Quantitative Comparison of White Blood Cells Produced with Heat and UV Killed C. Albicans in Winster Albino Rats
Ahmad Ibrahim1,*, Dauda Ben2 and Sade Sani Muhammad1
1Biochemistry Department, Federal University Lokoja, Nigeria ii. Industrial Chemistry Department, Federal University Lokoja- Nigeria
2Industrial Chemistry Department, Federal University, Nigeria
Received Date: 03/03/2023; Published Date: 16/05/2023
*Corresponding author: Ahmad Ibrahim, Biochemistry Department, Federal University Lokoja, Nigeria ii. Industrial Chemistry Department, Federal University Lokoja- Nigeria
Abstract
Systemic Candida albicans infection is continuously posing a serious threat to human health. Despite the increasing number of immunocompromised patients, toxicity or adverse drug reactions, drug resistance and other limitations related to drug use, efforts are being made to produce a novel preventive alternative. Candida albicans killed with Heat or UV methods have been used in animals to elicit immune response to prevent these infections. This study was aimed at comparing the quantitative difference of antibodies, granulocytes, lymphocyte between the two methods under consideration. Winster albino rats were grouped into Heat, UV and Control and received subcutaneous injection of Heat killed, UV killed and normal saline respectively at two intervals. The treatment groups were subsequently challenged with viable cells and sacrificed to collect blood sample for analysis. The result showed a statistically insignificant difference (p>0.05) in antibody titres, lymphocytes, granulocytes and white blood cells, monocyte, eosinophils and basophils between the heat and UV methods. However, more immune cells were elicited in the heat method as compared to the UV method.
Keywords: C. Albicans; Antibodies; Granulocytes; Lymphocytes
Introduction
Treatment or prevention of Candida albicans infections are considered one of the most difficult tasks of human health. These infections have caused serious detrimental effect on all and particularly patients with immune-suppressive conditions. Mortality rates for invasive candidiasis are very high and account for about 46 – 75% [1,2] Moreso, treatment options have proven ineffective due to a number of setbacks such as developing resistance [3] side-effects [4] and the specific nature of C. albicans: morphogenesis, pathogenesis, biofilm formation [5] and poorly understood complex anti-fungal immune responses [2]. These limitations necessitate the development of a novel preventive options that include Heat and UV – killed C. albicans among other strategies. Several studies conducted by [6-11] have strongly suggested that heat – and UV - killed C. albicans have the potential of eliciting substantial immune responses capable of conferring protection against C. albicans infections in animal models.
Therefore, comparing the number of immune cells produce by Winster albino rats immunized with Heat and UV- killed C. albicans would give an insight in selecting and improving on better strategy for effective protection
Material and Methods
Heat-Killing method for Candida albicans
This was achieved using Evron’s method, (1980) [8]. Whole C. albican cells were suspended in 0.85% sterile normal saline and inactivated by heating for 6 hours at 65oC. This heating was repeated three times and subsequently refrigerated to prevent contamination.
Preparation of Ultraviolet (UV) Inactivated Candida albicans
UV inactivation of C. albicans was prepared as described by Wheeler and Fink (2006). Petri dish containing C. albicans suspended in 0.85% sterile normal saline was directly exposed to a source of UV (Vilber Lourmat) at a wavelength of 254 nm for 30 minutes and checked for inactivation or non-viability every 10 minutes using microscopic and sub-culturing methods.
Experimental Procedure
All the methods were performed in accordance with the ARRIVE guidelines. The procedure used is explained below:
Experimental Animals
Normal male albino rats, 6-7 weeks old were used for the study. This study was approved by National Institute for Pharmaceutical Research and Development (NIPRD/01/03/CCPF/278). Ethical guidelines for the use of the rats were followed in accordance with the animal use regulation at NIPRD. The rats were provided with water and food ad libitum and were allowed to acclimatize for one week prior to the commencement of the experiment. After this acclimatization, blood samples from their tails were collected and pre-experimental values (baselines) determined.
Experimental Design
Winster albino rats were randomly divided into six (6) groups of five rats each. The immunization protocol used was as described by Thomas et al. (2006) [12].
A1 and A2 (Heat killed method)
B1 and B2 (UV killed method)
C1 and C2 (Control)
The killer cells described in 2.1 and 2.2 were subcutaneously injected into the Heat and UV groups after acclimatization respectively. This is followed by a booster injection of same antigen and concentration at day 21. After 14 days of booster injection, a lethal intravenous challenge of 0.2ml (1 x 106 cells of C. albicans) was administered to the experimental groups. However, the negative control group received only a sterile normal saline. Finally, the rats were sacrificed and their blood collected for analysis. The concentrations of both inactivated and viable C. albicans were determined using Mcfarland standard. The preventive effects of the vaccine were assessed as follows:
- Variation of white blood cells differentials data and;
- some immunological parameters
Determination of White Blood Cells Differentials
Groups A1, B1 and C1 were used to determine White Blood Cells Differentials. Blood samples in properly labeled EDTA bottles were used for the determination of White Blood Cell differentials with the aid of an automated hematology analyzer (Abacus 380) to determine the frequency of abnormalities.
Determination of Serum Antibody
Groups A2, B2 and C2 were used to determine serum agglutination titres as described by [13]. Blood samples collected were centrifuged at 2500 rpm for 10 minutes and sera obtained. As much as 100μl of serum was heat- inactivated at 56oC in water bath for 30 minutes. About 50μl of phosphate buffered saline (PBS) was then added to all 12 tubes. The first well or tube was considered the control hence it only received 50µl of PBS, while the second well received 50μl of heat-inactivated serum. Therefore, from the second well using a micropipette, 50μl of the mixture was taken after completely mixing it with the pipette and is serially diluted by 2 fold in the subsequent wells. Finally, 50 μl of inactivated C. albicans with a cell density of 1.6x106cells/ml was added to all the wells and the tubes were gently tapped to mix the cells and incubated at 37oC for 2 hours. The values of antibody titre were assigned to the highest serum dilution showing at least 50% of visible agglutination.
Statistical Analysis
The data collected from this study were subjected to statistical analysis. Analysis of variance (ANOVA) was used to determine the white blood cell differentials of controls and treatment groups. Analyses were performed using VassarStat Software (USA).
Results
Table 1: Antibody titres of albino rats immunized with Heat and Ultraviolet Radiation Inactivated Candida albicans.
Baseline Value: 25.56 ± 0.8298
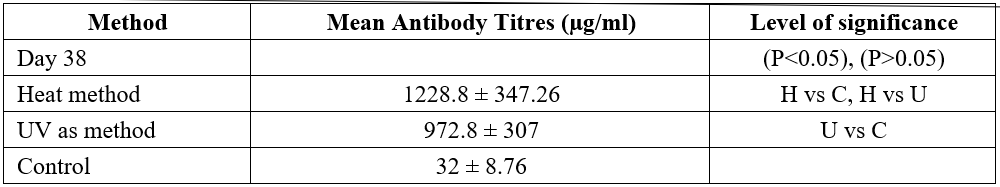
Keys: C- Control; H- Heat Method; U- UV Method
The mean values of antibody titers (Table 1) of the rats taken after 7 days of acclimatization (at baseline) was 25.6 ±10.55 for all the groups. This slightly increases to 32 ± 8.76 after 38days of the study for the control group. This increase is statistically not significance (P>0.05). Moreover, a statistically significant difference (P<0.05) was observed when the immunized rats were compared to the control group.
The heat method appears to stimulate more antibody titres, 1228.8 ± 347.26 compared to the UV method with 972.8 ± 307 antibody titres. However, this difference is not statistically significant (P>0.05).
White Blood Cells produced with Heat and UV radiation methods
The mean white blood cell (WBCs) counts at baseline was 7.6 ± 0.3195 and increased slightly (p>0.05) to 8.8 ± 0.3458 after 38 days of the study (Table 2). The WBCs produced from heat method 10.58 ± 0.7702, was higher than that of the UV method with a count of 10.48 ± 0.3434 even though this increase is not statistically significance (p>0.05). Therefore, comparing the two methods with the control showed that a statistically significant (p>0.05) WBCs were stimulated
Table 2: hite blood cell count of rats vaccinated with inactivated Candida albicans and controls.
Baseline Value: 7.6 ± 0.3195
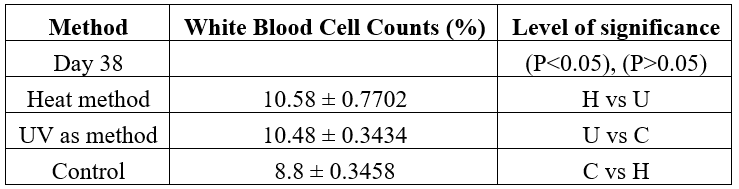
Keys: C- Control; H- Heat Method; U- UV Method
Lymphocytes Profile of Albino Rats Vaccinated with Heat and Ultraviolet Radiation Inactivated C. albicans
Albino rats in all the three groups recorded an increase in mean lymphocyte counts after 38 days as follows: Heat method (74.98%), UV method (73.18%) and Control (66.32%) (Table 3). Although the increase in lymphocyte counts relative to the baseline counts (67.42%) in all the groups was not significant (P>0.05), the mean lymphocyte counts of rats in the experimental groups shows a significant difference (P<0.05) when compared to the control group after 38 days.
Table 3: Mean Percentage Lymphocyte Counts of Rats Vaccinated with Inactivated Candida albicans and Controls.

Keys: C- Control; H- Heat Method; U- UV Method
Granulocytes Profile of Rats Vaccinated with Inactivated C. albicans and Control
The mean percentage granulocytes from the two methods and control group is presented in Table 4. At baseline, the mean percentage granulocytes were 25.94 ±1.061. At day 38, the control group recorded an insignificantly slight increase (25.94 ± 1.061) in the level of granulocytes (P>0.05). However, albino rats in Heat and UV inactivated and positive control groups recorded significantly lower (P>0.05) granulocyte values of 21%, 19.88% and 15.7% as compared to the initial (baseline) granulocyte values of 25.56% at day 7.
Table 4: Mean Percentage Granulocytes of Rats Vaccinated with Inactivated Candida albicans and Controls.
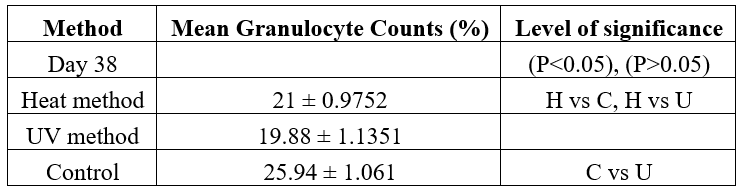
Keys: C- Control; H- Heat Method; U- UV Method
Mid- Range Cells Profile of Rats Vaccinated with Inactivated C. albicans and Control
The mean percentage Mid-range cells (monocytes, eosinophils and basophols) for the treatment groups and control at baseline was 3.66± 0.16 (Table 5). Rats in Heat and UV inactivated and negative control groups recorded MID ranging between 4.32 and 5.82%. Although the increase in MID at day 38 in the three groups was not significantly different from the MID recorded at day 7, and between the three treatment groups at day 38, there was a significant difference in MID recorded when compared to positive control groups (P<0.05).
Table 5: Mean Percentage MID (monocyte, eosinophils and basophils) of Rats Vaccinated with Inactivated Candida albicans and Controls.
Baseline value: 3.66 ± 0.16
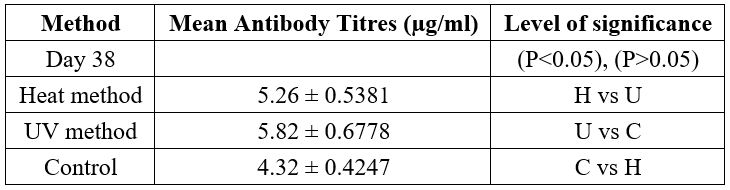
Keys: C- Control; H- Heat Method; U- UV Method
Discussion
Killed C. albicans have demonstrated in many studies the potential of preventing systemic candidiasis. In this study, the statistical difference observed from comparing the Heat and UV methods with control is as a result of the immunogenicity of Killed C. albicans to elicit preventive immunological response [12].
The heat method was observed to be a reliable strategy even though the higher quantity of total white blood cells, granulocytes and lymphocytes were not statistically significant when compared to the UV method. This is more likely because the heat method exposes more of the β – glucan on the cell wall surface of C. albicans, hence aiding better recognition by dectin – 1 and subsequent exposure to natural immunity [14]. However, the MID cells which are majorly monocytes/kupffer cells were slightly higher in the UV method. Monocytes are recruited to ingest invading microbes and eliminate them in the bloodstream. Therefore, more of these cells are only needed when circulating monocytes mature to form macrophages at the tissue where the microorganism has triggered inflammation [15,16] or are being continuously killed by C. albicans to escape phagocytosis through piercing [17-19]. This explains the higher mean percentage of MID cells in the UV method.
Acknowledgment
I want to thank Muhammad I. Odaki for his unwavering support towards a positive completion of this study.
References
- Brown GD, Denning DW, Gow NAR, Levitz SM, Netea MG, White TC. Hidden killers: human fungal infections. Science Translational Medicine, 2012; 4(165): 165rv13-165rv13.
- Thompson A, Davies LC, Liao C-T, da Fonseca DM, Griffiths JS, Andrews R, et al. The protective effect of inflammatory monocytes during systemic C. albicans infection is dependent on collaboration between C-type lectin-like receptors. PLoS Pathogens, 2019; 15(6): e1007850.
- Su S, Shi X, Xu W, Li Y, Chen X, Jia S, et al. Antifungal activity and potential mechanism of panobinostat in combination with fluconazole against Candida albicans. Frontiers in Microbiology, 2020; 11.
- Zhou L, Zhang P, Chen Z, Cai S, Jing T, Fan H, et al. Preparation, characterization, and evaluation of amphotericin B-loaded MPEG-PCL-g-PEI micelles for local treatment of oral Candida albicans. International journal of nanomedicine, 2017; 12: 4269.
- Lohse MB, Gulati M, Johnson AD, Nobile CJ. Development and regulation of single-and multi-species Candida albicans biofilms. Nature Reviews Microbiology, 2018; 16(1): 19-31.
- Evron R. In Vitro Phagocytosis of Candida albicans by Peritoneal Mouse Macrophages. infection and immunity, 1980; 28(3): 963-971.
- Hua X, Yuan X, Tang X, Li Z, Pflugfelder SC, Li DQ. Human corneal epithelial cells produce antimicrobial peptides LL-37 and β-defensins in response to heat-killed Candida albicans. Ophthalmic research, 2014; 51(4): 179-186.
- Evron R. In Vitro Phagocytosis of Candida albicans by Peritoneal Mouse Macrophages. infection and immunity, 1980; 28(3): 963-971.
- Ibrahim A, Umar AY, Suleiman AB. Immunological Response of Albino Rats Immunized with Heat-Killed Candida Albicans for the Possible Prevention of Candidemia. Global Journal of Animal Scientific Research, 2018; 6(4): 9-24.
- Ibrahim A, Umar YA, Busu MS, Ibrahim A. Immunological Response of Albino Rats Immunized with UV- Killed Candida albicans, 2019. https://doi.org/10.3844/ajbbsp.2019.
- Tamai R, Kiyoura Y. Heat-killed Candida albicans augments synthetic bacterial component-induced proinflammatory cytokine production. Folia microbiologica, 2019; 64(4), 555-566.
- Thomas DP, Viudes A, Monteagudo C, Lazzell AL, Saville SP, Lopez-Ribot JL. “A proteomic-based approach for the identification of Candida albicans protein components present in a subunit vaccine that protects against disseminated candidiasis”. Proteomics, 2006; 6: 6033-6041.
- Bin-Hafeez B, Ahmad I, Haque R, Raisuddin S. “Protective effect of Cassia occidentalis L. on cyclophosphamide -induced suppression of humoral immunity in mice”. J Ethnopharmacol, 2001; 75: 13-18.
- Gow NAR, Netea GM, Munro CA, Ferwerda G, Bates S, Héctor Mora-Montes HM, et al. Immune Recognition of Candida albicans β-glucan by Dectin-1. J Infect Dis, 2007; 196(10): 1565–1571.
- Visvanathan K, MacIsaac CM, Hall WW, Fischetti VA. Immunological Aspects of Infection: Essential of Clinical Immunology.Cambridge University Press, USA, 2009; 45-56.
- Fu J, Ding Y, Wei B, Wang L, Xu S, Qin P, et al. Epidemiology of Candida albicans and non-C. albicans of neonatal candidemia at a tertiary care hospital in western China. BMC Infectious Diseases, 2017; 17(1): 1–6.
- Cooney EL, Collier AC, Greenberg PD, Coombs RW, Zarling J, Arditti DE, et al. Safety of and immunological response to a recombinant vaccinia virus vaccine expressing HIV envelope glycoprotein. The Lancet, 1991; 337(8741): 567–572.
- Haurowitz F, et al. The Mechanism of the Immunological Response. Biological Reviews, 1952; 27(3): 247–280.
- Hua X, Yuan X, Li Z, Coursey TG, Pflugfelder SC, Li DQ. A novel innate response of human corneal epithelium to heat-killed candida albicans by producing peptidoglycan recognition proteins. PLoS One, 2015; 10(6): e0128039.

