Phytochemical and Antioxidant Activities of a Polyherbal Formulation from Selected Medicinal Herbs
Ahonsi CO*, Ovobovwori SU and Etatuvie SO
Quality Control Unit, Department of Product Development/Quality Assurance, Nigeria Natural Medicine Development Agency Lagos, Nigeria
Received Date: 15/02/2023; Published Date: 24/04/2023
*Corresponding author: Ahonsi CO, Quality Control Unit, Department of Product Development/Quality Assurance, Nigeria Natural Medicine Development Agency Lagos, Nigeria
Abstract
The present study aimed to investigate the phytochemical and antioxidant activities of a polyherbal formulation (PHF) obtained from the Nigerian Natural Medicine Development Agency (NNMDA) using In vitro methods. Ethanolic extract of the polyhebal formulation was prepared by the Soxhlation process, Phytochemical screening, Total Phenolic Content (TPC) and Total Flavonoid Content (TFC) of the extracts were estimated using standard methods. Extracts were analyzed for its antioxidant potential using DPPH (1, 1-diphenyl-2-picrylhydrazil), FRAP (Ferric Reducing Antioxidant Power), OH (Hydroxyl radical scavenging activity), NO (Nitric oxide scavenging activity), ABTS {2, 2’-azino-bis (3- ethylbenzothiazoline-6-sulfonic acid)}, and SRS (Superoxide Radical Scavenging activity) methods to assay their free scavenging activity.
Results shows that PHF contains Flavonoids (QE) 15.61±1.95, Total phenol (GAE) 25.24±3.56, preliminary phytochemical screening revealed the presence of tannins, alkaloids, flavonoids, glycosides, Anthraquinone, Terpenoid, Steroid, Phlobatannins and Coumarins. Antioxidant study for DPPH, FRAP, OH, NO, ABTS, and SRS of PHF were 86.09±1.58, 66.95±0.03, 80.89±0.10, 68.13±0.16, 65.54±0.55, and 69.69±1.12, at 250 µg/mL respectively. When compared with known standards PHF possess high antioxidant activity in a concentration dependant manner. IC50 values of drug combination exhibited higher antioxidant potential in DPPH (26.40± 0.56 µg/mL) and ABTS (28.91±0.34 µg/mL), which may be due to the combined activity of the individual plant extracts with its high phenolic and flavonoid content. these findings may provide efficient, supportive data on the use of medicinal herbs for treatment of numerous health ailments associated with the accumulation of harmful free radicals and reactive oxygen species.
Keywords: Polyherbal formulation (PHF); Total Phenolic Content (TPC); Ferric Reducing Antioxidant Power (FRAP); 1, 1-diphenyl-2-picrylhydrazil; Hydroxyl (OH) radical scavenging activity
Introduction
Plants have been a major source of medicines, either in the form of extemporaneous preparations or as pure active molecules [1]. Plant-based compounds and formulations for treatment of various ailments are becoming common place in society. Moreover, 75–80% of the world's population in developing countries continues to use herbal medicines for primary healthcare. Plants are the potential source of natural antioxidants, antioxidants are molecules that inhibit harmful free radicals and Reactive Oxygen Species (ROS) and delay or inhibit cellular damage [2]. Free radicals are molecular species or atoms which contain one or more unpaired electrons, these are constantly produced in the human body as a result of cell metabolism (Halliwell and Gutteridge, 2006). Free radicals play important roles in gene expression and activation of receptors as well as in regulation of signal transduction [3]. However, an excess of free radicals can become toxic to living cells [4] and may cause many degenerative diseases. Under normal circumstances, the rate and amplitude of oxidant formation is balanced by the rate of their removal. However, loss of balance between pro-oxidants and antioxidants results in oxidative stress. High levels of ROS in biological cells have a large impact on their functioning, leading to deficient cell operation, aging, or disease (Rodrigo, 2009). It is evident that plant extracts have diverse bioactivities such as anti-allergic, anti-inflammatory, antioxidant, anti-microbial, anti-fungal, antiviral, antidiabetic and anti-cancer properties (Friedman, 2007) [5].
Antioxidants inhibit the chain reaction of oxidation, acting as hydrogen donors or acceptors of free radicals, generating stabler radicals. The antioxidants in this group mainly have a phenolic structure and include the following: antioxidant minerals, antioxidant vitamins and phytochemicals, among which are flavonoids, catechins, carotenoids, β-carotene, lycopene, diterpene and their derivatives. These compounds interact by a variety of mechanisms including quenching ROS, binding of metal ions, scavenging free radicals, inhibiting oxidative enzymes, converting hydroperoxides to non-radical species, absorbing UV radiation or deactivating singlet oxygen. The efficiency of antioxidant compounds depends on several factors such as; structural properties, temperature, the characteristics of the substrate susceptible to oxidation, concentration and localization in the system, the presence of synergistic and pro-oxidant compounds and the physical state of the compounds. Antioxidants modulate the oxidant–antioxidant profile of body systems by nullifying pro-oxidant molecules [6]. Antioxidant use is associated with reduced production of ROS, free radicals and reduced pre-disposition to lipid peroxidation, oxidative stress, post-translational modification of proteins, and DNA damage. Most of these defensive antioxidants are available from plant and dietary sources [7]. Synthetic antioxidants for controlling undesirable redox events are available at high cost, and mostly suffer set-backs of unavailability and side effects. As a result of these challenges, natural antioxidants have received attention over time, since they do not possess the side effects associated with their synthetic counterparts, are cheaper and ever-present in many plants [8]. ROS including superoxide radicals, hydrogen peroxide, hydroxyl radicals and singlet oxygen are generated as byproducts of normal metabolisms [9]. Accumulation of ROS, results in a high level of free radicals in the body which causes oxidative stress [10]. Oxidative stress is associated in the development of various pathological processes, such as aging, cancer, coronary heart disease, Alzheimers disease, neurodegenerative disorders, atherosclerosis, cataracts, inflammation and diabetes mellitus, etc [11].
Post-mortem brains of patients with neurodegenerative diseases have shown the presence of biomarkers of oxidative stress including protein carbonyl, trans-4-hydroxy-2-nonenal (4-HNE) and malondialdehyde (MDA) [12]. Diabetes is associated with a high level of free radicals and many plants show antihyperglycemic property attributed to their antioxidant potential [13]. The remedial effects of several medicinal plants are usually due to antioxidant phytochemicals present in it such as polyphenols, flavonoids and phenolic compounds [14]. Consequently, these phytochemicals have many favourable effects on health, such as inhibition of low-density protein oxidization, anti-inflammatory, anti-carcinogenic properties, antidiabetic activity and are well known to function as chemo-preventive agents against oxidative damage by counteracting the deleterious effects of ROS [15]. According to [16], the use of biochemical assays to measure the antioxidant power of plants has become the best reliable and readily available methods. There are several assay methods and it is important to use more than one method because of the variable response engendered by a specific antioxidant in various testing systems in order to understand the mechanism of action of the bioactive component involved [17]. The objective of the study is to assess the antioxidant potential of a Polyherbal Formulation (PHF) which consists of extracts of five medicinal plants and to find an optimized ratio to explore its application on diseases complications resulting from accumulation of ROS and free radicals.
Materials and Methods
Preliminary phytochemical screening: Ethanolic extracts (80%) of the polyherbal drug was screened for various phytoconstituents such as Steroids, Tannin, Saponin, Alkaloid, Flavonoid Glycoside, Anthraquinone, Terpenoid, Phenols, Phlobatannins Coumarins, and triterpenoids [17].
Quantitative Phytochemical Screenings
Quantitative phytochemical assessments of the extracts and fractions were performed to estimate the Total Phenolic Content (TPC) using the Folin–Ciocalteu method, as previously reported [18] and Total Flflavonoid Content (TFC) using the aluminum chloride colorimetric assay, as described in [19]
In Vitro Antioxidant Studies: The extracts and their different combination proportions were tested for their free radical scavenging property using different in vitro models. All experiments were performed thrice. Ascorbic acid was used as standard control in each experiment. Results were expressed in IC50 values.
DPPH radical scavenging assay (1,1‑diphenyl‑2‑picryl hydrazyl):
The antioxidant activity of the sample was determined in terms of hydrogen donating or radical scavenging ability, using the stable radical DPPH, according to the method of Alam et al 2013 [20]. The sample extracts at various concentrations (200 - 1000μg) was taken and the volume was adjusted to 100 μl with methanol. 5 ml of 0.1 mM methanolic solution of DPPH was added and allowed to stand for 20 min at 27°C. The absorbance of the sample was measured at 517 nm. Percentage radical scavenging activity of the sample was calculated as follows: % DPPH radical scavenging activity = (control OD-sample OD / control OD) × 100 The analysis was performed in triplicate. The sample concentration providing 50% inhibition (IC50) under the assay condition was calculated from the graph of inhibition percentage against sample concentration.
Ferric Reducing Anti-Oxidant Property (FRAP ASSAY): The reducing power of both extracts was evaluated according to the method of Amir. The mixture containing 2.5ml of 0.2M phosphate buffer (pH 6.6) and 2.5ml of potassium ferricyanide (K3Fe (CN)6) (1%w/v) was added to 1.0 μg ml of the extract dissolved in 1ml of distilled water. The resulting mixture was incubated at 500C for 20 min, followed by the addition of 2.5ml of trichloroacetic acid (TCA) (10%w/v). The mixture was centrifuged at 3000 rpm for 10min to collect the upper layer of the solution (2.5ml) and 0.5ml of (FeCl3) ferric chloride (0.1%w/v). The absorbance was then measured at 700nm against Phosphate buffer (pH 6.6) blank sample. Ascorbic acid was used as a reference standard.
Hydroxyl radical scavenging activity:
To 0.5ml of the plant extract at various concentrations (15.625, 31.25, 62.5, 125 and 250), 60µl of FeCl3 (1mM), 90uL of 1,10-phenanthrolin (1mM), 2.4mL of phosphate buffer (0.2M; pH 7.8) and 150µl of H2O2 (0.17 M) were added. The mixture was homogenized using vortex and incubated at room temperature for 5 mins. Absorbance was read at 560nm against blank and the HO scavenging activity was calculated from equation below [21].
HO scavenging activity (%) = [(Ao− A1)/Ao] x 100
Where Ao is the absorbance of the absorbance of the blank and A1 is the absorbance of the sample.
Nitric Oxide Radical Scavenging Activity:
The modified method as described by Oyedemi (2009) was used to determine the nitric oxide radical scavenging activity of both extracts. 2 mL of 10 mM sodium nitroprusside in 0.5 mL of phosphate buffer saline (pH 7.4) was added to 0.5 mL of the plant extracts, ascorbic acid, and BHT at different concentrations (0.2–1.0 mg/mL). The mixture was then incubated for 150 min at a temperature of 250C. After incubation, 0.5 mL of the mixture was mixed with 0.5 mL of sulfanilic acid reagent and thereafter incubated for 5 min at room temperature. One millilitre of naphthyl ethylenediamine dihydrochloride (0.1% w/v) was finally added to the mixture and again incubated at room temperature for another 30 min, after which the absorbance was measured at 540 nm with a spectrophotometer.
ABTS•+2, 2’-azino-bis (3-ethylbenzthiazoline-6-sulphonic acid) Free radical scavenging activity: ABTS radical cation (ABTS +) was produced by reacting 2,2-azinobis-(-3ethyl benzothiozoline-6-sulphonate) ABTS solution (7mM) with 2.45 M ammonium per sulfate and the mixture was allowed to stand in dark at room temperature for 12-16 hours before use. For the study method of Shirwaiker et al. (2006) was used. Different concentrations (200µg -1000 µg) of herbal preparation (0.5ml) were added to 0.3 ml of ABTS solution and the final volume was made up with ethanol to make 1ml. The absorbance was read at 745nm and the experiment was performed in triplicate. And compared with sodium ascorbate Trolox and Galic acid as Standards.
Superoxide radical scavenging activity:
Superoxide anions were generated in a non-enzymatic phenazine methosulfate-nicotinamide adenine dinucleotide (PMS-NADH) system through the reaction of PMS, NADH, and oxygen. It was tested with the reduction of nitroblue tetrazolium (NBT). In these experiments, the superoxide anion was generated in 3 ml of Tris-HCl buffer (16 mM, pH 8.0) containing 0.75 ml of NBT (50 µM) solution, 0.75 ml of NADH (78 µM) solution, and 0.3 ml of different concentrations of the extract. The reaction was initiated by adding 0.75 ml of PMS (10 µM) to the mixture, the absorbance at 560 nm was measured in spectrophotometer after 5 min of incubation at room temperature. The superoxide anion scavenging activity was calculated according to the following equation:
% Inhibition = [(A0 –A1 )/A0 ×100], Where, A0 was the control absorbance (blank, without extract) and A1 was the absorbance of the extract [22].
Results
The medicinal plants, which contain high quantities of polyphenols, are considered to be good source of natural antioxidant compounds and more often possess higher antioxidant potential than that of dietary fruits and vegetables. Consumption of these plant products certainly prevents the free radical mediated damage in the cell and therefore protects the body from several health problems. These antioxidant compounds can be used as natural antioxidant additives or nutritional supplements in the food products. As of natural origin, these antioxidants are much safe to use. Thus, much attention has been focused on the investigation of natural antioxidant compounds from plants, which can effectively scavenge ROS. Invitro antioxidant studies on polyherbal extract shows high amount of polyphenol and flavonoids (25.24±3.56 and 15.61±1.95) as presented in Table 1. Flavonoids and phenol in small quantities are free radical scavengers, which prevent oxidative cell damage. They have been known to produce anti-allergic, anti-inflammatory, antimicrobial, and anticancer activities. other phytoconstituents identified in the study are tannins, alkaloids, Flavonoids, glycosides, Anthraquinone, Terpenoid Phenol, Steroid, Phlobatannins and Coumarins.
Invitro Antioxidant Activity
Table 1: Quantitative Phytochemical Screening of Polyherbal Herbal Formulation.
DPPH radical scavenging activity
DPPH is a stable free radical and accepts an electron or hydrogen radical to become a stable diamagnetic molecule. In the DPPH assay, the antioxidants are able to reduce the stable radical DPPH to non-radical form, DPPH-H. The purple-colored alcoholic solution of DPPH radical changes to yellow in the presence of hydrogen donating antioxidant which could be measured at 517nm, the activity is expressed as effective concentration IC50, which is the concentration of the sample leading to 50% reduction of the initial DPPH concentration.
The antiradical scavenging activity of the extracts was measured by their ability to scavenge DPPH free radicals and was compared with standard Ascorbic acid and tocopherol. It was observed that the herbal formulation scavenging power for DPPH was seen in a concentration dependent manner. A maximum scavenging activity for DPPH radicals (86.09±1.58) was recorded at concentration 250 μg/ml for herbal formulation, and compared with the standard ascorbic acid (86.09±1.58) and tocopherol (67.37±0.25) respectively, after which the scavenging capacity declined for other lower concentrations as presented in Table 2. Values are expressed as mean ± SEM. ANOVA followed by PostHoc (LSD) multiple range tests. Values not sharing a common superscript differ significantly at P<0.05.
Table 2: DPPH radical scavenging activity of herbal formulation compared to known standards.

Ferric Reducing antioxidant Power (FRAP)
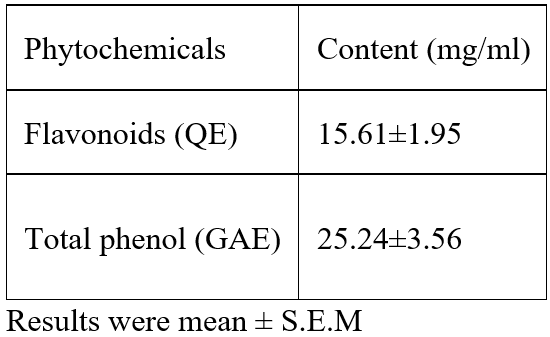
The Ferric reducing antioxidant activity of the polyherbal formulation is presented in Table 3. The 80% ethanolic extract of polyherbal formulation had a strong ferric reducing antioxidant power of 66.95±0.03 mmol Fe II/ g at 250µg/ml when compared with the standard ascorbic acid (70.05±0.07 mmol Fe II/ g). Ferric reducing power of herbal formulation was seen in a concentration dependent manner at 250 µg/ml there was a maximum value thereafter it decreases as the concentration reduces with herbal formulation having a significant maximum ferric reducing power when compared with ascorbic acid at all concentration.
Values are expressed as mean±SEM. ANOVA followed by PostHoc (LSD) multiple range tests. Values not sharing a common superscript differ significantly at P<0.05.
Table 3: Ferric Reducing antioxidant Power (FRAP) of herbal formulation compared to known standards.
Hydroxyl (OH) Radical Scavenging Potential
The ability of herbal formulation to scavenge hydroxyl radical was dependent on the concentration of herbal formulation. High hydroxy antioxidant activities were recorded for herbal formulation (80.89±0.10, 66.29±0.08, 53.79±0.49 and 46.23±0.42) at 250, 125,62.5 and 31.25 µg/ml respectively, but declined at 15.625µg/m (23.66±0.10). When compared with the standard Manitol at 250 µg/ml (92.20±0.24) the extract shows a significant scavenging activity as presented in Table 4.
Values are expressed as mean±SEM. ANOVA followed by PostHoc (LSD) multiple range tests. Values not sharing a common superscript differ significantly at P<0.05.
Table 4: Hydroxyl (OH) Radical Scavenging Potential of herbal formulation compared to known standards.

Nitric Oxide Radical Scavenging Activity
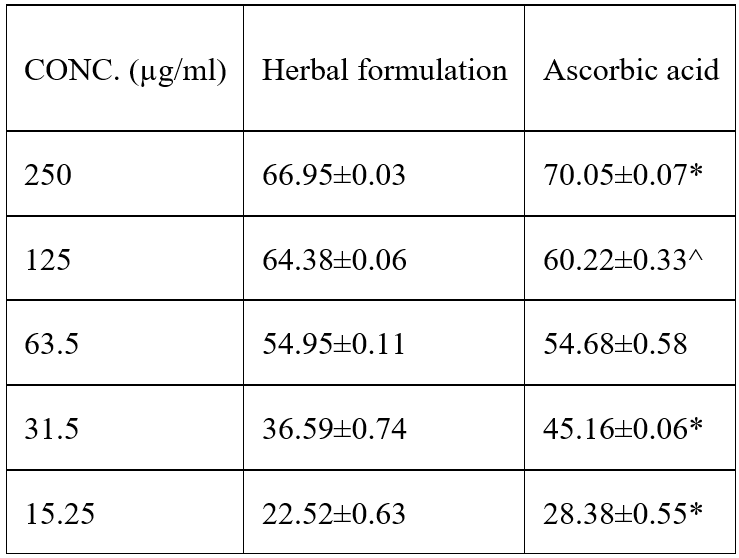
Nitric oxide scavenging ability of herbal formulation caused a dose dependent inhibition of nitric oxide compared to the reference antioxidant ascorbic acid. At high concentrations 250, 125 and 62.5g/mL PHF had significant inhibition of (68.13±0.16, 54.07 ±0.05 and 47.94±0.04) when compared with the standard ascorbic acid. Values are expressed as mean±SEM. ANOVA followed by PostHoc (LSD) multiple range tests. Values not sharing a common superscript differ significantly at P<0.05.
Table 5: Nitric Oxide Radical Scavenging Activity of herbal formulation compared to known standards.
ABTS Radical Scavenging activity
The antiradical scavenging activity of the extracts was measured by the ability to scavenge ABTS free radicals and was compared with three standards: sodium ascorbate, Trolox and Galic acid. It was observed that the Herbal formulation and reference antioxidants showed significant scavenging activity in a concentration’s dependent manner on ABTS free radicals. The maximum scavenging activity in Herbal formulation was observed at 250μg/ml and compared to the reference antioxidants, and this activity decreased as the concentration reduces. the scavenging activity of PHF at 250 and 125µg/m concentration studied ranged from 65.54±0.55 - 54.59±0.24, results were significant when compared with Sodium ascorbate (65.32±0.33 - 57.10±0.51) and Galic acid (67.49±.72 - 64.85±0.32) however when compared to Trolox herbal formulation was not significant in terms of its radical scavenging activities as presented in Table 6. Values are expressed as mean±SEM. ANOVA followed by PostHoc (LSD) multiple range tests. Values not sharing a common superscript differ significantly at P<0.05.
Table 6: ABTS radical scavenging activity of herbal formulation compared to known standards.

Superoxide Radical Scavenging (SRS) Activity
The Herbal formulation inhibited superoxide generation in concentration dependent manner the maximum scavenging activity recorded for PHF was 69.69±1.12 at 250 μg/ml compared to the standard reference Ascorbic acid (85.19±0.79) as presented in Table 7.Values are expressed as mean±SEM. ANOVA followed by PostHoc (LSD) multiple range tests. Values not sharing a common superscript differ significantly at P<0.05
Table 7: Superoxide Radical Scavenging (SRS) Activity of Herbal Formulation Compared to known Standards.

Discussion
In a normal cell, there is an appropriate balance between free radicals and antioxidants. However, this balance can be shifted towards free radicals when the production of reactive oxygen species is increased or when the antioxidants level is declined. This state is ‘oxidative stress’ and can lead to serious cell damage if stress is massive or prolonged [23,24]. Oxidative stress plays a major role in degenerative or pathological processes, such as diabetes mellitus, hypertension, cancers, heart failure, neurodegenerative disorders, etc [25] (Huang et al., 2005). Antioxidants from natural sources have attracted considerable attention from researchers and users on account of adverse toxicological reports of some synthetic antioxidants. In this context, medicinal plants are considered safer, cost-effective and cure the disease from its root cause [26]. Among these, medicinal plants are being viewed as an easily available and potent source of antioxidants as they contain a mixture of numerous different chemical compounds that may act individually or in synergy to cure disease and improve health [27]. In the majority of traditional systems like Ayurveda, African traditional medicine, Chinese system of medicine, etc., many diseases are better managed by herbs combination (Polyherbal) instead of single herb because of synergism and fewer side effects [28].
Polyherbal formulations magnify the therapeutic action and reduce the concentrations of single herbs, thereby reducing the adverse effects. In this study, 80% ethanolic extracts of a polyherbal formulation was evaluated. Phytochemical screening of the polyherbal extract revealed the presence of glycosides, alkaloids, flavonoids, steroids, phenolic compounds and saponins, suggesting its usage for various medicinal purposes in folk medicine. Most of the phytochemicals identified serve as natural antibiotics, which assist the body system in fighting microbial invasion and infections (Lillehoj et al., 2018). Flavonoids are known to have antioxidant effects and have shown to inhibit the initiation, promotion and progression of tumors [29]; this is done by either scavenging or quenching free radicals or by inhibiting enzymatic systems responsible for free radical generation [30]. Thus, as antioxidant present in this plant suggest its use as hepatoprotective, nephroprotective, antimicrobial, anti-inflammatory and anticarcinogenic [31,32]. Alkaloids may be responsible for its anti-malarial, analgesic properties and stomach disorder [33]. Many alkaloids such as morphine, codeine, sanguinarine etc are known for their psychotropic and stimulant, antihyperglycemic, antibacterial, antimalarial and anticancer activities [34,35]. While tannins have been reported to possess astringent properties, which hastens the healing of wounds [36].
Total phenol content of PHF was calculated as 25.24±3.56mg/ml (expressed as GAE % w/w), Similarly Total flavonoid content was 15.61±1.95mg/ml, (expressed as QE % w/w). The results obtained demonstrated that the combination of the herbal extracts increase significantly the amount of TPC and TFC. Williams et al., 2004 reported that Phenolic compounds and flavonoids are reported to have antioxidant and free-radical scavenging activity. They perform scavenging activity by stabilizing free radicals via their conjugated ring structures and hydroxyl groups (Amic et al., 2003). the high quantity of phenolic and flavonoid contents of PHF may contribute to its high antioxidant property. DPPH radical has been used extensively as a free radical to test the reductive ability of extracts or compounds as free radical scavengers or hydrogen donors and to evaluate the antioxidant activity of plant extracts and foods [37,38]. Antioxidants react with DPPH by providing electron or hydrogen atom, thus reducing it to 1,1-diphenyl-2-hydrazine (DPPH-H) or a substituted analogous hydrazine. The deep violet colour of DPPH at maximum absorption of 517 nm is changed to light yellow, colourless or bleached product, resulting in decrease in absorption [39,40]. The scavenging effect of plant extracts on DPPH has been shown to be related to the phenolic concentration of the extracts [38,41-43], which is believed to contribute to their electron transfer /hydrogen donating ability. It could therefore be suggested that PHF contains flavonoids with hydroxyl groups that could stabilize free radicals or scavenge their activities and that the method employed is reliable. The antioxidant activity of PHF for DPPH at 250µg/mL was significant when compared with ascorbic acid and tocophenol as represented in Table 2.
This show that the scavenging activities of the crude extracts on DPPH was increased with increasing concentrations of the extract. Investigating the fifty percent inhibitory concentration (IC50) of the ethanolic extract of the polyherbal formulation was found to be 86.09±1.58 µg/mL at 250µg/mL compared with standard ascorbic acid and tocopherol 56.56±0.39 and 67.37±0.25µg/m. These results revealed the capability of the polyherbal formulation to quench the DPPH radical, which suggested that the extract may be a good antioxidant with high radical scavenging activity. This is in agreement with the study of Arun et al., 2020. they investigated the antioxidant activity of a polyherbal formulation containing three medicinal plants (Mormodica charantia, Andrographis paniculata and withania somnifera. It was reported in their study that the extract possessed free radical scavenging activity in a concentration dependant manner, individual extracts of the drugs show antioxidant potentials comparable to ascorbic acid but in combination their action got boosted dramatically. They reported an IC50 values of drug combination (MC:AP:WS) in DPPH (26.40± 0.56 µg/mL) and ABTS (28.91±0.34 µg/mL), which may be due to synergistic effect of polyherbal formulation with higher phenolic and flavonoid content.
ferric radical antioxidant assay depends on the capability of an antioxidant to reduce Fe3+ to Fe2+ in the presence of TPTZ, developing an intense blue Fe2+-TPTZ complex with an absorption maximum at 593 nm. The absorbance decrease is proportional to the antioxidant content (Hou et al., 2003). The tendency for ferric ions reducing activities of ethanolic extract at different concentrations is shown in Table 3. The IC50 value was found to be 66.95±0.03 µL/mg at 250 µg/mL compared with the standard ascorbic acid (70.05±0.07) the result of the present study showed significantly ferric reducing property and thereby indicates the potential hydrogen-donating capacity of the extract.
Also in the current study, the hydroxyl radical scavenging activity of the ethanolic extract of herbal formulation showed maximum activity of 80.89 at 250 µg/mL, whereas Manitol at the same concentration exhibited 92.20±0.24µg/mL. Thus, the ethanolic extract shows a scavenging property of hydroxyl radicals Hydroxyl radicals are the major active oxygen species causing lipid peroxidation, damage to DNA and proteins (Spencer et al., 1994). According to Diplock 1997, the antioxidant action may be attributed to various mechanisms, some of which may be prevention of chain initiation, decomposition of peroxides, prevention of continued hydrogen abstraction and radical scavenging action. The reducing capacity of the compound/compounds in a mixture serve as a significant indicator of its potential antioxidant activity [44-49]. The reducing capacity of PHF was concentration dependent as presented in (Table 4). Phenolic compounds are powerful chain breaking antioxidants (Shahidi and Wanasundara 1992). The phytochemicals identified in the study may chelate the transition metals and inhibit the formation of free radicals at the initial level itself, Hence the various degree of inhibition of various oxidants can be seen in this aspect in the various scavenging models used in this study. It is reported that the crude extracts of plants are pharmacologically more active than their isolated active principles due to the synergistic effects of various phytochemicals present in the whole extract (Hamberger and Hastettman 1991). It has been reported that administration of C.sativus, A.sativum and C.longa together have shown increase in antigenotoxic effects against cyclophosphamide induced genotoxicity in mice against administration of individual agents separately, Premkumar et al 2004.
Similarly, PHF exhibited scavenging effect on nitric oxide radical (NO•) but concentration dependent. The antioxidant activity of PHF for Nitric oxide scavenging activity at 250µg/mL was significant when compared with the standard ascorbic acid, the IC50 of PHF was 68.13±0.16 and 72.134±.13 for the standard ascorbic acid as presented in Table 5. However, the PHF activity may be due to antioxidant principles in which they compete for oxygen to react with nitric oxide (Ilalenti et al. 1996), and thus inhibit the generation of nitrite. Similar mechanism was reported for the antioxidant action of Sphaeranthus indicus (Linn) by Shirwaiker et al., 2006.
ABTS radical scavenging activity renders a redox functioned proton ion to unstable free radicals and performs a key role in stabilizing detrimental free radicals in the human body (Lee et al., 2015) [50-55]. The PHF powerfully scavenged ABTS radicals produced by the reaction between ABTS and ammonium persulfate, as shown in Table 6. The antiradical activity was found to increased in a dose-dependent manner. The extract showed an IC50 value of 65.54±0.55µg/mL while 65.32±0.33, 96.13±0.79, 67.49±.72 were reported for sodium ascorbate, Trolox and Galic acid respectively at 250 µg/ml. Hence, the ABTS radical scavenging activity of ethanol extract of polyherbal extract indicates its capability to scavenge free radicals, thus preventing lipid oxidation through a chain-breaking reaction [56-61].
Superoxide radical scavenging activity: Superoxide anion is the first reduction product of oxygen which is measured in terms of inhibition of generation of O2 (Kamalakkannan and Stanley 2003). Superoxide dismutase catalyses the dismutation of reactive superoxide anion to oxygen and hydrogen peroxide. The effect of PHF-5 in scavenging anions may be due to inhibition of generation of superoxide. Such an effect was reported for ethanolic extract of B. monnieri (Ghosh et al., 2007; Meena et al., 2012; Tripathi et al., 1996).
Conclusion
In the present study, antioxidant activities of the ethanolic extract of herbal formulation obtained from the medicinal plants Garcinia kola (bitter kola), Allium sativum (Garlic), Zingiber officinale (Ginger) and other Nigeria plants were investigated [62-64]. The potent free radical scavenging activity present in the ethanolic extract of the developed herbal formulation could be due to the presence of the phytochemical constituent present in the polyherbal formulation. The combination of multiple medicinal plants was shown to have higher antioxidant potentials than individual plant extracts which may be due to the combined activity of the individual plant extracts, it is accomplished from the current findings that the herbal formulation could be an effective and alternative treatment regimen for various diseases
Conflicts of Interest: The authors declare no conflict of interest
Acknowledgement: The authors are thankful to the Management of Nigeria Natural Medicine Development Agency and Staffs of Emma-Maria Scientific Research Laboratories Delta State Nigeria
References
- Gill, N.S., Bajwa, J., Sharma, P., Dhiman, K., Sood, S.Evaluation of antioxidant and antiulcer activity of traditionally consumed cucumis melo seeds. J. Pharmacol. Toxicol., 2011; 6(1): 82-89.
- Poonia, P., Niazi, J., Chaudhary, G., Kalia, A.N (2011). In vitro antioxidant potential of Jasminum mesnyi Hance (leaves) extracts. Res. J. Pharm. Biol. Chem. Sci.; 2(1): 348-57.
- Ajith T. A and. Janardhanan K. K (2007), “Indian medicinal mushrooms as a source of antioxidant and antitumor agents,” Journal of Clinical Biochemistry and Nutrition, vol. 40, no. 3, pp. 157–162.
- Liu H and Visner,G. A (2008)“Oxidants and antioxidants,” Molecular Pathology of Lung Diseases, vol. 1, 2008
- Pisoschi AM, Pop A (2015) The role of antioxidants in the chemistry of oxidative stress: a review. Eur J Med Chem 97:55-74.
- Ajith, Y., Dimri, U., Dixit, S. K., Singh, S. K., Gopalakrishnan, A., Madhesh, E., Rajesh, J. B., Sangeetha, S. G. (2017). Immunomodulatory basis of antioxidant therapy and its future prospects: An appraisal. Inflammopharmacology, 25(5), 487–498.
- Sagin, F. G., & Sozmen, E. Y. (2004). Anti-inflammatory effects of dietary antioxidants. Current Medicinal Chemistry - Anti-Inflammatory & Anti-Allergy Agents, 3(1), 19–30.
- Rivera, J. O., Loya, A. M., & Ceballos, R. (2013). Use of herbal medicines and implications for conventional drug therapy medical sciences. Alternative & Integrative Medicine, 2(6), 1–6.
- Wang, S.Y., Jiao, H. (2000) Scavenging capacity of berry crops on superoxide radicals, hydrogen peroxide, hydroxyl radicals and singlet oxygen. J. Agric. Food Chem., ; 48 (11): 5677-84.
- Sreeramulu, D., Reddy, C.V.K., Chauhan, A., Balakrishna, N.,Raghunath, M (2013). Natural antioxidant activity of commonly consumed plant foods in India: effect of domestic processing. Oxid. Med. Cell. Longev., 1-12.
- Sen, S., Chakraborty, R., Sridhar, C., Reddy, Y.S.R., De, B. (2010) Free radicals, current status and future prospect. Int. J. Pharma. Sci. Rev. Res. 3: 91-100.
- Jomova, K.; Vondrakova, D.; Lawson, M.; Valko, M.(2010) Metals, oxidative stress and neurodegenerative disorders. Mol. Cell. Biochem. , 345, 91–104.
- Borar, S., Punia, P., Kalia, A.N. (2011) Antioxidant potential of n-butanol fraction from extract of Jasminum mesnyi Hance leaves. Indian J. Exp. Biol., 49: 39-43.
- Mukhija, M., Singh, M.P., Dhar, K.L., Kalia, A.N (2015) Cytotoxic and antioxidant activity of Zanthoxylum alatum stem bark and its flavonoid constituents. J. Pharmacogn. Phytochem., 2015; 4(4): 86-92.
- Miyake, K., Mickley, L., Litman, T., Zhan, Z., Robey, R., Cristensen, B., Brangi, M., Greenberger, L., Dean, M., Fojo, T., Bates, S.E. (1999) Molecular cloning of cDNAs which are highly overexpressed in mitoxantrone-resistant cells: Demonstration of homology to ABC transport genes. Cancer Res., ; 59(1): 8-13.
- Moukette, B.M., Constant, A.P., Jacques, R.N., Cabral, P.N.B., Bravi, M and Jeanne, Y.N (2015): In vitro antioxidant properties, free radicals scavenging activities of extracts and polyphenols composition of a non-timber forest product used as spice: Monodora myristica. Biological Research.45 (15): 1-17.
- Bhakta, D. and Siva, R. (2012): Amelioration of oxidative stress in bio-membranes and macromolecules by non-toxic dye from Marinda tinctoria (Roxb) roots. Food Chemistry Toxicology. 50:2062-9.
- Agbo, M.O.; Uzor, P.F.; Nneji, U.N.A.; Odurukwe, C.U.E.; Ogbatue, U.B.; Mbaoji, E.C. Antioxidant, total phenolics and flavonoid content of selected Nigerian medicinal plants. Dhaka Univ. J. Pharm. Sci. 2015, 14, 35–41
- Biju, J.; Sulaiman, C.T.; Satheesh, G.; Reddy, V.R.K. (2014) Total phenolics and flavonoids in selected medicinal plants from Kerala. Int. J. Pharm. Pharm. Sci., 6, 406–408.
- Alam, M.N., Bristi, N.J., Rafiquzzaman, M (2013). Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharma. J; 21: 143-152.
- Yu, W., Zhao, Y. and Shu, B. (2004): The radical scavenging activities of radix puerariae isoflavonoids: A chemiluminescence study. Food Chemistry.86:525-529.
- Bajpai VK, Agrawal P, Bang BH, Park YH. (2015). Phytochemical analysis antioxidant and antilipid peroxidation effects of a medicinal plant, Adhatoda vasica. J Front Life Sci ;8:305-12.
- Singh, H., Sidhu, S., Khan, M.U (2016). Free radical scavenging property of β-Aescin and Trans- chalcone: In Vitro study. Eur. J. Pharm. Med. Res.; 3(2): 309-312.
- Panchawat, S., Rathore, K.S., Sisodia, S.S(2010). A review on herbal antioxidants. Inter. J. Pharmtech. Res., ; 2(1): 232-239.
- Singh, H., Sidhu, S., Chopra, K., Khan, M.U(2017). The novel role of β-aescin in attenuating CCl4- induced hepatotoxicity in rats. Pharm, Biol.; 55(1): 749-757.
- Sylvie, D.D., Anatole, P.C., Cabral, B.P., Veronique, P.B. Comparison of in vitro antioxidant properties of extracts from three plants used for medical purpose in Cameroon: Acalypha raceosa, Garcinia lucida and Hymenocardia lyrata. Asian. Pac. J. Trop. Biomed., 2014; 4(2): 625-32.
- Miguel, M.G. Antioxidant activity of medicinal and aromatic plants. A review. Flavour Frager. J., 2010; 25(5): 291-312.
- Spinella, M. The importance of pharmacological synergy in psychoactive herbal medicines. Altern. Med. Rev., 2002; 7(2):130-137.
- Batra, P and Sharma, A.K. (2013): Anti-cancer potential of flavonoids: recent trends and future perspectives. 3Biotech. 3(6): 439- 459.
- Deepak, M.K., Surendra, S.K., Mahabaleshwar, V.H. and Hanhong, B. (2015): Significance of Antioxidant Potential of Plants and its Relevance to Therapeutic Applications. International Journal of Biological Science. 11(8):982–991.
- Kassuya, C.A., Silerstre, A.A., Rehder, V. and Calixto, J.B. (2003): Anti allodynic and antiedematogeni properties of lignin from Phyllanthus amarus in models of persistent inflammatory and neuropathic pain European Journal of Pharmacology, 478,145-153
- Adeneye, A.A., Benebo, A.S. and Agbaje, E.O. (2006): Protective effect of the aqueous leaf and seed extract of phyllanthus amarus on alcohol induced hepatotoxicity in rats. West African Journal of Pharmacological Drug Research. 23:42- 50.
- Okwu, D.E. and Josiah, C. (2006): Evaluation of the chemical composition of two Nigerian medicinal plants. African journal of Biotechnology. 5(4):357-361.
- Kaisa, AS., Achim, M., Lenka, J., Laura, E., Korhonen, Minna,R and Risto, O.J.(2011): Inhibition of human drug metabolizing ctyochrome P450 enzymes by plant isoquinoline alkaloids. Phytomedecine: International Journal of Phytotherapy and phytopharmacology. 18(6): 533-538.
- Noureddine, B. (2018): Pharmacuetical activity of alkaloids: A review. Asian Journal of Botany. (1): 1-6.
- Okwuonu, U., Baxter-Grillo, D.C., Njoya, H. and Iyemene, P.T. (2017): Proximate and elemental constituents of Aspilia Africana (wild flower). Journal of Medicinal Plants Studies. 5(4), 22-27.
- Porto, C.D., Calligaris, S., Celloti, E. and Nicoli, M.C. (2000). Antiradical properties of commercial cognacs assessed by the DPPH•test. J. Agric.Food Chemistry. 48: 4241-4245.Prakash, A. Antioxidant activity. Anal. Prog. 19: 1-6.
- Manian, R., Anusuya, N., Siddhuraju, P. and Manian, S. (2008). The antioxidant activity and free radical scavenging potential of two different solvent extracts of Camellia sinensis (L.) O. Kuntz, Ficus bengalensis L. and Ficus racemosa L. Food Chem.107: 1000-1007.
- Miliauskas, G., Venskutonis, P.R. and Van Beek, T.A. (2004). Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chemistry. 85: 231-237.
- Koksal, E., Gulcin, I., Beyza, S., Sarikaya, O. and Bursal, E. (2009). In vitro antioxidant activity of silymarin. J. Enz. Inh. Med. Chem. 24(2): 395-404.
- Akinmoladun, A.C., Obuotor, E.M. and Farombi, E.O. (2010). Evaluation of Antioxidant and Free radical scavenging capacities of some Nigerian Indigenous medicinal plants. J. Med. Food. 13(2): 1-8.
- Sen, S., De, B., Devanna, N. and Chakraborty, R. (2013). Total phenolic, total flavonoid content, and antioxidant capacity of the leaves of Meyna spinosa Roxb., an Indian medicinal plant. Chin J. Nat. Med. 11(2): 149-157.
- Das, N., Islam, M.E., Jahan, N., Islam, M.S., Khan, A., Islam, M.R. and Parvin, M.S. (2014). Antioxidant activities of ethanol extracts and fractions of Crescentia cujete leaves and stem bark and the involvement of phenolic compounds. BMC Complement Altern. Med. 14: 45.
- Chang, C.C., Yang, M.H., Wen, H. M., Chern, J.C (2002) Estimation of total flavonoids content in propolis by two complementary colorimetric methods. J. Food Drug Anal., 10, 178-182.
- Antwi-Boasiako C, Abubakari A (2011). Antimicrobial and phytochemical properties of crude extracts of Garcinia kola heckels stems used for oral health. Res. J. Pharmacol. 5(5):68-76.
- Anbudhasan, P., Surendraraj, S., Karkuzhali, S. and Sathishkumaran, S. (2014): Natural antioxidants and its benefits. International Journal of Food and Nutritional Sciences 3(6). 225-232.
- Akinmoladun AC, Ibukun EO, Afor E, Akinrinlola BL, Onibon TR, Akinboboye AO, Obutor EM. Farombi EM. (2007) Chemical constituents and antioxidant activity of Alstonia boonei. Afr J Biotechnol 6(10):1197-1201.
- Anwar, F.; Ashraf, M.; Bhanger, M.I(2005). Interprovenance variation in the composition of Moringa oleifera oilseeds from Pakistan. J.Am. Oil Chem. Soc., 82, 45–51.
- Dhakad, A.K.; Ikram, M.; Sharma, S.; Khan, S.; Pandey, V.V.; Singh, A. (2019) Biological, nutritional, and therapeutic significance of Moringa oleifera Lam. Phytotherapy Res. 33, 2870–2903.
- Fernandez-Lopez, N. Zhi, L. Aleson-Carbonell,J.A. Perez- Alvarez and V. Kuri, Antioxidant and antibacterial activities of natural extracts: application in beef meatballs, Meat Sci., 69, 2005, 371-380.
- Fadzai, B., Elaine, C., Stanley, M. (2014): Evaluation of Nitrite radical scavenging properties of selected Zimbabwean plant extracts and their phytoconstituents. Journal of Food Processing. 1 (8 )2356-7384.
- Farombi EO, Owoeye O (2011). Antioxidative and chemopreventive properties of Vernonia amygdalina and Garcinia biflavonoid. Int. J. Environ. Res. Public Health 8(6):2533-2555.
- Goyal BR, Agrawal BB, Goyal RK, Mehta AA (2007). Phytopharmacology of Moringa oleifera Lam. an overview. Natural Product Radiance 6(4):347-353.
- Nepolean P, Anitha J, Emilin Renitta R. (2009) Isolation, analysis and identification of phytochemical and antimicrobial activity of Moringa oleifera Lam. Current Biotica; 3 (1):33-39.
- Ide N, Lau BH. (1997) Garlic Compounds Protect Vascular Endothelial Cells from Oxidized Low Density Lipoprotein-induced Injury. J. Pharm. Pharmacol. ;49(9):908–911.
- Ibikunle GF, Ogbadoyi EO (2011). Pharmacological evaluation of Garcinia kola nuts for antitrichomonal activity. Int. J. Pharmacol. Bio. Sci. 2(2):264-269.
- Jayaprakasha G. Kand . Patil, B.S (2007) In vitro evaluation of the antioxidant activities in fruit extracts from citron and blood orange, Food Chem., 101, 410-418.
- Madaan, R., Bansal, G., Kumar, S., Sharma, A. Estimation of total phenols and flavonoids in extracts of Actaea spicata roots and antioxidant activity studies. Indian J. Pharm. Sci., 2011; 73: 666-669.
- Nepolean P, Anitha J, Emilin Renitta R. (2009) Isolation, analysis and identification of phytochemical and antimicrobial activity of Moringa oleifera Lam. Current Biotica; 3 (1):33-39.
- Omage K, Erifeta OG, Esosa US, Sunday JJ, Ajeigbe OK (2011). Evaluation of hypoglycemic and antioxidative properties of aqueous extract of Garcinia kola seeds in Wistar Rats. Curr. Res. J. Bio. Sci. 3(4):326-329
- Sanchez RM, Rojas-Graü MA, Odriozola-Serrano I, González-Aguilar GA, Martín-Belloso O (2009). Effect of minimal processing on bioactive compounds and antioxidant activity of fresh Cola nitida plant. Postharv. J. Biol. Technol. 51(3):384-390.
- Saravanan G, Prakash J (2004). Effect of garlic (Allium sativum) on lipid peroxidation in experimental myocardial infarction in rats. J Ethnopharmacol.;94(1):155–158.
- Stadtlander, T.; Becker, K. (2017) Proximate Composition, Amino and Fatty Acid Profiles and Element Compositions of Four DifferentMoringa Species. J. Agric. Sci. 9, 46.
- Ujah, O. F., 2 Mohammad, Y. Y. and 1 Dewua, C. M (2021) Quantification, Antioxidant and Free Radical Scavenging Potentials of Polyphenols from Crude Extracts of Phyllanthus amarus leaves ChemSearch Journal 12(1): 97 – 107.

