Oxidative Stress Markers and Lipid Profile Among Female Residents in Oil Impacted Communities of Rivers State, Nigeria
Blessing Dum-Awara L1, Solomon M Uvoh1,*, Emily Kiridi Ge2 and Onokpite Emmanuel3
¹Department of Human Physiology, Faculty of Basic Medical Sciences, College of Health Sciences University of port Harcourt, Nigeria
2Department of Human Physiology, Faculty of Basic Medical Sciences, College of Health Sciences Niger Delta University, Nigeria
3Department of Anaesthesia and Intensive Care, Faculty of Clinical Sciences, College of Medicine, Delta State University Abraka, Nigeria
Received Date: 03/04/2023; Published Date: 06/07/2023
*Corresponding author: Dr Solomon M Uvoh, Department of Human Physiology, Faculty of Basic Medical Sciences, College of Health Sciences University of port Harcourt, Rivers State, Nigeria
Abstract
This study examined serum oxidative stress markers such as glutathione (µg/ml), Malondialdehyde (nmol/ml), duration of exposure to petroleum impacted environment and lipid profile among female subjects between the ages of 18-50yrs in Khana, Gokana local government area of Rivers state as test group and Ogoja, Boki in Cross rivers state as control group in Nigeria. A total of 244 subjects (123 as control and 125 test) make up the study population groups. The results from this study indicate significant (p-value<0.05) increase in glutathione and Malondialdehyde (0.69µg/ml and 4.29nmol/dl) among the test group compared with the control of (0.17µg/ml and 0.84nmol/dl). With regards to the duration of exposure to petroleum impacted environment in years, the subject’s residents of stay between 41-50yrs had significant higher glutathione and Malondialdehyde ((0.82µg/ml and 4.83nmol/dl) among test group compared with control (0.16µg/ml and 0.86nmol/dl) group. Further findings from this study reveal an increase in the lipid profile ie serum total cholesterol (109mg/dl), triglycerides (59.42mg/dl), HDL (31.67mg/dl), LDL (65.93mg/dl) among test group compared with the control (71.53, 35.88,28.63, 35.72 mg/dl) group respectively. A similar trend of significant increase in lipid profile among test group subjects exposed between 41-40yrs was observed except in low density lipoprotein compared with the control. Findings from this research study have shown a true reflection of imbalance between free radicals and antioxidants (oxidative stress) among female residents in petroleum exploration environment which could leads to damages of tissues and organs resulting in various diseases whose immediate aetiology may not be fully known during their initial visitation to the hospital.
Keywords: Low density lipoprotein; High density lipoprotein; Oxidative stress, Triglycerides; Total cholesterol; Malondialdehyde; Glutathione
Introduction
Low-density lipoprotein is a major lipoprotein subgroup that carries fat molecules and extracellular water (CDC, 2017). It provides fat molecules to cells and is oxidized in arterial walls of atherosclerosis. The size of an individual LDL particle varies on the kind and number of fat molecules it carries, and it transports between 3,000 and 6,000 fat molecules per particle. (Segrest et al., 2001). The two sizes of LDL particles are pattern A, which are large and low density, and pattern B, that are tiny and high density. Pattern B connects a higher risk of coronary heart disease [1]. In one research, LDL diameters 20.6-22 nm classified pattern A, whereas sizes 19.0-20.5 nm classifies as pattern B. (Bhalodkar et al., 2005). Some research suggested that the correlation between Pattern B and coronary heart disease is stronger than correlation between the LDL readings obtained from conventional lipid profile test.
Oxidized LDL used to describe LDL particles with structural components that have undergone oxidation. Free radical damage can cause the lipid and protein parts of LDL to oxidize in the vascular wall. Dietary oxidized lipids may potentially be the source of LDL oxidized lipids in addition to the oxidative processes taking place in the artery wall (Ahotupa, 2017). The atherogenicity of oxidized LDL has been attributed to the LDL receptors' inability to recognize oxidation-modified LDL structures, which hinders the normal metabolism of LDL particles and eventually leads to the development of atherosclerotic plaques (Stocker, Roland; Keaney and John, 2004). The final atherogenic species of the lipid content in LDL are a variety of lipid oxidation products (Birukov, 2006). Another way that LDL might raise the risk of atherosclerosis is by serving as a transporter of these harmful chemicals (Shao and Heinecke, 2009).
Triglyceride: Triglycerides are the primary components of body fat in humans and other vertebrates, as well as vegetable fat (Nomenclature of Lipids 2007). Triglycerides are ester produced from glycerol and fatty acids [2]. The key component of human skin oils and is present in the blood to allow the bidirectional transference of adipose fat and blood glucose from the liver [3].
Triglycerides are broken down in the stomach into monoacylglycerol and free fatty acids, a process known as lipolysis. The absorptive enterocyte cells that line the gut get them next. In the enterocytes, the triglycerides are pieced back together and then bundled with proteins, cholesterol, and cholesterol to form chylomicrons. These are evacuated by the cells, collected by the lymphatic system, transported to the large blood veins nearby the heart, and then mixed with the blood. Liver cells have the capacity to make and store triglycerides. The hormone glucagon triggers hormone-sensitive lipase to break down the triglycerides to liberate free fatty acids when the body requires fatty acids as a source of energy. Since fatty acids are not utilised by brain as an energy source (White and Balasubramanian 2011). The glycerol component of triglycerides is broken down into dihydroxyacetone phosphate, which is converted into glyceraldehyde 3-phosphate, and used as a source of glucose for brain energy. Cell membranes are impermeable to triglycerides. Triglycerides must be converted into free fatty acids and glycerol by lipoprotein lipases, specialized enzymes found on the walls of blood arteries. After then, cells may absorb fatty acids through the Fatty Acid Transporter (FAT).
Triglycerides serve as energy sources and transporters for dietary fat in the body. High blood triglyceride levels in humans are linked to atherosclerosis, heart disease, and stroke [4].
Total cholesterol: Cholesterol is an organic substance known as sterol and is a kind of lipid. It is an essential component of the structural makeup of animal cell membranes. With the aid of cholesterol, bile acid, vitamin D, and steroid hormones may all be biosynthesized [5]. The stomach has a difficult time absorbing cholesterol since it gets esterified when swallowed. Compensate for the absorption of dietary cholesterol, the body also reduces the creation of its own cholesterol [6]. Cholesterol makes up about 30% of the membranes of animal cell membranes. One of the Physiologic functions of cholesterol is its role in forming many hormones to keep the cell membrane insoluble in water and in forming bile salts [7]. Creation and upkeep of membranes regulates their fluidity over the range of physiological temperatures. Each cholesterol molecule's hydroxyl group, together with the polar heads of the membrane's phospholipids and sphingolipids, interacts with the water molecules around the membrane. Cholesterol alters membrane fluidity, maintains membrane integrity so that animal cells do not need to be built with cell walls, and increases membrane packing by interacting with phospholipid fatty-acid chains [8]. The membrane is stable, durable without being rigid, enabling animals to change their shape and move. Because the molecule is Trans conformation, which renders all cholesterol stiff and planar except for the side chain, the structure of the cholesterol's tetracyclic ring contributes to the fluidity of the cell membrane (Ohvo-Rekilä et al., 2002).
Cholesterol also lowers the plasma membrane's permeability to neutral solutes like sodium ions and hydrogen ions [9].
According to Harvard Health Publishing, (2018), about 80% of cholesterol generated is produced in the liver and intestines.
High-Density Lipoprotein (HDL)
The five main forms of lipoproteins include high-density lipoprotein (HDL) (CDC, 2017). HDL particles remove fat molecules from cells. Triglycerides, phospholipids, and cholesterol are among the lipids transported; the quantities of each vary [10]. HDL particles are known as "good cholesterol." Because HDL has the largest protein to lipid ratio among the lipoprotein particles, it is also the smallest and densest. It contains the two apolipoproteins A-I and A-II most often. It has been shown that the uncommon genetic variation ApoA-1 Milano can improve the prevention and treatment of atherosclerosis, an arterial disease.
The liver produces these apolipoprotein and phospholipid complexes, which resemble cholesterol-free flattened spherical lipoprotein particles [10]. To intercept internal cholesterol carried by cells, the complexes interact with the ATP-binding cassette transporter A1 (ABCA1) [11]. Free cholesterol is changed by the plasma enzyme Lecithin-Cholesterol Acyltransferase (LCAT) into cholesteryl ester, which is then trapped inside the core of the lipoprotein particle, giving the newly created HDL a spherical form. For instance, by interacting with the ABCG1 transporter and the phospholipid transport protein (PLTP), HDL particles may take up more cholesterol and phospholipid molecules from cells and other lipoproteins as they circulate through the blood [10].
Through both direct and indirect channels, HDL mostly delivers cholesterol to the liver or steroidogenic organs such the testicles, ovary, and adrenals. Scavenger receptor BI (SR-BI), which permits the selective absorption of cholesterol from HDL, is one of the HDL receptors that destroy HDL. For instance, by interacting with the ABCG1 transporter and the phospholipid transport protein (PLTP), HDL particles may take up more cholesterol and phospholipid molecules from cells and other lipoproteins as they circulate through the blood [10].
Additionally, negligible quantities of HDL offer defense against the protozoan parasite Trypanosoma bruceibrucei. Through both direct and indirect channels, HDL mostly delivers cholesterol to the liver or steroidogenic organs such the testicles, ovary, and adrenals. Scavenger receptor BI (SR-BI), which permits the selective absorption of cholesterol from HDL, is one of the HDL receptors that destroy HDL. Inhibition of oxidation, inflammation, endothelial activation, coagulation, and platelet aggregation is one way that HDL and the lipid and protein that make up the molecule function. All of these characteristics may help HDL in its battle against atherosclerosis, even if it is not yet apparent which one is most crucial. Additionally, negligible quantities of HDL offer defense against the protozoan parasite Trypanosoma brucei. Trypanosome Lytic Factor (TLF), an HDL subfraction, is made up of particular, highly active proteins that are peculiar to the TLF molecule (Stephens et al., 2012). In the course of the stress response, HDL particles transport serum amyloid A, one of the acute-phase proteins and an apolipoprotein, to the wounded tissue where it is activated by cytokines and cortisol produced in the adrenal cortex. Leukocytes are attracted to the site of inflammation and become active there. Amyloidosis, brought on by its buildup in tissues, is a sign of ongoing inflammation. The concentration of large HDL particles, rather than the concentration of all HDL particles, is thought to more accurately reflect the protective impact [12].
Glutathione (GSH): Glutathione serves as an antioxidant in plants, animals, fungi, certain bacteria, and archaea. Reactive oxygen species, such as free radicals, lipid peroxides, and heavy metals, can damage crucial cellular components. Glutathione can prevent this from occurring [13]. In this tripeptide, cysteine and the carboxyl group of the glutamate side chain are connected by a gamma peptide. The carboxyl group of the cysteine residue is linked to glycine by conventional peptide linkage. There are two adenosine triphosphate-dependent stages in glutathione biosynthesis:
Gamma-glutamyl cysteine is initially made from cysteine and L-glutamate. This process requires the enzyme glutamate-cysteine ligase (GCL, glutamate cysteine synthase).
Second, glycine is added to the C-terminal of gamma-glutamyl cysteine. This condensation is catalysed by glutathione synthetase. Although all animal cells can make glutathione, it has been that liver-specific glutathione synthesis is essential [14]. With concentrations ranging from 0.5 to 10 mmol, glutathione the most common thiol in animal cells. It is protected from peptidase hydrolysis by a special gamma amide bond. Both the cytoplasm and the organelles contain it [15]. Glutathione in two states, reduced (GSH) and oxidized (GSSG). A higher GSSG-to-GSH ratio, according to [16], indicates increased oxidative stress.
The thiol group of the cysteinyl residue provides a source of one reducing equivalent in the reduced state. Thus, glutathione disulphide (GSSG) is produced. NADPH transforms the oxidized state into the reduced state [17].
Glutathione reductase is the enzyme responsible for this conversion:
NADPH + GSSG + H2O → 2 GSH + NADP+ + OH−
GSH protects cells by neutralising reactive oxygen species (Michael, 2005). This conversion is illustrated by the reduction of peroxides:
2 GSH + R2O2 → GSSG + 2 ROH (R = H, alkyl) and with free radicals:
GSH + R. → 0.5 GSSG + RH
Glutathione supports thiol protection and redox management of cellular thiol proteins under oxidative stress by protein S-glutathionylation, a redox-regulated post-translational thiol modification. As part of the whole process, the protectable protein (RSH) and GSH produce an asymmetrical disulphide [18].
RSH + GSH + [O] → GSSR + H2O
Additionally, glutathione is used to detoxify the dangerous oxidative stress metabolites formaldehyde and methylglyoxal. This detoxification procedure is controlled by the glyoxalase system. Vitamins C and E, two exogenous antioxidants, are maintained at low levels [19]. Glutathione enhances the nitric oxide cycle component citrulline (Ha et al, 1999). Influence of a cofactor on glutathione peroxidase [20]. Glutathione facilitates the easier metabolization of xenobiotics. The Glutathione S-transferase enzymes' conjugation of glutathione to lipophilic xenobiotics aids their excretion or subsequent metabolism [21].
Malondialdehyde (MDA): A chemical substance having the nominal formula CH2 (CHO) 2 is Malondialdehyde (MDA). It occurs as the enol, a white liquid and a highly reactive molecule. It is a naturally occurring indicator of oxidative stress.
Malondialdehyde mainly exists as the enol [22]
CH2 (CHO)2 → HOC(H)=CH-CHO
The cis-isomer is preferred in organic solvents, but the trans-isomer predominates in water. The sodium salt of the enolate (m.p. 245 °C), produced by simple deprotonation of malondialdehyde. Lipid peroxidation of polyunsaturated fatty acids results in the production of malondialdehyde [23]. It plays a crucial role in the creation of thromboxane A2, which is created when platelets and a number of other cell types and tissues use cyclooxygenase 1 or cycloxygenase 2 to convert arachidonic acid to prostaglandin H2. This molecule is still being broken down by thromboxane synthase, which results in the production of thromboxane A2, 12-hydroxyheptadecatrienoic acid, and malonyldialdehyde. Malondialdehyde levels in tissues used to gauge the degree of lipid peroxidation [23]. When reactive oxygen species degrade polyunsaturated lipids, malondialdehyde is produced. This molecule, which reactive aldehyde and one of the several reactive electrophile species that cause toxic stress in cells and create covalent protein adducts known as Advanced Lipoxidation End-products (ALE), is similar to Advanced Glycation End-products (AGE) (Farmer and Davoine, 2007). The production of this aldehyde functions as a biomarker to gauge an organ's level of oxidative stress (Del Rio et al.,2005).
Materials and Methods
Study area
This study was carried out in Rivers and Cross Rivers states in Nigeria. In each of the states, two local government areas were considered. The Rivers State Local Government Areas of Khana and Gokana LGA served as a testing ground.
Cross Rivers State local government areas of Ogoja and Boki serve as control, which are non-petroleum exploitation and exploration zones.
Study population
The participants in this study were women between the ages of 18 to 50 years in good health that gave their written or verbal consent to participate in the study.
Ethical approval: This study was duly approved by the ethical committee of the University of port harcourt and the cross rivers state ministry of health with approval numbers as follows: UPH/CEREMAD/REC/MM67/019,22/11/2019 and CRCMOH/REC/2020/113.
Determination of Total serum Cholesterol (TC)
Principle
The method and theory of Richmond (1973) was used to measure the serum total cholesterol concentration. Enzymatic hydrolysis and oxidation were followed by the measurement of cholesterol levels. In the presence of phenol and peroxidase, the indicator quinoneimine was created from hydrogen peroxide and 4- aminoantipyrene.
Reaction Principle

Procedure:
Test tubes with the designations TT1 (blank), TT2 (standard), and TT3 were used (sample). TT1 received 10 l of distilled water, whereas TT2 and TT3 received 10 l of standard cholesterol and 10 l of plasma, respectively. Randox cholesterol reagent in an amount of 1.0 ml was applied to each of the test tubes (TT1, TT2, and TT3). The tubes were combined and left at 37 OC for 5 minutes. At 500 nm, the absorbance (concentration) of the sample and the standard were measured.
Determination of Total serum Cholesterol (TC)
Principle
Glycerokinase Peroxidase- Peroxidase method according to Tietz colorimetric method (Tietz, 1990) was used to determine the level of Triglyceride in the samples.
Reaction Principle
Triglycerides + 3H2O ↔ Glycerol + fatty acid
Glycerol + Adenine triphosphate (ATP) ↔ Glycerol-3-phosphate + ADP
Glycerol-3-phosphate + O2 ↔ Dihydroxyacetone Phosphate + Hydrogen peroxide (H2O2)
H2O2 + 4-Aminoantipyrine + 4-Chlorophenol ↔ Quinoneimine + HCl + H2O
Procedure
The TT1 (blank), TT2 (standard), and TT3 (sample) test tubes were utilized. TT1 contained 10 l of distilled water, TT2 and TT3 each contained 10 l of plasma, and TT1 contained 10 l of triglyceride standard. One (1.0) ml of the Randox triglyceride reagent was added to each of the three test tubes with the designations TT1, TT2, and TT3. After thoroughly combining, TT1, TT2, and TT3 were incubated at 37°C for 5 minutes. The absorbance was measured at 500nm.
Determination of High-Density Lipoprotein
Principle
Serum HDL levels were analysed using the principle and method developed by Lopes-Virella et al., (1977.)
In the presence of phosphotungstic acid, magnesium ions quantitatively precipitate the LDL, VLDL, and chylomicron fractions, but the HDL remains in the supernatant after centrifugation.
Procedure:
Several test tubes (TT) bearing the labels TT1 (blank), TT2 (standard), and TT3 (sample) were used in this experiment. TT1 contained 0.5 ml of distilled water. TT2 had 0.5 ml of Randox standard cholesterol solution and TT3 had 0.5 ml of plasma. Each tube received 1 ml of Randox precipitant. The materials were well blended and allowed to stand at room temperature for 10 minutes before centrifuging at 4000 x g for 10 minutes. The cholesterol levels in the supernatants were determined, as can be seen in the table below. With the labels TT1 (blank), TT2 (Randox standard cholesterol), and TT3 in place, three test tubes were used (sample). TT1, TT2, and TT3 each containing 0.1 ml of the supernatant from the blank tube previously described, 0.1 ml of the Randox standard cholesterol solution, and 0.1 ml of the sample tube, respectively. One (1 ml) of Randox cholesterol reagent was added to the tube. After 10 minutes in a water bath at 25 oC, the mixture was taken out, and the absorbance at 546 nm was measured.
Determination of Low-Density Lipoprotein
Estimation of low density –cholesterol (LDL-C) was calculated using the equation described by Friedewald (Friedewald et al., 1972). According to the equation, LDLC was calculated as follows:
LDL-cholesterol = total cholesterol-(triglyceride)/5-HDL.
Estimation of Malondialdehyde
Principle: A spectrophotometric approach was used to measure malondialdehyde (MDA), (Ohakawa et al., 1979). Thiobarbituric acid condenses with malondialdehyde and other thiobarbituric reactive compounds (TBARS), resulting in a bright red derivative that may be measured spectrophotometrically.
Procedure: There were two test tubes set up with the labels T1 (reagent blank) and T2 (test sample). Compared to T2, which included 0.5 ml of normal saline, 0.5 ml of the sample, and 2 ml of the thiobarbituric acid/trichloroacetic acid mixture, T1 contained only 0.5 ml of normal saline. Following an hour of boiling, the mixture was allowed to cool to ambient temperature before being centrifuged at 4000 g for five minutes. The absorbance of the clear supernatant was determined at 535 nm.
It was calculated as; MDA concentration (Umol/ml) =Absorbance of sample x 1.75 / 0.156
Estimation of Glutathione (GSH)
Principle:
Spectrophotometric technique (Sedlak and Lindsay, 1968) states that the Ellman's reagent, 5,5-dithio-bis (2-Nitrobenzoic acid) DTBN, combines with glutathione to produce 5-thio-2-nitrobenzoic acid (TNB).
Procedure:
Multiple test tubes (TT) bearing the markings TT1 for the reagent blank and TT2 for the test sample were used. 0.5 ml of 50% trichloroacetic acid (TCA) and 0.5 ml of distilled water, respectively, were included in TT1 and TT2. The TT was mixed together and centrifuged at 2000 g for 5 minutes. The supernatant (1 ml) was then placed into a separate test tube that already contained 2 ml of 0.01 m DTBN reagent (Ellman's reagent). The solution's absorbance at 412 nm was assessed after being protected from direct light for 15 to 20 minutes.
Results
Table 1: Oxidative stress markers among subjects in study population.
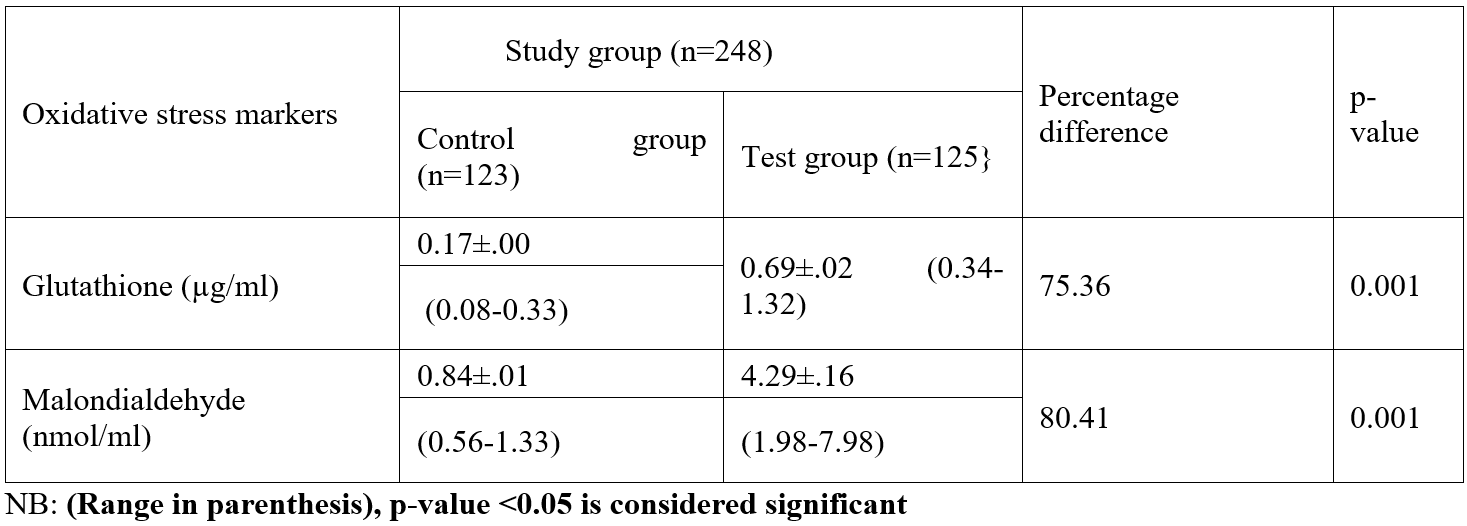
Table 2: Duration of exposure of oxidative stress markers among study population.
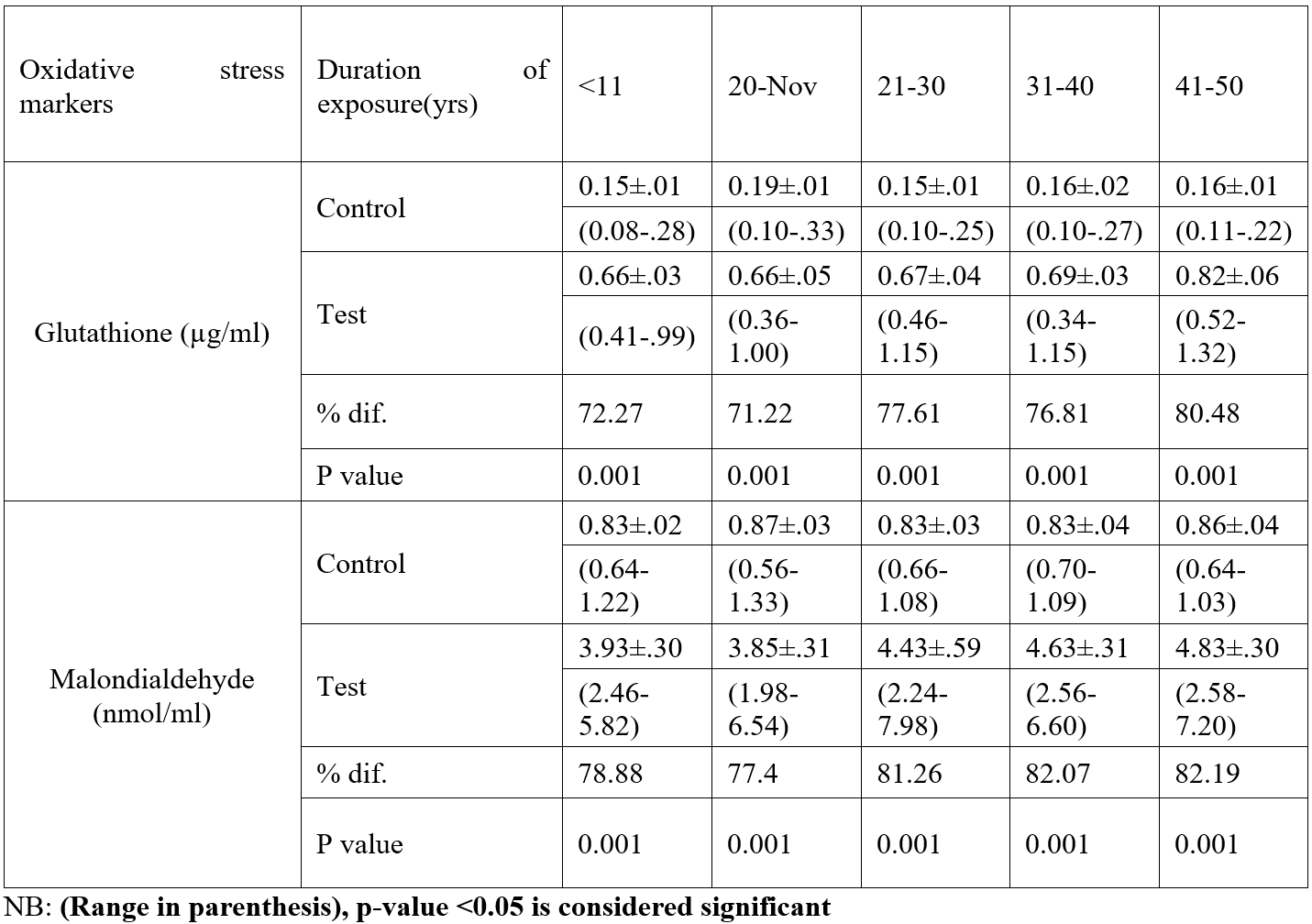
Table 3: Lipid profile among subjects in study population.
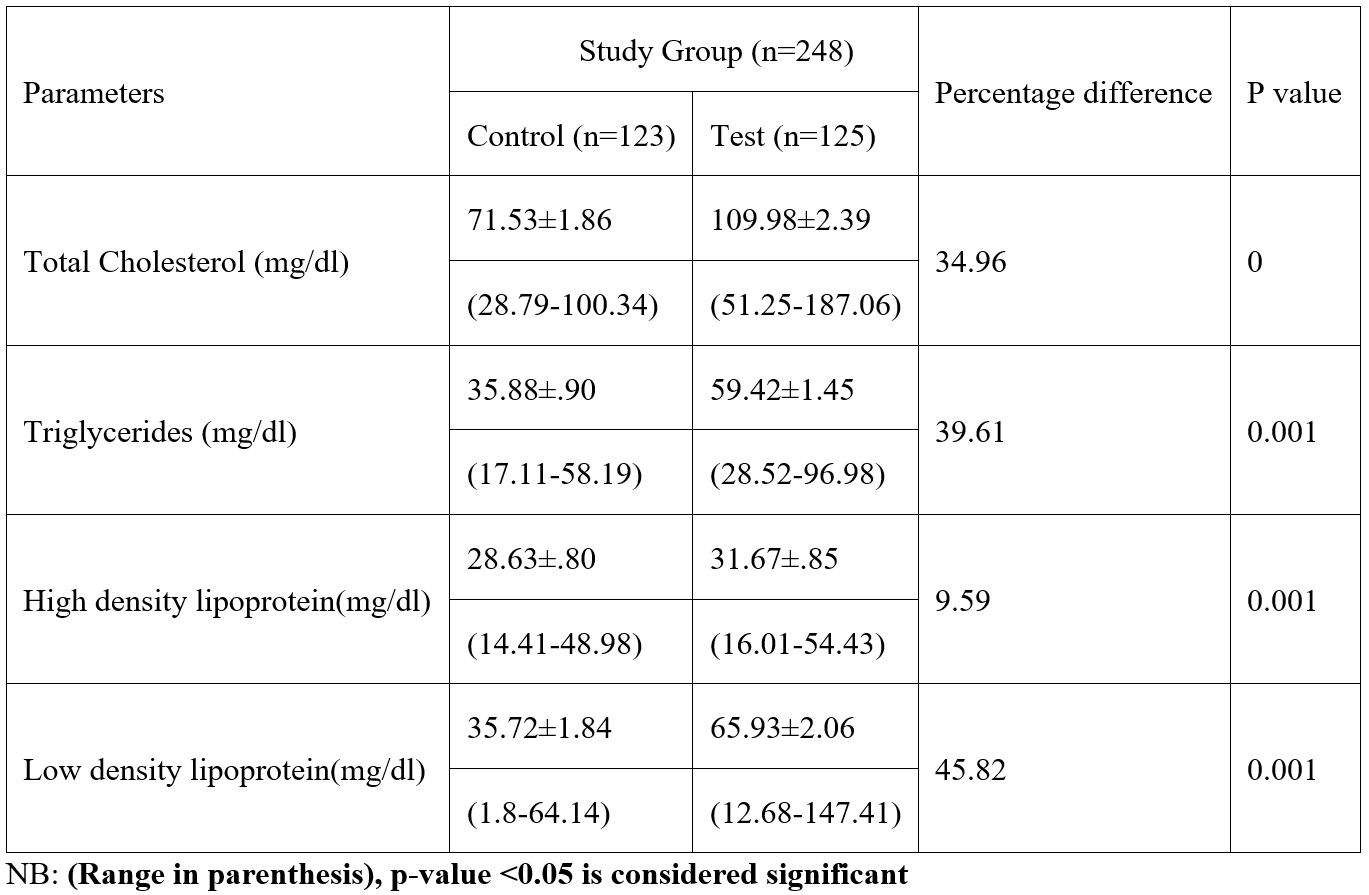
Table 4: Duration of exposure with lipid profile among study subjects.

Discussion
According to this study, adult female inhabitants of petroleum-bearing communities in Ogoni Rivers State had considerably higher levels of glutathione compared with those of non-petroleum-bearing communities in Ogoja Cross Rivers State., the blood level of glutathione significantly increased as exposure time progresses. In the body, glutathione functions as a crucial antioxidant that aids in the fight against free radicals. According to this study, participants who lived in neighbourhoods affected by petroleum had considerably greater levels of Malondialdehyde than those who lived in the control group. This study shown that, when the test group was compared to the control group, the levels of total cholesterol, triglycerides, and low-density lipoproteins were all significantly higher among the test group. The study also showed that, when the test group was compared to the control group, the serum blood level of HDL increased significantly. There were variations among the test group blood levels of total cholesterol, triglycerides, and low-density lipoprotein compared with the control. The study revealed no variation in High Density Lipoprotein levels according to the duration of exposure time. All test group parameters had considerably greater serum levels than the control group. Malondialdehyde synthesis is utilized as a diagnostic to gauge an organism's level of oxidative stress (Del Rio et al., 2005). Malondialdehyde is a marker for oxidative stress and higher levels of MDA indicates a greater lipid peroxidation.
High triglyceride levels may lead to atherosclerosis, or the thickening of the arterial walls, which raises the risk of heart disease, heart attack, and stroke. Acute pancreatic inflammation can gradually develop by very high triglycerides (pancreatitis). The risk of heart disease might rise if subjects have high cholesterol levels due to fatty deposits in the intima of blood vessels. Low-density lipoprotein (LDL) cholesterol levels above normal are linked to a higher risk of cardiovascular disease. The primary roles of cholesterol are to preserve the fluidity and integrity of cell membranes and act as a precursor in the creation of chemicals essential for the body (Antonis and Emmanuella, 2019). Triglycerides give the body energy while storing extra calories. HDL, on the other hand, takes cholesterol in and transports it to the liver. In the human circulation, low-density lipoprotein (LDL) particles are the primary transporters of cholesterol. It has been established that a high amount of LDL in human blood is a major, independent risk factor for the development of artherosclerosis (Ren and Jia, 2011). This study has shown that women who live in petroleum-impacted areas have elevated lipid profiles. This research is in line with that of [24,25], which found that residents of Nigeria's Niger Delta region exposed to oil and gas sector operations have higher blood levels of TC, TG, LDL, TC/HDL, LDL/HDL, and HDL/VLDL. Anthony et al, (2015) previous studies observed that the blood levels of depot employees have considerably higher concentrations of total cholesterol, diacylglycerol, and very low-density lipoprotein. According to the study, women who live in regions affected by petroleum may have altered lipid metabolism and an increased risk of cardiovascular diseases. Cadmium exposure increased pro-inflammatory lipid levels, enhanced lipid synthesis, and blocked lipid breakdown. It also caused intracellular lipid build-up. The weight of inflammatory bioactive lipids increases as a result of cadmium exposure, which encourages inflammation and high blood pressure (Huihui and colleagues,2012), [26]. Ionic and oxidative stress-related mechanisms are used by lead metal to generate toxicity in living cells. According to research, oxidative stress in living cells results from an imbalance between the formation of free radicals and the synthesis of antioxidants, which are needed to either detoxify reactive intermediates or repair the harm they cause. The presence of glutathione in the cell protects it against free radicals, but when lead a heavy metal is present, the number of ROS rises and the level of antioxidants falls. Since glutathione may be reduced (GSH) or oxidized (GSSG), the reduced form of glutathione converts its thiol groups in cystein into reducing equivalents (H+ + e) that ROS can use to stabilize themselves. Reduced glutathione rapidly combines with another molecule of glutathione in the presence of the enzyme glutathione peroxidase and generates glutathione disulfide (GSSG). Under typical circumstances, 90% of the total glutathione concentration is in the reduced form (GSH), while 10% is in the oxidized form (GSSG). However, in oxidative stress conditions, GSSG concentrations are higher than GSH concentrations [27]. Lipid peroxidation is another biomarker for oxidative stress. Lipid peroxidation is caused when a free radical collects an electron from lipid molecules in the cell membrane. At high concentrations, ROS may damage cells, proteins, nucleic acids, membranes, and lipids, creating a stressed state at the cellular level (Mathew et al., 2011).
Conclusion
Results from this study have shown significant higher levels of glutathione and Malondialdehyde among the test group's serum blood compared with the control. There was also a substantial increase in the lipid profile among the study group compared with the control group among female residents in petroleum impacted environment in Rivers state, Nigeria. Increased free radicals (oxidative stress markers) in the body than can be kept in balance by antioxidants can result in fatty tissues, DNA damages and sudden increase aging process.
Conflict of Interest: The authors have declared that there is no conflict of interest.
References
- Ivanova EA, Myasoedova VA, Melnichenko AA, Grechko AV, Orekhov AN. Small Dense Low-Density Lipoprotein as Biomarker for Atherosclerotic Diseases. Oxidative Medicine and Cellular Longevity, 2017; 1273042.
- Nelson DL, Cox MM. Lehninger, Principles of Biochemistry(3rd ed.). New York: Worth Publishing. Nomenclature of Lipids". IUPAC-IUB Commission on Biochemical Nomenclature (CBN), 2000.
- Lampe MA, Burlingame AL, Whitney J, Williams ML, Brown BE, Roitman E, et al. Clinical review: Ketones and brain injury. Critical Care, 2011; 15(2): 219.
- Drummond KE, Brefere LM. Nutrition for Foodservice and Culinary Professionals (8th ed.), 2014.
- Hanukoglu I. "Steroidogenic enzymes: structure, function, and role in regulation of steroid hormone biosynthesis". The Journal of Steroid Biochemistry and Molecular Biology, 1992; 43(8): 779–804.
- Lecerf JM, de Lorgeril M. "Dietary cholesterol: from physiology to cardiovascular risk". The British Journal of Nutrition, 2011; 106 (1): 6–14.
- Solomon MU, Asara AA, Bruno C, Bonnie KG. Correlation of serum cholesterol, electrolytes and body mass index with cardiovascular status of selected adults in Bayelsa state, Nigeria.Eurpean Journal of Pharmaceutical and Medical Research, 2014; 7: 110-117.
- Sadava D, Hillis DM, Heller HC, Berenbaum MR. Life: The Science of Biology 9th Edition. San Francisco: Freeman, 2011; pp. 105–114.
- Haines TH (2001). "Do sterols reduce proton and sodium leaks through lipid bilayers?” Progress in Lipid Research, 2001; 40(4): 299–324.
- März Winfried, Kleber Marcus E, Scharnagl Hubert, Speer Timotheus, Zewinger Stephen, Ritsch Andreas, et al. "HDL cholesterol: reappraisal of its clinical relevance". Clinical Research in Cardiology, 2017; 106(9): 663–675.
- Huang CX, Zhang YL. "The target of regulating the ATP-binding cassette A1 protein (ABCA1): promoting ABCA1-mediated cholesterol efflux in different cells". Current Pharmaceutical Biotechnology, 2013; 14(6): 623–631.
- Kwiterovich PO. "The metabolic pathways of high-density lipoprotein, low-density lipoprotein, and triglycerides: a current review". The American Journal of Cardiology, 2000; 86(12A): 5L–10L.
- Pompella A, Visvikis A, Paolicchi A, De Tata V, Casini AF. "The changing faces of glutathione, a cellular protagonist". Biochemical Pharmacology, 2003; 66 (8): 1499–1503.
- Chen Y, Yang Y, Miller ML, Shen D, Shertzer HG, et al. "Hepatocyte-specific Gclc deletion leads to rapid onset of steatosis with mitochondrial injury and liver failure". Hepatology, 2007; 45(5): 1118–1128.
- Guoyao Wu, Yun-Zhong F, Sheng Y, Joanne RL, Nancy DT. Glutathione Metabolism and its Implications for Health. Journal of Nutrition, 2004; 134 (3): 489–492.
- Lu SC. "Glutathione synthesis". Biochemical et Biophysica Acta (BBA) – General Subjects, 2013; 1830(5): 3143–3153.
- Couto N, Malys N, Gaskell SJ, Barber J. "Partition and turnover of glutathione reductase from Saccharomyces cerevisiae: a proteomic approach". Journal of Proteome Research, 2013; 12(6): 2885–2894.
- Dalle-Donne I, Rossi R C, Graziano G, Daniela MA "Protein S-glutathionylation: a regulatory device from bacteria to humans". Trends in Biochemical Sciences, 2009; 34(2): 85–96.
- Dringen R. "Metabolism and functions of glutathione in brain". Progress in Neurobiology, 2000; 62(6): 649–671.
- Grant CM. "Role of the glutathione/glutaredoxin and thioredoxin systems in yeast growth and response to stress conditions". Molecular Microbiology, 2001; 39(3): 533–541.
- Hayes John D, Flanagan Jack U, Jowsey Ian R. "Glutathione transferases". Annual Review of Pharmacology and Toxicology, 2005; 45: 51–88.
- Nair V, O'Neil CL, Wang PG. Malondialdehyde, Encyclopedia of Reagents for Organic Synthesis, 2008.
- Davey MW, Stals E, Panis B, Keulemans J, Swennen RL. "High-throughput determination of malondialdehyde in plant tissues". Analytical Biochemistry, 2005; 347(2): 201–207.
- Egwurugwu JN, Nwafor A, Chinko BC, Oluronfemi OJ, Iwuji SC, Nwankpa P. Effects of Prolonged Exposure to Gas Flares on the Lipid Profile of Humans in the Niger Delta Region, Nigeria, American Journal of Research Communication, 2013; 1(5).
- Solomon MU, Charles NN, Kiridi Emily GE. Blood serum lead and cadmium among pregnant women in gas flaring communities in Bayelsa state Nigeria. International Journal of Scientific Research publication, 2021; 11(5): 11316.
- Solomon MU, Asara AA, Kiridi Emily GE, Alagha BE, Dum-Awara BL. Total serum cholesterol and selected electrolytes during pregnancy among women residents in Yenagoa metropolis, Bayelsa state Nigeria. The Journal of Medical Research, 2021; 7(6): 191-194
- Jaishankar Monisha, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN. Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol, 2014; 7(2): 60-72. doi: 10.2478/intox-2014-0009.
- Elias M. Human stratum corneum lipids: characterization and regional variations". J. Lipid Res, 1983; 24(2): 120–130.

