Electrochemical Behaviour of Ketamine at Solid Electrodes
Inam ul Haque*
Department of Chemistry, University of Engineering and Technology, Pakistan
Received Date: 26/03/2023; Published Date: 29/06/2023
*Corresponding author: Inam ul Haque, Department of Chemistry, University of Engineering and Technology, Lahore 54890 Pakistan
Abstract
This work deals with the preliminary studies of ketamine by using several solid electrodes such as platinum, gold, nickel, compact graphite, titanium, glassy carbon and copper and the response was observed by comparing the results with the background observations. Cyclic, differential pulse, normal pulse and square wave voltammetry of ketamine hydrochloride was carried out at glassy carbon electrode. The results show a first sharp peak followed by a broad peak, which might have resulted from the products of first oxidation peak which is suggested for the electrochemical determination of ketamine in biological samples.
Keywords: Ketamine; Solid electrodes; Cyclic voltammetry; Recreational drug
Introduction
Ketamine is considered a dissociative anesthetic drug. The drug also has anesthetic properties that have been used in both human and veterinary medicine [1]. Along with anesthetic benefits, there are certain reactions to ketamine that make it appealing to illicit users [2].
In social situations ketamine is often used intranasally and orally and it can be injected, the rapid onset of effects from oral or nasal use makes it more convenient [1]. There are two isomers of ketamine: S(+) ketamine and R(-) ketamine. Ketamine metabolism is mediated by hepatic microsomal enzymes. Ketamine causes increased intracranial pressure, bronchodilation and stimulation of cardiovascular system. It is used for premedication, sedation, induction and maintenance of general anesthesia. Ketamine is an ideal anesthetic agent for trauma victims, patients with hypovolemic and septic shock, patients with pulmonary diseases [3]. Ketamine and its metabolites in biological samples (blood, urine, hair, and so on specimens) are determined and detected by different methods include GC, GC/MS, HPLC, etc [4].
Later on, it was being used by recreational users. The increase in illicit use prompted ketamine’s placement in schedule III of the United States controlled Substance act in August 1999 [5]. A new color test for the identification of ketamine is reported by treating ketamine with alkaline bromide produces a deep purple color [6]. There are very little studies on its electrochemistry except few. Ion-selective membrane electrode to the drug ketamine hydrochloride has been constructed using a modified PVC membrane which has ionic end-groups as ion-exchanger sites and which was cast using plasticized with ortho-nitrophenyloctyl ether (o-NPOE) as plastisizer. This drug electrode shows excellent Nernstian responses (59 mV per decade) in the concentration range 1×10-5–1×10-2 M with a detection limit of 5×10−6 M. Response time was about 10 s for ketamine concentrations between 1×10−5 and 1×10−2 M. The response is not affected by pH between 4.0 and 8.5. Selectivity coefficients against various organic and inorganic cations were evaluated. The electrode was applied for determination of ketamine hydrochloride in pharmaceutical preparations using direct potentiometry. The drug electrode has also been used to study the interaction of bovine serum albumin with ketamine in buffer solution (phosphate, pH 7). The saturated quantities of ketamine binding were 114, 32 and 25 mol/mol in 0.01, 0.02 and 0.1% of protein, respectively. The Hill equations were applied to obtain co-operative drug bindings to bovine serum albumin at 27 °C [7].
The kinetics of methylamine oxidation at a gold electrode in contact with an alkaline electrolyte solution was studied. The adsorptive behavior of substrate molecules was determined by changes in the potential range of gold oxide layer formation. The general reaction pathways of methylamine oxidation on the gold electrode are proposed [8]. A series of alcohols and amines have been oxidized at oxide-covered nickel, silver, copper, and cobalt anodes in aqueous alkaline solutions. The kinetics of these electrode processes have been studied and it is shown that the mechanism of these oxidations involves hydrogen abstraction from the substrate by an oxide species rather than direct electron transfer to the anode. The mechanism put forward is compared with those commonly suggested for the chemical reactions of transition-metal oxides and for heterogeneous catalysis [9]. A liquid chromatography/atmospheric pressure ionization mass spectrometry has been developed for the determination of ketamine, norketamine, and dehydronorketamine in human urine. A separation of these analytes in urine samples without tedious pretreatment procedures has been achieved within 10 minutes linear calibration curves of these analytes with coefficients better than 0.998 have been obtained over a wide range from 12.5 to 200 ng/mL. The accuracy was between 2.1% and 7.2% with detection limits at levels of 0.02 ng/mL, 0.02 ng/mL and 0.93 ng/mL for ketamine, norketamine and dehydronorketamine, respectively. The results demonstrate the suitability of the liquid chromatography/atmospheric pressure ionization mass spectrometry approach to analyze trace ketamine, norketamine and dehydronorketamine in urine. Urinary ketamine and norketamine levels were relatively low at 4-24 hours intervals and were difficult to assay in a normal laboratory. In the present study, the determination of urinary dehydronorketamine levels at 2-24 hours appears to have a great potential for use in Schedule III controlled drugs management [10].
Experimental
Chemicals and Materials
Ketamine hydrochloride (in the form of 5 mL injection vial with the name ketasol manufactured by Indus pharma Karachi contains 250 mg of ketamine, (Courtesy of Dr. M. Sarwar, Foreign Faculty, Forensic Research Laboratory, Center for Excellence in Molecular Biology, University of the Punjab Lahore, Pakistan).
Sodium sulphate E Merk Germany
Sodium hydroxide E Merk Germany
pH of the vial was checked it was 5.
pH of the Background solution was checked it was 6.
Cell Configuration
All experiments were performed in a three-electrode cell containing different working electrode, platinum short-wire or platinum spiral counter electrode and silver/silver chloride, chloride reference electrode.
Instrumentation
Voltammetry was carried out using EG & G, Princeton Applied Research Corp, Versastat II. Data were acquired using M270 Electrochemistry Research Software on a dedicated P II micro–processor coupled to the potentiostat.
Background electrolyte was sodium hydrooxide dissolved in doubly distilled water. Potentials were measured against silver/silver chloride, chloride reference electrode.
Other experimental conditions are same [12].

Ketamine (2-(2-chlorophenyl)-2-methylamino-cyclohexan-1-one)
Results and Discussion
Voltammetry of Ketamine at Metal Anodes
Voltammetry at platinum electrode
Figure 1 show the behavior of ketamine towards platinum electrode in basic conditions as it is obvious there is no oxidation peak is observed and the cathodic peak which was observed in case of background is suppressed in the presence of ketamine.

Figure 1: Cyclic voltammograms of the platinum electrode in the solution of 0.01 M NaOH (-----) and with 10 mM ketamine (____) at sweep rate 0.1V s-1. Potential in volts vs. silver/silver chloride, chloride.
Voltammetry at compact graphite electrode
Figure 2 shows very little response in both oxidation and reduction sides, only a wave appears in case of compact graphite electrode.

Figure 2: Cyclic voltammograms of the of the compact graphite electrode in the solution of 0.01 M NaOH (----) and with 10 mM ketamine (____) at sweep rate 0.1V s-1. Potential in volts vs. silver/silver chloride, chloride.
Voltammetry at nickel electrode
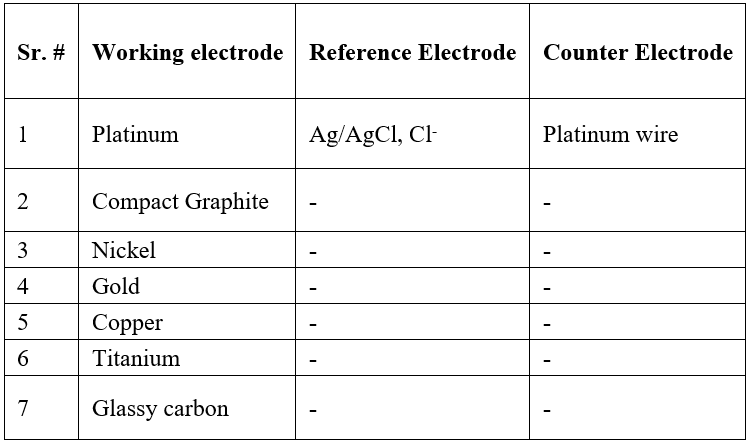
Figure 3 is indicative of chemical oxidation of ketamine by a surface resident higher-valent form of nickel oxide. Circumstantial formation of methylamine in such a reaction would lead to enhancement of cathodic peak current [13] with respect to the background, indicated by arrows as observed in Figure 3 starting -0.4.
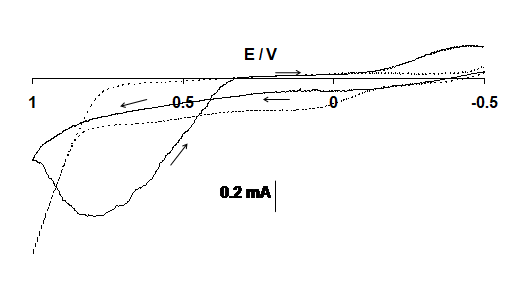
Figure 3: Cyclic voltammograms of the nickel electrode in the solution of 0.01 M NaOH (----) and with 10 mM ketamine (____) at sweep rate 0.1V s-1. Potential in volts vs. silver/silver chloride, chloride.
Voltammetry at gold electrode
In case of gold electrode Figure 4 it is observed that gold oxide is formed and then it reduces in case of background but when ketamine was added to the solution no such peaks were observed. Formation of methylamine is also believed to b involve in the chemical oxidation of ketamine by gold sesquioxide. [11].
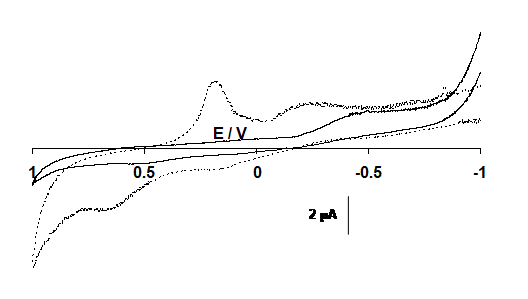
Figure 4: Cyclic voltammograms of the gold electrode in the solution of 0.01 M NaOH (----) and with 10 mM ketamine (____) at sweep rate 0.1V s-1. Potential in volts vs. silver/silver chloride, chloride.
Voltammetry at titanium electrode
As Figure 5 shows that no response was observed at titanium electrode.
Voltammetry at glassy carbon electrode
In case of glassy carbon increase in current indicates that oxidation of ketamine takes place (Figure 6).
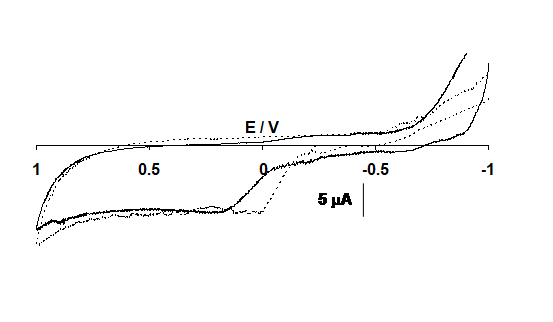
Figure 5: Cyclic voltammograms of the titanium electrode in the solution of 0.01 M NaOH (----) and with 10 mM ketamine (___) at sweep rate 0.1V s-1. Potential in volts vs. silver/silver chloride, chloride.

Figure 6: Cyclic voltammograms of the glassy carbon electrode in the solution of 0.01 M NaOH (----) and with 10 mM ketamine (____) at sweep rate 0.1V s-1. Potential in volts vs. silver/silver chloride, chloride.
Voltammetry at copper electrode
In case of copper electrode, the response is much pronounced as seen in Figure 7. After that, crystals appear in the solution which might be free base ketamine and when the scan was run in presence of those crystals no such type of response was observed as in Figure 7 but rather the response was same as was that of background.

Figure 7: Cyclic voltammograms of the copper electrode in the solution of 0.01 M NaOH (----) and with 10 mM ketamine (____) at sweep rate 0.1V s-1. Potential in volts vs. silver/silver chloride, chloride.
Voltammetry of Ketamine at Glassy Carbon Anode
Figure 8 shows the cyclic voltammogarm of ketamine hydrochloride at gassy carbon electrode. The anodic applications are mild and highly reproducible and may have potential for synthetic application, particularly for the synthesis of metabolites [15].

Figure 8: Cyclic voltammogram of 10 mM ketamine hydrochloride in 0.05 M sodium sulphate at glassy carbon electrode (0.125 cm2). Potential in volts vs. silver/silver chloride, chloride, current measured at sweep rate 0.1V s-1.
References
- Dotson JW, Ackerman DL, West LJ. J. Drug Iss., 1995; 25: 751.
- Drug Enforcement Administration, 2001.
- Radovanovic D, Pjevic M. Med. , 2003; 56: 439.
- Chen LL, Liao LC, Wang ZL. Forensic Med., 2005; 21: S5.
- Ketamine - Schedule III of the Controlled Substances Act (CSA). Anestesiología Mexicana en Internet, 2006.
- Sarwar M. Microg. J., 2006; 4: 24.
- Alizadeh N, Mehdipour R. J. Pharm. Biomed. Anal., 2002; 30: 725.
- Luczak T. J. Appl. Electrochem., 2007; 37: 461.
- Fleischmann M, Korinek K, Pletcher D. Chem. Soc., Perkin Trans., 1972; 2: 1396.
- Wu G-J, Chan H, Lee M-R, Chen C-Y, Yang D-Y, Cheng F-C. J. Chin. Chem. Soc., 2007; 54: 351.
- Sarwar M. personal communication
- Haque IU, Sadaf S, Fatima G. ECS Transactions, 2007; 6: 79.
- Robertson PM, J. Electroanal. Chem., 1980; 111: 97.
- Hall LR, Iwamoto RT, Hanzlik RP. J.Org. Chem., 1989; 54: 2446.
- Zaky R E-S M, Mohamed FZ, Amin AS, Gouda A A E-F. J. Chin. Chem. Soc., 2006; 53: 831.
- Jeremiah A, Morris BS. J. Forensic Sci., 2007; 52: 84.
- Uslu B, Ozkan SA. Comb. Chem. High Through. Screen., 2007; 10: 495-513.
- Haque IU, Sadaf S, Idrees S. Meet. – Electrochem. Soc.USA, 2008; 801: 543.
- Sergi M, Compagnone D, Curini R, D’Ascenzo G, Carlo MD, Napoletano S et al. Anal. Chim. Acta, 2010; 675: 132-137.
- Smith KM, Larive LL, Romanelli F. Soc. Health-Syst. Pharm, 2002; 59: 1067-1076.
- Brown SD, Melton TC. Chromatogr., 2011; 25: 300-321.
- Reinke LA, Kotake Y, Moore DR, Nanji AA. Free Rad. Biol. Med., 1998; 24: 1002-1006.
- Kovacic P, Somanathan R. ISRN Anesthesiology, doi:10.5402/2011/402906.
- Leung LY, Baillie TA. Med. Chem., 1986; 29: 2396-2399.
- Elhawi H, Eini H, Douvdevani A, Byk G. Molecules, 2012; 17: 6784-6807.
- Morgan CJA, Curran HV. Addiction, 2012; 107: 27-38.
- Ling Y, Yang Y, Bian S, Tu Y. Drug Test Anal., 2010; 2: 388-391.
- He J-N, Liu D-X. Changchun Normal Univ., 2007; 26: 69-71.
- Oelschlaeger H, El-Hossny T. Arch. Pharm. (Wienheim), 1983; 316: 412-421.
- Tamagawa RE, Miranda EA, Santana CC, Giulietti M. J. Chem. Eng. Data., 2009; 54: 16-21.
- Kalgutkar AS, Gardner I, Obach RS, Schalter CL, Callegari E, Henne KR, et al. Curr. Drug Metab., 2005; 6: 161-225.
- Chiuminatto U, Gosetti F, Dossetto P, Mazzucco E, Zampieri D, Robotti E, et al. Anal. Chem., 2010; 82: 5636-5645.
- Josey J, Gamage SA, Harvey MG, Voss LJ, Sleigh JW, Denny WA. Bioorg. Med. Chem., 2013; 21: 5098-5106.
- Yokoyama R, Matsumoto S, Nomura S, Higaki T, Yokoyama T, Kiyooka S-I. Tetrahed., 2009; 65: 5181-5191.
- Miksa IR, Cummings MR, Poppenga RH. J. Anal. Toxicol., 2005; 29: 544-551.
- Zhang X, Feng J, Zhu P, Zhao Z. Inflammation, 2013; 36: 1094-1099.
- Chen Y, Yang Y, Tu T. Sens. Actuat. , 2013; 183: 150-156.
- Zhou J, Xu Y, Wang L, Liu J, Li Y, Ye B. J. Chin. Chem. Soc., 2012; 59: 879-883.
- Wang F, Dong S. Yaowu Fenxi Zazhi, 2007; 27: 1295-1299.
- Okide GB, Odoh UE. Ind. J. Pharm. Sci., 1998; 60: 368-370.
- Sellers G, Lin HC, Riddell MG, Ravis WR, Lin YJ, Duran SH, et al. J. Vet. Pharmacol. Ther., 2010; 33: 480-484.

