inaccessible heterochromatin part and an accessible euchromatin part, both in the normal cell.

Figure 2: Epigenetic Patterns in Normal and Canccr Cells (Source: Bornstein0275; Wikipedia).
In the first part: Figure 2(A1) shows a repetitive sequence of a methylated CpG site in which transcription does not take place whereas Figure 2(A2) shows the hypometylation of DNA in a repetitive sequence with allowed transcription. In Figure 2(B1) for a normal cell, histone modifications are illustrated in a closed chromatin configuration with no transcription allowed whereas Figure 2(B2) shows the same for a cancerous cell with loss of histone methylation and allowed transcription. In the second part, Figure 2(C1) shows a normal cell with umethylated CpG island and methylated gene body with initial transcription before exon 1 but no transscriptions thereafter at the exons 1, 2 or 3 illustrated whereas, for a cancer cell, Figure 2(C2) illustrates hypermethylation of a promoter with the opposite transcription pattern. Lastly, Figure 2(D1) for a normal cell shows histone modifications in an open chromatin modification with transcription whereas, for a cancer cell, Figure 2(D2) shows the .loss of histone acetylation with the absence of transxcription.
Drug development has focused mainly on Histone Acetyl Transferase (HAT) and Histone DeACetylase (HDAC), and has included the introduction to the market of the new pharmaceutical Varinostat, an HDAC inhibitor. HDAC has been shown to play an integral role in the progression of oral squamous cancer. Current front-runner candidates for new drug targets are Histone Lysine Methyl Transferase (HLMT) and Protein Arginine Methyl Transferase (PAMT).
Applications of epigenetics to cancer sub-types (cervical, leukemia, prostate, sarcoma, etc.) will not be discussed here. More epigenetic aspects remain to be discussed such as functional epigenomics (or the engineering of the epigenome), RNA and beyond RNA-epigenetics. Such a discussion would go far beyond the scope of this Chapter and will not likewise be presented here.
DNA Repair Epigenetics
Germ line (familial) mutations have been identified in 34 different DNA repair genes that cause a high risk of cancer, including, for example BRCA1 and ATM. However, cancers caused by such germ line mutations make up only a very small proportion of cancers. For instance, germ line mutations cause only 2-5% of colon cancer cases.
Epigenetic reductions in expression of DNA repair genes, however, are very frequent in sporadic (non-germ line) cancers, as shown among some representative cancers in Table 1, while mutations in DNA repair genes in sporadic cancer are very rare.
Deficiencies in expression of DNA repair genes cause increased mutation rates and cause genome instability, which is likely the main underlying cause of the genetic alterations leading to cancer. In fact, the first event in many sporadic neoplasias is a heritable alteration that affects genetic instability and epigenetic defects in DNA repair are somatically heritable.
Epigenetic Carcinogens
A variety of toxic compounds or pathogens (diethylstilbestrol, arsenite, hexachlorobenzene, nickel) are considered as epigenetic carcinogens in that they increase the incidence of tumors. However, they do not show mutagen activity. By epigenetic mechanisms, many teratogens (such as diethylstilbestrol) exert specific effects on the fetus and throughout the life of an affected child. However, the possibility of birth defects resulting from the child's parents and their parents' exposures have not been observed. Nonetheless, a range of male-mediated abnormalities have been demonstrated.
The Ecogenetics of Cancer
Ecogenetics is concerned with the identification of “polymorphisms” in genes involved in environmentally-induced diseases, specifically cancer for our present purpose. Ever since the origin of DNA-based life, genomes have been subjected to environmental stresses. Every second, the genome of each of our cells is altered, broken, and reassembled. The survival of cells, humans, and species depends on mechanisms to repair this damage and reconstitute genomes.
Evolution has been at work for billions of years to produce exquisite DNA repair systems that patrol the genome, fixing or replacing damaged, altered, and miscoded nucleotides. The ability of the cell to maintain its genetic integrity is crucial for, without this ability, there would ensue a cascade of mutations reaching an error threshold at which point the genetic information could no longer be maintained. In order to correct this damage before it affects cellular functionality or triggers apoptosis, the cell has evolved multiple repair mechanisms (reversal of damage, base excision repair, nucleotide excision repair, mismatch repair, recombination with restoration of DNA sequences, and bypass of lesions by special DNA polymerases). Genetic variations in the human population can affect the efficiency and accuracy of these repair mechanisms and can lead to greater disease susceptibility. Unrepaired DNA damage can reduce the overall fitness of a cell, triggering cell cycle arrest, apoptosis, unchecked growth, or other diminished functionality. Therefore, loss of any of these repair pathways in humans can result in mutations, cancer, and death. Variability in the ability of these repair systems to perform may ultimately lead to an increase in mutations in somatic cells (any cells that are not egg or sperm) and to a higher risk for disease. Polymorphisms in DNA repair enzymes may increase the risk of disease.
Environmental Agents with Known Ecogenetic Variation
In 1997, as part of the Human Genome Project (HGP), the (U.S.) National Institute of Environmental Health Sciences (NIEHS) started the Environmental Genome Project (EGP) -- a comprehensive effort to identify “polymorphisms” in genes involved in environmentally-induced diseases. The key objective is to identify alleles that confer susceptibility to the adverse effects of environmental agents. Classes of environmental agents with known ecogenetic variation have been summarized in Table 2.
Table 2: Classes of Environmental Agents with Known Ecogenetic Variation.

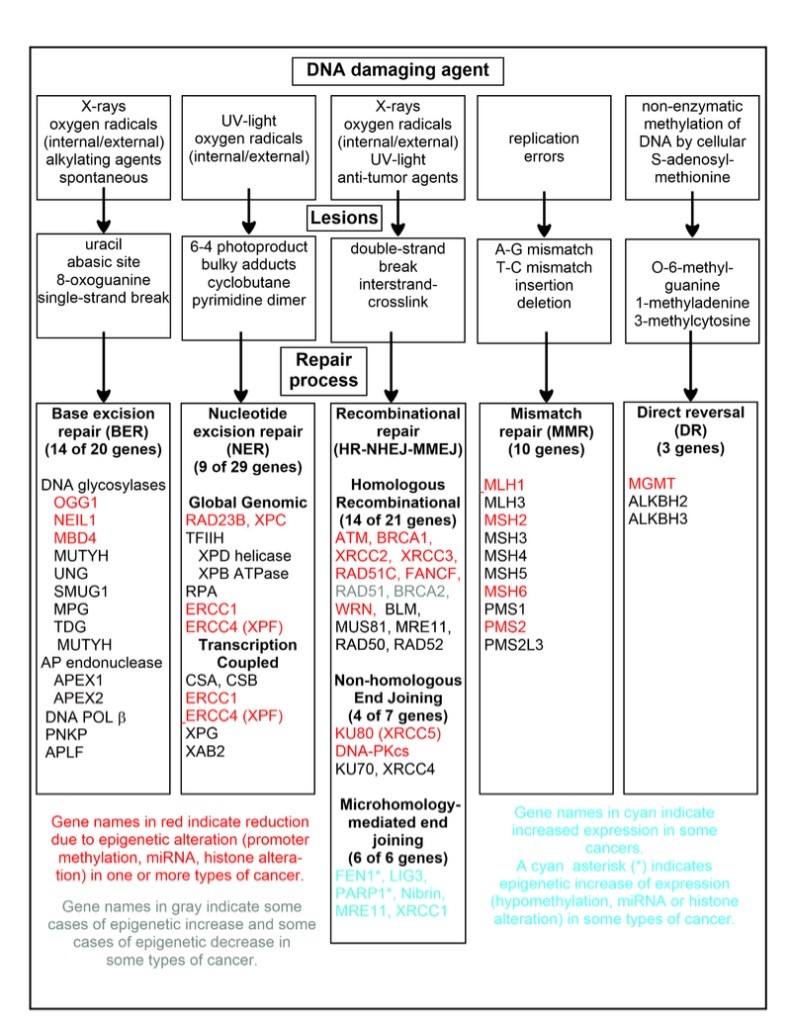
Figure 3: Common DNA Damaging Agents, Legions and Repair Processes (Source: Bornstein)
Of particular note in Table 2 are the effects of environmental/chemical agents such as inhaled pollutants that can induce lung and bladder cancer and likewise for high-energy electromagnetic radiation. Similarly, lifestyle and behavioral agents (such as smoking) can induce lung cancer.
In Figure 3, common DNA damaging agents are charted including examples of lesions they cause in DNA, and pathways used to repair these lesions. Also shown are many of the genes in these pathways, an indication of which genes are epigenetically regulated to have reduced (or increased) expression in various cancers. It also shows genes in the error prone microhomology-mediated end joining pathway with increased expression in various cancers.
Role of Enzymes in Processing Toxic Substances
A major area of focus in pharmacogenetics and pharmacoecogenetics research to date is the role of enzymes in processing toxic substances. There are many enzymes in the body that participate in the metabolism and elimination of endogenous compounds and xenobiotics. These biotransformations usually aid in the ultimate elimination of such compounds, although in some cases the reactions bioactivate parent compounds. Individual differences here can limit their efficacy, or lead to severe adverse reactions.
Because of the need to recall several widely utilized drugs due to unexpected severe toxic effects, pharmacogenetic research is now focused toward the definition of individualized therapies that take into account individuals' genetic makeup. In addition, evidence is emerging that these same biotransformation enzymes can also modulate an individual's susceptibility to environmental and occupational toxicants – the specific area of ecogenetics research.
The toxic effects of many chemicals result from the ability of the activated chemical to damage DNA. DNA damage from both endogenous and exogenous sources occurs frequently in every living cell in our bodies. Fortunately, our cells possess remarkably efficient repair processes that remove and correct such DNA damage.
Exogenous And Endogenous DNA Damage
There are two main categories of DNA damage: exogenous (environmental) and endogenous (internal, spontaneous). With all of this damage occurring within the cell, it is no wonder that drugs that reduce the damage load on cells, such as anti-oxidants, are being promulgated for cancer prevention.
Exogenous DNA damage can be caused by many environmental agents, including (a) natural chemicals found in food (e.g., aflatoxins); (b) synthetic (human-made) chemicals (e.g., benzopyrine found in cigarette smoke); and (c) chemicals used in chemotherapy of cancer (e.g., cisplatin); (d) exposure to UV radiation produced naturally by the sun or artificially by tanning booth lamps; and (e) ionizing radiation such as γ-rays and X-rays (during diagnosis and therapeutic treatment; occupational exposure).
The damages result in (a) the chemical instability of DNA, which can manifest as depurination and depyrimidation events resulting in the loss of a base from the DNA strand. It can be estimated that 10,000 bases per cell per day are lost spontaneously and subsequently repaired; and (b) the production of reactive molecules by normal cellular processes. The damage in cells by reactive oxygen (e.g., hydroxyl radicals, superoxide anion) is likewise estimated to be 10,000 events per cell per day. On the other hand, endogenous DNA damage is caused by chemical alterations such as methylation, and incorporation of incorrect bases during DNA synthesis.
Gene-Environment and Gene=Gene-Environment Interactions in The Etiology of Diseases
All the following cancers (lung, gastro-intestinal, and others) [and also neurodegenerative diseases (NDDs) including Alzheimer, AD; Parkinson PD; and Amyotrophic Lateral Sclerosis, ALS or Lou Gehrig disease); cardiovascular diseases (CVDs); type 2 T2D diabetes; infectious diseases such as malaria and HIV/AIDS] clearly have a genetic and an environmental component to their etiology. The complexity of the diseases poses substantial difficulties in establishing clear-cut associations. Most often, the end point is the result of an array of multifactorial aspects involving both the individual's genome and the environment. In some instances, Gene-Environment (GE) interactions must be enlarged to Gene-Gene-Environment interactions (GGE).
Well over 100 types of cancer have been observed to occur in humans, each with its own unique constellation of risk and protective factors. However, no matter how strong a particular risk factor might be, whether an environmental exposure such as tobacco or alcohol, or an inherited predisposition to cancer, it is quite rare that one factor completely determines the development of cancer. Multiple factors must therefore play a role in causing a normal cell to develop the myriad genetic abnormalities characteristic of the malignant phenotype. In some instances, risk factors may act through different biological pathways, and are therefore said to act independently of each other. In other instances, the effect of one risk factor may depend on the presence of another risk factor, that is, they may interact. The interacting risk factors might both be environmental in nature. In other instances, environmental risk factors may interact with genetic factors in modifying disease risk. Finally, multiple gene products typically act in complex metabolic pathways, and they may interact with each other in determining disease risk (e.g., gene-gene interactions). Notwithstanding the wide variation in etiology of different cancers, it is important to consider each tissue or organ system in its own particular context.
Genetics Versus Epigenetics and Ecogenetics
It is of interest to contrast, at least in part, genetics with epigenetics and ecogenetics. This is the tentative purpose of Table 3 below:
Table 3: Comparison Between Genetics, Epigenetics and Ecogenetics.
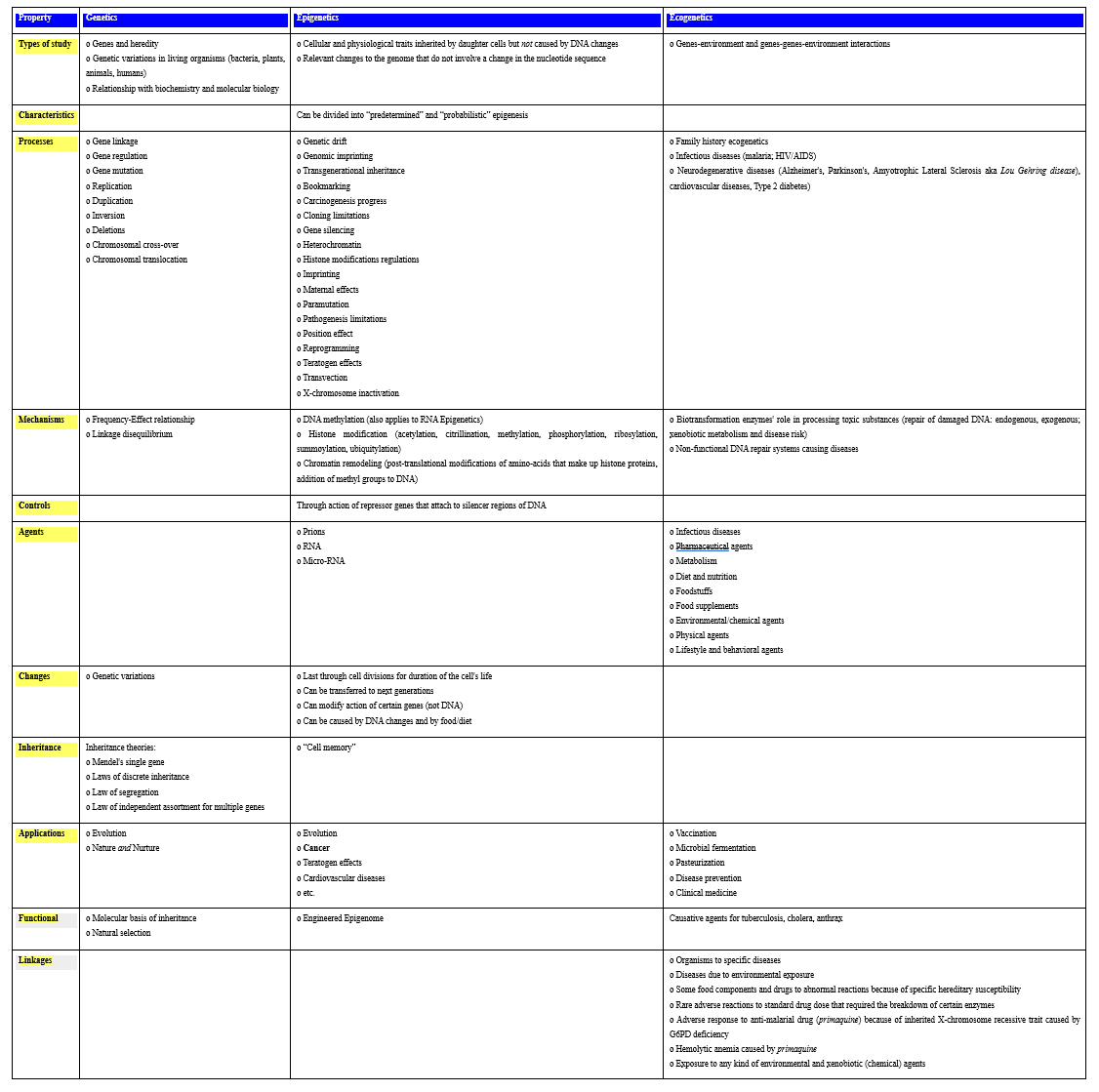
The several epigenetic and ecogenetic processes and mechanisms listed in Table 3 provide tools that will be helpful in the development and implementation of oncoepigenomics and oncoecogenomics.
Cancer Theories and the Road to Cancer's Personalized Treatment
Various theories (hypotheses) of cancer and corresponding therapies were propounded over time:
Blood Suppuration
John Bennett (erroneously) characterized leukemia as a blood suppuration (1845). This was disproved by Michael Anton Bermer who described that illness as neoplasia...not a suppuration of blood (1860). William H. Coley, James Ewing and Ernest Codman treated bone sarcoma with a mixture of bacterial toxins (the so-called “Coley toxin”) but with unpredictable results due probably to immune stimulation, highlighting the important role of the immune system in cancer therapy.
Somatic Mutation
The somatic mutation hypothesis posited that environmental carcinogens (soot, smoke, radium, X-rays, etc.) permanently altered the cell and thus caused cancer (early 20th century).
Viral Propagation
In 1910, experimenting with spindle-cell sarcoma, Peyton Rous concluded that the cause of cancer was not a cell or an environmental carcinogen but rather some tiny particle within a cell...a virus, later called “Rous sarcoma virus” (RSV). As an endogenous causative factor, RSV dealt a deep blow to the exogenous somatic mutation theory. In 1948, Rous reinforced the conclusion of a virus as the cause of cancer and Denis Burkitt concluded that “Burkitt's lymphoma” (an aggressive form of lymphoma) was caused by a human virus: the Epstein-Barr virus (EBV) that is responsible for infectious mononucleosis. The notion that cancer was an infectious disease was then resurrected (as hypothesized in the blood suppuration hypothesis), but this condemned the somatic mutation theory of cancer to its death. By the mid-1970s, a revival of the viral origin theory took place.
Retroviral Propagation
Howard Temin had disproved the central “dogma” (a misnomer at best; rather a hypothesis) of molecular biology which posited a unidirectional flow of information: DNA → RNA (so-called transcription)→ proteins. Rather, viruses possessed the reverse capacity: RNA → DNA → (so-called reverse transcription). Thus, a cancer-causing virus could become a physical part of a cell's genes. This and like viruses (or retroviruses) have genes that exist as RNA outside cells. When these RNA viruses infect cells, they make a DNA copy of their genes and attach this copy to the cell's genes. This DNA copy, called a provirus makes RNA copies, and the virus is regenerated to form new viruses ad infinitum, unleashing pathological mitosis, that is cancer. Unfortunately, retroviruses are not the cause of cancer but of another disease – HIV.
Anti-Vitamin and The Beginnings of Chemotherapy
Before the advent of modern imaging techniques, it was not possible to quantify cancer and measure its progression, the only exception being for leukemia. In leukemia, a simple blood draw and its analysis could inform on the white blood cell count and its changes as a result of drug treatment. Sidney Farber was kindled by two contemporaneous hematological findings; (a) George Minot's discovery that “pernicious” anemia was caused by a lack of vitamin B12 and could be treated by administrating that vitamin, and (b) Lucy Wills' treatment of “Indian-workers” anemia by the vitamin-like folic acid or folate (a crucial DNA building block). He was likewise kindled by the link between vitamins, bone marrow, and normal blood. Unfortunately, neither of these two vitamins could treat cancer (actually, quite the contrary in the case of folic acid) so Farber searched for “anti-vitamins” (which do not exist). Nonetheless, this was the beginning of the search for a chemical to treat cancer, that is “chemotherapy”. In the 1920s, while working for an antidote to the anemia of folate deficiency, Yellapragada Subbarao developed decoy molecular structures that nearly mimicked natural molecules. These structures can bind to enzymes and receptors and block their actions. They behave like antagonists to folic acid (anti-folates),...that is the very anti-vitamins Farber was looking for. In 1947-8, Farber successfully treated, at least temporarily, a patient presenting with acute lymphoblastic leukemia (ALL) with the antifolate drug Aminopterin supplied to him by Subbarao - an unprecedented remission in the history of leukemia. Unfortunately, the remission was short-lived (a few months) and the leukemia came roaring back. This could also truly be thought of as the beginning of chemotherapy – the disappearance (even though temporary) of an aggressive systemic cancer via a chemical drug.
Combination Chemotherapy
Particular combinations of cytotoxic drugs would cure the cancer, which marked a new beginning of chemotherapy. In 1974, the survival rate from metastatic testes cancer was less than 5%. With a three-drug cocktail called BVP (Bleomycin, Vinblastine, Cisplatin abbreviated P for platinum), Larry Einhorn cured this solid cancer. The notion that even relatively indiscriminate cytotoxic agents discovered largely by accident would cure cancer captivated oncology. An avalanche of such drugs poured in: Taxol, Adriamycin, Etoposide, Bleomycin (an antibiotic), and an alphabet soup of other combinations (ABVD, BEP, C-POMP, ChlaVIP, CHOP, ACT). At about the same time, using a high dose combination chemotherapy (a cocktail of seven drugs), Ian Magrath and John Ziegler cured Burkitt's lymphoma. Yet, despite the escalation of drugs and doses, the efficacy of the drug regimen remained minimal. The pattern repeated itself regularly for many forms of cancer. Over the decades that followed, a large array of drugs was synthesized for the fight on cancer. Hodgkin's disease is now cured with multi-drug chemotherapy; locally advanced lung cancer is controlled with the triadic treatment (surgery + chemotherapy + radiation); lymphoblastic leukemia can be induced into a prolonged remission after intensive chemotherapy with cure rates of 80% routinely achieved. The mortality for every form of cancer (lung, breast, colon, prostate, etc.) has continuously dropped for fifteen straight years. For some other cancers (colon, cervix), the decline is almost certainly due to the success of secondary prevention – cancer screening. For leukemia, lymphoma, and testicular cancer, the decline is the result of successful chemotherapeutic treatments. The death rate for breast cancer has been dramatically brought down by the combination (mammography + surgery + adjuvant chemotherapy).
Proto-Oncogene
In the mid-1970s, Harold Varmus and J. Michael Bishop showed that a precursor of a cancer-causing gene - which they called the “proto-oncogene”, was a normal cellular gene existing inside cancer cells. Mutations induced by chemicals or X-rays caused cancer, not by inserting foreign genes, but by activating such endogenous proto-oncogenes. Thus, cancer genes come from within the human genome.
Two-Hit Hypothesis
In the early 1970s, Alfred Knudson proposed the two-hit hypothesis of cancer, initially for retinoblastoma. He proposed that genes come in two flavors: “positive” genes that are mutant-activated versions of normal cellular genes (they accelerate cell division, but only when the cell receives an appropriate growth signal) and “negative” genes that suppress cell division when the cell receives appropriate signals, having been inactivated by mutations. Both abnormalities activate proto-oncogenes and inactivate tumor-suppressors. When this activation/inactivation falls out of equilibrium, cancer then develops.
Metastatic Mechanism
In developing cancer, individuals differ in both their inherited tendency and exposure to the environment, a multi-event combination process. At the cellular level, mutations occasionally occur as the cells divide and, although not heritable by any offspring (somatic mutations), they can affect cell behavior, sometimes causing more frequent growth and division. Normally, cell division responds to growth factors and stops when encountering growth inhibitory signals from surrounding cells. After a number of divisions, the cell dies and remains within the epithelium from which it is unable to migrate to other organs. To become cancerous, it would have to bypass these signals and accumulate new genetic mutations in a number of genes (3-7), the most frequent being a loss of function of the p53 protein (a tumor suppressor) or in the p5 pathway and/or a gain of function through mutations in the protein or in other oncogenes. It will then keep growing, escape from the epithelium and the primary tumor, cross the endothelium of a blood vessel, be transported by the blood stream and colonize (a) new organ(s) to give rise to metastases.
Thus, after a circuitous detour through several hypotheses and theories (elements of which are nonetheless valuable), we have now come to the conclusion that cancer is stitched into our genome; oncogenes arise from mutations in essential genes that regulate the growth of cells and accumulate in these genes when DNA is damaged by carcinogens (theoretically preventable), but also by seemingly random errors in copying genes when cells divide (a flaw deeply entrenched in ourselves and therefore unpreventable). Thus, we can rid ourselves of cancer only in as much as we can rid ourselves of the processes in our physiology that depend on growth – aging, regeneration, healing, and reproduction. It becomes paramount to understand how cancer cells develop drug resistance, a topic I shall address in the next section.
Major Recent Developments in Cancer Treatment
The nine major recent developments in cancer treatment are recounted below:
Nanochemotherapy
Nanochemotherapy (NCT) uses Nanodevices (NDs) to deliver Nanoparticles (NPs) containing cytotoxic drugs to tumors. NPs include nutshells (120 nm in diameter coated with gold), platelet-coated NPs (~ 100 nm in diameter), gelatin NPs for delivering multiple drugs to the brain, shape-shifting engineered NPs, bioavailability-improved NPs and molecules, and others. The basic process to use drug delivery here involves at least three steps: (a) encapsulation of the drugs; (b) successful delivery of said drugs to the targeted region of the body; and (c) successful release of that drug there. The drugs are delivered employing engineered NDs. Because of their diverse capabilities, NDs can contain both targeting and therapeutic agents (in both single and multi-drug approaches). They can deliver high drug levels in several situations, including anticancer drugs at the tumor site that can increase chemotherapeutic efficacy. They can also be “smart" nanotherapeutics to "time" the release of any given drug or to deliver multiple drugs sequentially in a timed manner or at several locations in the body. There are numerous clinical advantages:
- The nNPs circulate throughout the bloodstream without being attacked by the immune system;
- They preferentially bind to damaged blood vessels and certain pathogens such as MRSA (Methycillin Resistant Staphylococcus Aureus) bacteria, allowing them to deliver and release their drug payloads specifically to these body sites in the body;
- They are non-toxic as the platelet membranes are nanoparticle cores made of a biodegradable polymer that can be safely metabolized by the body; and
- They can be packed with many small drug molecules that diffuse out of the polymer core and through the platelet membrane onto their targets.
Innate Immunotherapy with Neutrophil-Mediated Drug Delivery
A neutrophil-mediated anticancer nanotechnological drug delivery has been proposed for the suppression of postoperative malignant glioma recurrence. Neutrophils are white blood cells in the granulocytic series of blood cell development. Formed by myelopoietic tissue of the bone marrow and released into the circulating blood, they can penetrate inflamed brain tumours and, although they are not typically attracted to glioblastomas (GBMs), they are recruited at sites where tumors had been removed in response to post-operative inflammation. Liposome capsules have been developed that encased Paclitaxel (PTX), a traditional chemotherapy drug, with lipids, loaded into neutrophils and injected in the blood. The neutrophil-carrying drugs are able to penetrate the blood-brain barrier (BBB), destroy residual cancer cells, and slow the growth of new tumors. Inflammatory factors released after tumour resection guide the movement of the neutrophils into the inflamed brain. The highly concentrated inflammatory signals in the brain trigger the release of liposomal PTX from the neutrophils, allowing delivery of PTX into the remaining invading tumour cells. This neutrophil-mediated delivery of drugs efficiently slows the recurrent growth of tumours, significantly improving survival rates, but does not completely inhibit the regrowth of tumours. While tumor recurrence is not completely prevented, overall, this treatment prolongs life significantly. The technique can be used in other inflammation-mediated disorders and any other diseases that naturally attract neutrophils.
Synthetic Immunotherapy
Immunotherapy represents a paradigm shift in cancer treatment in that it targets the immune system, not the cancer itself. In 2013, the Science magazine declared it as that year's breakthrough! Nonetheless, today's immunotherapies do not help everyone (e.g., the odds remain long for patients with metastatic cancer) and biomarkers that might offer answers remain to be designed as well as experimenting with ways to make therapies more potent. In clinical trials, new immune system-boosting cancer drugs have saved lives in seemingly untreatable melanoma or lung cancer cases, but the drugs seem useless against colon cancer. Nonetheless, even cancers impervious to the new drugs (3-4%) could be treated if those malignancies have the right error-riddled DNA signature. The idea of boosting the body’s own defenses is elegant and appealing. Further, once their task completed, the engineered cells remain in the body, offering protection against recurrence or re-infection for years to come. Immunotherapy can use either natural or synthetic chimeric antigen receptors (CAR). We distinguish the following:
1. PD-1 inhibition: Tumor cells hide from T-cells by activating PD (programmed-death)-1 receptors on the surface of the immune system's T- cells and can attack them if the receptors are blocked by PD-1 inhibitors. There are two new types of drugs (including Pembrolizumab [Keytruda]) to harness the immune system, keeping tumors at bay for years. In clinical trials, they generally have worked in less than half the cases, and work best on tumors with lots of Advanced cancers seem more likely to respond if they have so-called mismatch repair mutations, which explains why the best outcomes have been for the heavy mutation tumors of lung cancer and melanoma. The hypothesis is that some of these mutations may alter genes to code for abnormal (or foreign) proteins or antigens so that the more of them, the more antigens to launch an attack from T-cells unleashed by a PD-1 inhibitor. Clinical trials found that mutations in mismatch repair genes can lead to cancer-promoting mutations and would respond to PD-1 inhibitors. In the case of few mutations, one implication is that the tumors might respond better to PD-1 inhibitors if they first receive radiation or chemotherapy that create new mutations.
2. Chimeric Antigen Receptor T-cells: Combining gene therapy, synthetic biology, and cell biology, engineering T-cells involves the following steps: (a) Extracting from the blood T-cells known to respond best to a given disease; (b) implanting them with new genes using a custom-built virus; (c) creating cells that target a molecule (CD19) found on surfaces of few cancers; and (d) returning to the body the modified cells where their new DNA gives them a fresh set of targets to attack. The technique can be refined by overcoming the treatment's toxic effects that may occur in most advanced cancers. Here, a runaway reaction (called a cytokine storm) can be fatal so the dose is brought down to its lowest to reduce this immune system's overreaction. Unfortunately, besides CD19, we know of no other chemical target that is specific to cancer alone. A modified technique has been proposed in which cells are tweaked to attack when they sense not one but two different target chemicals. The idea is that whereas neither target may be unique, the combination might be, allowing the immune system to be unleashed on tumors whilst sparing healthy tissue. Engineered T-cells (and perhaps also B-cells, another part of the immune system) might be used to treat a wide range of diseases besides cancer, including HIV, immune deficiencies, and autoimmune disorders.
DNA Origami/Trojan
In the original origami technique, to foil drug resistance in solid tumors, a cancer drug is packaged in a capsule made of folded-up DNA. A chemotherapeutic drug [Daunorubicin in the case of Acute Myeloid Leukemia (AML)] upon entering cancerous cells recognizes them and pumps them back out through openings in the cell wall. In its refined version, the genome of a common bacteriophage and synthetic strands that were designed to fold up its DNA are encapsulated and do not encode any proteins or do any of the normal DNA functions. Potentially, the technique should work on most any form of drug-resistant cancer.
mnk-2 Conversion to Overcome Drug Resistance
Breast, lung and colon cancer cells change the structure of the enzyme mnk-2, which is involved in the transmission of information from the environment/body into the cell. This enzyme is binary with a “normal” form that inhibits cancer development and an “abnormal” form that promotes it. The balance between these two forms will determine whether the cancer is arrested or promoted. To overcome drug resistance, molecules have been developed that can convert the abnormal to the normal form of mnk-2. The underlying mechanism elucidates how cancer cells eliminate the anti-cancer form, and provides a means to reverse it.
Antiangiogenesis
Cancer cells hijack and feed off blood vessels. In the brain, this results in a weakened BBB and this may provide one reason for the rapid spread of GBMs. This observation may lead to new ways to kill brain tumors using the BBB weakness to get targeted drugs into the brain during the early stages of the cancer. Outside the main tumor mass, nearly all GBM cells gather in the space between the astrocytic end feet and the outer surface of blood vessels. The cancer cells use the network of small blood vessels as a scaffold to guide their migration along the blood vessels as they extract nutrients from the blood inside. The GBM cells hijack control over blood flow in the BBB away from the astrocytes, loosening the tight junctions, and resulting in a breakdown in the barrier. Very small groups of GBM cells – even individual cells – could weaken the BBB in the early stages of the disease. At an early stage of the disease, as they invade the blood vessels, the tumor cells are not completely protected by the BBB, thus making them more vulnerable to targeted drugs delivered via the bloodstream to the brain. If the above findings hold true in humans, treatment with anti-invasive agents might be beneficial in newly diagnosed GBM patients.
Self-Eradication During Meiosis
During anaphase in mitosis, an “inherent death mechanism” can self-eradicate duplicating cancer cells without impairing healthy cells in both normally and rapidly proliferating human cancer cells. The faster cancer cells proliferate, the faster and more efficiently they will be eradicated. The mechanism involves the modification of specific proteins that affect the construction and stability of the spindle - a newly discovered mechanism that is able to arrest cancer cells from dividing and multiplying, thus stopping cancer progression in its track. It may be suitable for treating aggressive cancers that are not responsive to traditional chemotherapy. Three proteins can be specifically modified during mitosis to unleash an “inherent death mechanism”. Certain compounds (the Phenanthridine derivatives) are able to impair the activity of these proteins, distorting the spindle structure and preventing the segregation of chromosomes. Once the proteins are modified, the cells are prevented from dividing, inducing the cell’s rapid self-destruction and apoptosis. A variety of additional drugs that also modify these specific proteins could be developed.
Inflammation
Many malignancies arise in areas of chronic inflammation and inadequate resolution of inflammation could have a major role in tumor invasion, progression, and metastasis although it may not play a role in oncogenesis. Inflammation is of particular pathophysiological relevance in lung cancer in that chronic bronchitis, triggered by asbestos, silica, smoking, and other external inhaled toxins, results in a persistent inflammatory response. Inflammation in the tumor microenvironment mediated by interleukin IL-1β has a major role in cancer invasiveness, progression, and metastasis. This, in turn, suggests that IL-1β participates in the invasiveness of already existing malignancies. Thus, inhibition of IL-1β might have an adjunctive role in the treatment of cancers that have at least a partial inflammatory basis. Inhibition of IL-1β with the monoclonal antibody Canakinumab is associated with reduced incidences of fatal cancer, lung cancer, and fatal lung cancer. Anti-inflammatory therapy with Canakinumab targeting the IL-1β innate immunity pathway could significantly reduce incident lung cancer and lung cancer mortality.
Electrochemotherapy
Electrochemotherapy (ECT) delivers non-permeant drugs (e.g., Bleomycin) or low-permeant drugs (e.g., Cisplatin, an alkalyting agent) to the cell interior. The technique is based on the local application of short and intense electrical pulses that transiently permeabilize the cell membrane, thus allowing the transport of chemotherapeutic molecules through it (a phenomenon called electroporation or electropermeabilization). The pulse characteristics (amplitude, phase, pulse duration and repetition frequency, DC- or AC-currents, shape and position of the electrodes) are chosen depending on the tissues; however, the pulse amplitude has to be high enough to establish a 400 Volt/cm electrical field in the tumor area and yield eight 100 ms pulses at a 5000 Hz repetition frequency for patient comfort and shorter treatment duration. For deep-seated tumors in the relative vicinity of the heart, pulses are synchronized with the absolute refractory period of each heart beat so as to minimize the probability of their interaction with the heart function. The drugs can then freely diffuse into the cytoplasm and exert their cytotoxic effect. ECT has been widely used for cutaneous and subcutaneous tumors or their metastases, yielding an objective response rate > 80% and, when compared to standard chemotherapy, a faster and more efficient tumor size reduction. Patients with skin metastasis from melanoma, Kaposi sarcoma, squamous cell carcinoma, basal cell carcinoma, adenocarcinoma, or breast cancer have been successfully treated. ECT employs lower dosages of chemotherapeutic drugs than standard chemotherapy protocols resulting in lower patient's burden and more limited side effects.
With the momentous advances of the Human Genome Project (HGP) and its sequel the Human Cancer Genome Project (HCGP), we have now come to the realization that understanding more intimately the biology of cancer, gene by gene and pathway by pathway, will direct us into the right direction for cancer therapeutics. A second direction would obviously be cancer prevention, or at least prevention of those factors that may trigger cancer. The third and last direction would be to integrate our understanding of aberrant genes and pathways to explain the behavior of cancer. This would lead to a personalized approach to cancer treatment along the lines advocated for a new paradigm in medicine and health care, namely, personalized medicine. This brings us to “oncogenomics” as the newest approach to cancer treatment.
The Oncogenomic Approach
With the above in mind, we are now searching for all genomic processes and pathways that could evidence actionable treatment indicators utilizing a multi-drug chemotherapeutic tool. Unfortunately, as a sequel to various chemotherapies, patients develop resistance to drugs and therapies and, left without options, those who relapse enroll in a succession of generally unsuccessful clinical trials because they lack a clear biological rationale to guide them. Additionally, other cancer theories and therapies continue to be proposed.
Research about cancer causes now focuses on the following issues:
- What are the agents (e.g., viruses) and events (e.g., mutations) that cause or facilitate genetic changes in cells destined to become cancerous?
- What is the precise nature of the genetic damage, and the genes that are affected by it? And
- What are the consequences of those genetic changes on the biology of the cell, both in (a) generating the defining properties of a cancer cell, and (b) facilitating additional genetic events that lead to further progression of the cancer?
We distinguish between solid and non-solid (e.g., blood) tumors. In the former case, immunotherapy with autologous CAR-T cells engineered to target the appropriate cancer cells is employed. Standard DNA tests are conducted to zoom in on specific loci in the cancer cells' genome. Such DNA tests have been useful in identifying “actionable mutations”, that is, those that indicate the cells could be vulnerable to a particular drug. The idea of basing cancer treatments on DNA mutations is not new although it can be difficult to sift out “actionable findings” from DNA sequencing data. In non-solid tumors (e.g., myeloma, a blood cancer): autologous CAR-T cells engineered to target RCMA (a protein on the surface of myeloma cells) did not succeed. Research on multiple myeloma has lagged behind solid tumors in trying to implement a personalized approach and incorporating genomics data in the treatment.
Problems with multiple myeloma are multiple in that pathology reports do not identify clearly the drivers of the cancer. In addition, DNA-sequencing does not always give a clue as to how to manage the cancer. Possible solutions are then RNA-sequencing to have a peek not just at gene mutations, but also at other changes in the cancer cells (example: copy number variations, CNV) that might be treatment-relevant. The most recent application of genomics to cancer treatment looking beyond DNA has been conducted for multiple myeloma by a New York Mt Sinai team of researchers (Parekh, Perumal et al.). Their trial was limited and included 64 of the hospital's patients who had relapsed or not been responding to standard treatments. Cancer cells' DNA and messenger RNA were sequenced and searched for anything that might respond to any of the approved cancer drugs (even if not for multiple myelomas). Only 26 subjects received the recommended personalized treatment (other patients have died or enrolled in other drug trials, and for one patient no drug could be recommended). Out of the 21 evaluable subjects, 16 responded to treatment with the 5-drug cocktail (DCTVI), one of whom experienced complete remission, and 5 had side effects (fatigue or diarrhea). [DCTVI stands for Dexomethasone (an anti-inflammatory corticosteroid) + Carfizomib (a myeloma treatment) + Trametinib (a myeloma treatment) + Venetoclax (approved for chronic lymphocytic leukemia) + Ibrance (a breast cancer drug)]. The other 5 patients did not stay on the recommended treatment for long enough or did not complete the scans or/and the tests needed for evaluation.
Oncoepigenetics and Oncoecogenetics
Like for oncogenomics, the approach would be to sequence the epigenome and the ecogenome of the patient in order to evidence epigenetic and ecogenetic actionable treatment indicators (epiATIs and ecoATIs, respectively).
Actionable Treatment Indicators in the Epigenome
These are:
- Gene expression changes in the cancerous tissue: These can be analyzed by quantitative polymerase chain reaction (qPCR) wherein both positive and negative control PCR primer pairs are included to determine the fold enrichment, the latter amplifying a region of the genome not bound by the antibody target of interest. There are commercially available analysis kits (particularly the ones developed by the U.S. company ActiveMotif.)
Further, referring to Figure 1, other epiATIs are:
- Changes in DNA Methylation: as evidenced by methyl groups that have tagged DNA and activated or repressed genes. These can be shown, in particular, by enrichment followed by PCR analysis.
- Epigenetic factors that bind to histone tails; these alter the extent to which DNA is wrapped around histones, thus dictating the availability of genes in the DNA to be activated.
- Changes in chromatin structure: These can be shown by chromatin immunoprecipitation (ChiP) whose success depends on the quality of the ChiP antibody and the abundance of the target protein. Because it enables identification of the localization of proteins bound to specific DNA loci, ChIP is a powerful tool for studying protein/DNA interactions. Applications include:
- Transcription factors (TF: these are the regulatory proteins that bind to DNA to either promote or inhibit the transcription of a gene);
- Co-regulatory proteins;
- Modified histones;
- Chromatin-modifying enzymes; and
- Polymerases
When used in combination with whole-genome analysis, insights are possible into gene regulation, gene expression, mechanisms of chromatin modification and pathway analysis.
Actionable Treatment Indicators in the Ecogenome
These are the various chemical/environmental agents and the lifestyle & behavioral agents listed in Table 3. A person's likelihood of developing a particular cancer is determined largely by a complex interplay of risk factors involving environmental exposures, lifestyle factors, and inherited susceptibility. The challenge is to understand in sufficient detail how these specific factors interact in causing (or preventing) these cancers, so that more effective prevention and early detection programs can be developed and applied toward persons at highest risk of the disease. (Further, let us recall here, this author's admonition that “risk is not cause, and risk management is not cure, only palliation”.)
Manipulations of epigenetic mutations hold great promise for cancer prevention, detection, and therapy. Several medications which have epigenetic impact are now used in several of these diseases.
Current research has shown that epigenetic pharmaceuticals could be a replacement or adjuvant therapy for currently accepted treatment methods such as radiation and chemotherapy, or could enhance the effects of these current treatments. It has also been shown that the epigenetic control of the proto-onco regions and the tumor suppressor sequences by conformational changes in histones directly affects the formation and progression of cancer. Epigenetics also has the factor of reversibility, a characteristic that other cancer treatments do not offer.
Applications of epigenetics to cancer sub-types (cervical, leukemia, prostate, sarcoma, etc.) will not be discussed here. More epigenetic aspects remain to be studied such as functional epigenomics (or the engineering of the epigenome), RNA and beyond RNA-epigenetics. Such a discussion would go far beyond the scope of this Chapter and will not be presented here.
Summary and Conclusions
Africa is witnessing significant improvements in population health as witnessed by the declining infant mortality rates, plummeting HIV/AIDS fatality rates, rising life spans, and falling burden of communicable diseases. At the same time, morbidity and mortality from non-infectious diseases have been rising and cancer has become the fifth leading cause of death in Africa. From the GLOBOCAN 2020 data, it has been projected that by 2040 all neoplasms in that continent will increase to 2.1 million new cases and 1.4 million deaths. Cancer has not been cured, not in developed countries and even less in Africa despite more than a four-decade full-fledged “war” against the disease. Fortunately, with the more recent deeper understanding of cell biology, genetics, epigenetics, and ecogenetics, it now appears that cancer is less an organ disease and more a disease of molecular mechanisms caused by mutations of specific genes.
Brief primers on cancer, epigenetics, and ecogenetics have served as a background for the study and inter-comparison of the genetics, epigenetics, and ecogenetics of cancer. Likewise, a review of the theories of cancer, associated recent developments as well as the study of the oncoepigenomcs and oncoecogenomics have begun to evidence specific epigenetic and ecogenetic actionable treatment indicators eading the road to personalized cancer therapy. Other steps forward would consist in investigating other genomic information including the transcriptome and reverse transcriptome, and mechanisms of genetic changes. Whereas this application may be time and resource prohibitive, its process could and should be accelerated.
There are various applications of epigenetics in medicine and drug development, of particular importance in cancer and cancer treatment. Epigenetic pharmaceuticals could be a replacement or adjuvant therapy for currently accepted treatment methods such as radiation and chemotherapy, or could enhance the effects of these current treatments. The epigenetic control of the proto-onco regions and the tumor suppressor sequences by conformational changes in histones directly affects the formation and progression of cancer. Epigenetics also has the factor of reversibility, a characteristic that other cancer treatments do not offer. Ddiscussions of drug developments, applications to cancer sub-types, functional epigenomics (or epigenome engineering), and RNA and beyond RNA-epigenetics remain to be fully investigated in the future.
References
- Berger SL, Kouzarides T, Shiekhattar R, Shilatifard A. “Epigenetics as the Stably Heritable Phenotype Resulting from Changes in a Chromosome Without Alterations in the DNA Sequence”, 2009.
- Bishop JB, Witt KL, Sloane RA. “Genetic Toxicities of Human Teratogens”, Res, 1997; 396(1–2): 9–43. doi:10.1016/S0027-5107(97)00173-5. PMID 9434858.
- Egger G, Liang G, Aparicio A, Jones PA. “Epigenetics in Human Disease and Prospects for Epigenetic Therapy”, Nature, 2004; 429(6990): 457–463. Bibcode:429..457E. doi:10.1038/nature02625. PMID 15164071.
- Feinberg AP, Tycko B. “The History of Cancer Epigenetics”, Nature Reviews: Cancer, 2004; 4(2): 143–153. doi:1038/nrc1279. PMID 14732866.
- Friedler G. “Paternal Exposures: Impact on Reproductive and Developmental Outcome: An Overview”, Biochem. Behav, 1996; 55(4): 691–700. doi:10.1016/S0091-3057(96)00286-9. PMID 8981601.
- “Contributors to Sickness: Epigenetic Regulators of Many Gene Expressions”, presented at the 2nd International Scientific Conference of the Society for the Advancement of Science in Africa, Polokwane, South Africa, 2013.
- Fymat AL. “The Long Quest for Cancer Cures”, J Cancer Prev Curr Res, 20161; 6(2): 00201. doi: 10.15406/jcpcr.2016.06.00201.
- Fymat AL. “Recent Research Developments in Anti-Cancer Therapy”, J Cancer Prev Curr Res, 2016b; 5(2): 00155. doi: 10.15406/ jcpcr.2016.05.00155.
- Fymat AL. “Recent Developments in Nanomedicine Research”, J Nanomed Res, 2016c; 4(4): 00096. doi: 10.15406/jnmr.2016.04.00096.
- Fymat AL. “Nanotechnology and Cancer”, J Cancer Prev Curr Res, 2016d; 5(6): 00180. doi: 10.15406/jcpcr.2016.05.00180.
- Fymat AL. “Immunotherapy: An Emergent Anti-Cancer Strategy”, J Cancer Prev Curr Res, 2017a; 7(3): 00233. doi: 10.15406/jcpcr.2017.07.00233.
- Fymat AL. “Synthetic Immunotherapy with Chimeric Antigen Receptors”, J Cancer Prev Curr Res, 2017b; 7(5): 1-3. 00253: doi: 10.15406/jcpcr.2017.07.00253.
- Fymat AL. “Nanochemotherapy: An Emergent Anti-Cancer Modality”, Global J Nanomed, 2017c; 1(1): 555555.
- Fymat AL. “On Cancer Electro- and Nano-Chemotherapy”, J Cancer Prev Curr Res, 2017d; 7(2): 00232. doi: 10.15406/jcpcr.2017.07.00232.
- Fymat AL. “Antiangiogenic Targeting of Early Developing Glioblastoma Behind a Weakened Blood-Brain Barrier”, Journal of Anti-Tumor Medicine & Prevention, 2017e; 2(3): 1-6.
- Fymat AL. “Genetics, Epigenetics and Cancer”, Cancer Therapy & Oncology International Journal, 2017f; 4(2): 1-11. doi:10.19080/CTOIJ.2017.04.555634.
- Fymat AL. “Disrupting Cell Mitoses to Provoke Cancer Self-Destruction, Cancer Therapy & Oncology International Journal, 2017g; 5(1): 1-4. doi:10.19080/CTOIJ.2017.05.555655.
- Fymat AL. “On Cancer's Theories, True Nature, and Possible Self-Eradication”, Cancer Therapy & Oncology International Journal, 2017h; 7(2): 1-3. doi:10.19080/CTOIJ.2017.07.555709.
- Fymat AL. “On the Inflammation Theory of Cancer”, Cancer Therapy & Oncology International Journal, 2017i; 8(3): 1-7. doi:10.19080/CTOIJ, 2017.08.555740 (19 December 2017).
- Fymat AL. “Anti-Tumor Therapies: Cases of Breast and Prostate Cancers, Journal of Tumor Medicine & Prevention, 2017j; 1(2): 1-12.
- Fymat AL. “Glioblastoma Treatments: Where Do We Stand?”, MedPlus Journal of Cancer & Oncology Research, 2017k; 1(1): 1-12.
- Fymat AL. “Surgical and Non-Surgical Management and Treatment of Glioblastoma: I. Primary Tumors, Open Access Journal of Surgery, 2017l; 7(2): 1-8. doi: 10.19080/OAJS.2017 07 555706.
- Fymat AL. “Surgical and Non-Surgical Management and Treatment of Glioblastoma: II. Recurring Tumors”, Open Access Journal of Surgery, 2017m; 7(1): 1-7. doi: 10.19080/OAJS.2017.07.555703.
- Fymat AL. “Cancer Therapy with Chimeric Antigen Receptors – A Landmark Moment for Cancer Immunotherapy”, J of Cancer Prevention & Current Res, 2017n; 8(6): 1-7. doi: 10.15406/jcpcr.2017.08.00300.
- Fymat AL. “Innate Immunotherapy of Recurring Glioblastoma: Preliminary Trials with Neutrophils”, Journal of Current Opinions in Neurological Science, 2018a; 2(3): 480-482.
- Fymat AL. “Harnessing the Immune System to Treat Cancers and Neurodegenerative Diseases”, J of Clinical Research in Neurology, 2018b; 1(1): 1-14.
- Fymat AL. “Cancer: The clonally-evolving disease braided in our genome”, Tellwell Talent Publishers, 2021; pp 334. ISBN: 10-0228848857 (hardcover); -13:978-0228848851
- Glasspool RM, Teodoridis JM, Brown R. “Epigenetics as a Mechanism Driving Cancer”, British Journal of Cancer, 2006; 9(8): 1087–1092. doi:1038/sj.bjc.6603024. PMC 2361257. PMID: 16495912.
- Hanahan D, Weinberg R. “The Hallmarks of Cancer”. JCO (2018). Precis Oncol, 2001; doi:10.1200/PO.18.00019
- Jones PA, Baylin SB. The Fundamental Role of Epigenetic Events in Cancer, Nature Reviews Genetics, 2002; 3(6): 415–428.
- Karni, et al. “Structure Changes in the mnk-2 Enzyme and Patient Response to Chemotherapy”, Hebrew University Medical Center, Jerusalem, Israel, 2015.
- Novak K. “Epigenetic Changes in Cancer Cells”, Medscape General Medicine, 2004; 6(4): 17. PMC: 1480584. PMID: 15775844.
- Parekh S, Perumal D. “Omics-ing Cancer”, The Scientist, 2018; 16-17.
- Riggs AD, Russo VEA. Marthiessen RA. “Epigenetics as the Study of Mitotically and Meiotically Heritable Changes in Gene Function that Cannot be Explained by Changes in DNA Sequence”, 1996.
- Sharma R, Aashima, Nanda M, Fronterre C, Sewagudde P, Ssentongo AE, Yenney K, et al. “Mapping Cancer in Africa: A Comprehensive and Comparable Characterization of 34 Cancer Types Using Estimates from GLOBOCAN 2020”, Front. Public Health, 2022. https://doi.org/10.3389/fpubh.2022.839835.
- Spannhoff A, Sippl W, Jung M. “Cancer Treatment of the Future: Inhibitors of Histone Methyltransferases”, Int. Biochem. Cell Biol, 2009; 41(1): 4–11. doi:10.1016/j.biocel.2008.07.024. PMID 18773966.
- Wong NC, Craig JM. “Epigenetics: A Reference Manual.” Norfolk, England: Caister Academic Press, 2011. ISBN 1-904455-88-3.

Subscribe to newsletter
© 2020. All rights reserved.
- Scott RB, Robb-Smith A. "Histiocytic medullary reticulosis." The Lancet, 1939; 234(6047): 194-198
- Ishii E, et al. "Nationwide Survey of Hemophagocytic Lymphohistiocytosis in Japan." International Journal of Hematology, 2007; 86(1): 58-65.
- Meeths M, Bryceson YT. "Genetics and pathophysiology of haemophagocytic lymphohistiocytosis." Acta Paediatrica, 2021; 110(11): 2903-2911.
- Brissmophagocytic Lymphohistiocytosis: Experiences From 180 Patients with Severe Dengue, Clinical Infectious Diseases, 2020; 70(11): 2247–2255.
