Clinical Pharmacists: Essential Players in Times of Crisis Experience of Morroco
Chaimae Rhaymi1,2,* and Aicha CHAIBI1,2
1Department of Therapeutic Chemistry and Clinical Pharmacy, Faculty of Medicine and Pharmacy of Rabat, Mohammed-V University, Morocco
2Ibn Sina University Hospital Centre, Rabat, Morocco
Received Date: 18/02/2023; Published Date: 11/04/2023
*Corresponding author: Chaimae Rhaymi, Department of Therapeutic Chemistry and Clinical Pharmacy, Faculty of Medicine and Pharmacy of Rabat. Mohammed-V University, Rabat, Morocco
Abstract
Introduction: Tuberculosis (TB) remains one of the world’s deadliest communicable diseases. There is an increased risk of transmission of Tuberculosis (TB) in institutional settings and overcrowded areas. Early detection, rapid diagnosis and early initiation of treatment are the cornerstone for containing the spread of infection., This is particularly important during outbreaks as it interrupts the transmission chain. Screening of contacts allows detection of active disease and latent TB infection (LTBI) in order to initiate the right treatment.
Methods: This was an outbreak investigation of TB cases in Seolwane village, in the Maunatlala health catchment area in Palapye DHMT central district, Botswana., with a population of 1513 people an expert team was set-up in order to investigate the outbreak. The primary goal of the outbreak investigation was to control the disease within the affected population and to prevent the disease from spreading to other populations. The team responded to the outbreak using a systematic approach following the outbreak investigation steps.
Results: We report a cluster of 16 TB cases at Seolwane village. The median age was 36 years (IQR 17-50). Of the 16 patients, all were new notifications. 62.5% were males and 43.5% were HIV positive on treatment.The epidemiologic curve depicted a propagated source outbreak
Conclusion: The outbreak investigation afforded detection of cases who were probably living with the disease and transmitting it and also early identification of more patients who benefited from early management of TB, preventing further morbidity and transmission. Outbreak investigations are an important intervention in any communicable disease.
Keywords: Tuberculosis; Outbreak investigation; Botswana
Introduction
The ongoing COVID-19 pandemic has disrupted a globalized, highly interconnected and urbanized society, changing our habits and lifestyles [1]. The Moroccan health system had to adapt quickly and continuously to cope with the two waves of contamination, which arrived in Morocco in March/April and then in October/November 2020 [2].
Manifestations of the disease can range from asymptomatic infection to mild upper respiratory tract disease to severe viral pneumonia with respiratory failure and even death [3].
Population demographics and prevalence of comorbidities differ from country to country, but published studies have shown a high frequency of hypertension, diabetes [4] and coronary heart disease [5]. Obesity was particularly high in patients admitted to intensive care for SARS-CoV-2 [6].
Specialized hospitalization units for the management of this disease (COVID units) have been created within our hospital center. Clinical pharmacy activities have been implemented in these COVID units. Clinical pharmacy has been widely developed since 2017 within our institution. Their mission is to optimize the medication management of patients, in a way that complements the pharmaceutical validation activity performed by the hospital pharmacist.
Terms of Deployment of Clinical Pharmacy Activities in the COVID Unit
In March 2020, clinical pharmacists deployed clinical pharmacy in COVID units. Initially, this was a request for assistance in the use of Personal Protective Equipment (PPE). The hospital's clinical pharmacy department proposed a series of actions to standardize practices, train teams, and raise awareness about environmental health and PPE. It reinvented itself during this health crisis and adapted effectively to the new situation by making personal protection and environmental hygiene a top priority in addition to its usual missions [7].
Given that the Technical and Scientific Committee of the Ministry of Health has decided to prescribe a combination of chloroquine/hydroxychloroquine with azithromycin for all symptomatic patients confirmed with COVID-19 as of March 23, 2020 [8].
This off-label use exposes to an iatrogenic risk. The clinical pharmacy department of Ibn Sina Hospital has developed practical prescription assistance charts: two charts on the two molecules of the therapeutic protocol, one chart on drugs that prolong the QT interval, one chart on antiemetics that can be associated with the protocol [9].
A clinical pharmacist has joined one of these units, treating patients with COVID- 19, in order to bring their expertise to the nursing staff from different departments of the institution, who are not accustomed to this specific management.
The objective of this study was to analyze the pharmaceutical interventions performed on the prescriptions of patients in the COVID units of our institution.
Material and Method
Study design and patients:
The study is prospective and was within a COVID unit at Ibn Sina Hospital in Rabat, Morocco, over a four months period from November 2020 to February 2020. It is a 9-bed unit taking care of COVID patients.
All prescriptions were subject to pharmaceutical analysis and validation by a full-time clinical pharmacy resident who participates in the various COVID service meetings. All prescription analyses were performed according to the definition of the French Society of Clinical Pharmacy [10] and all pharmaceutical interventions (PI) were sent to the senior pharmacist.
The pharmacist attended the medical staff. Data collected included demographic information (age, gender and Body Mass Index (BMI)), comorbidities (hypertension and diabetes). For each hospitalized patient, medical prescriptions were analyzed daily throughout the stay by a clinical pharmacist. It exploits all the patient's data: medical history, hemodynamic and physiological functions (capillary glycemia, heart and respiratory rate, blood pressure and diuresis), biology (kidney function, liver function, blood chemistry, blood count, coagulation tests and microbiological data).
When the clinical pharmacist deems it necessary, he/she issues a pharmaceutical opinion regarding the therapeutic problems identified, which he/she communicates orally to the prescribing physician (resident or Professor). After discussion, the prescriber accepts or not the proposed change.
A pharmaceutical intervention chart (IP) is then systematically filled out in order to record various elements and to have, in this way, the information necessary for the analysis.
Data was entered into an anonymized Excel database.
Results
Patient characteristics
During this period, 818 prescriptions were analyzed, 56 of which were the subject of one or more pharmaceutical interventions. A total of sixty-three pharmaceutical interventions were performed. The IPs involved 41 women and 22 men. The average age of the patients was 62 years, with approximately 24% >75 years, as shown in Table 1.
The prevalence of patients with at least one comorbidity was 76%. Hypertension was the most prevalent (34%), followed by diabetes (28%), chronic kidney disease (20%), cardiovascular disease (17%) and chronic respiratory disease (1%).
Table 1: Demographic and clinical characteristics.

Pharmaceutical Activity and Drug-Related Problems
Sixty-three pharmaceutical interventions were performed by the clinical pharmacist.
Figure 2 and 3 show drug-related problems and PIs for patients with COVID-19-positive status.
- Therapeutic Category:
Systemic general anti-infective agents (ATC J) were the most responsive PIs with a rate of 25% (16/63), followed by corticosteroids 23% (15/63), hydroxychloroquine 19% (12/63) and anticoagulants 17% (11/63). (Figure 1)
- drug-related problems:
In our department, the problem of dosage, particularly overdose, ranked first with a percentage of 38% (Figure 2). Among them were overdosage of corticosteroids, non-adaptation of hydroxychloroquine to the kidney function of the patients, prescription of an antiplatelet agent to a patient who is already on an antiplatelet agent for his initial pathology.
Followed by drug use without indication (17 %): forgetting to stop vitamin therapy and antibiotics.
Non-conformity to guidelines (12%) was mainly due to the use of drugs with dosages that were not in accordance with the national protocol.
- Pharmaceutical interventions:
In total, dosage adaptation (40%) was the most prevalent PI, including adaptation of hydroxychloroquine to kidney function, followed by PIs that led to stopping the drug (29%). (Figure 3)
Medical Acceptance Rate:
The outcome of the PIs was as follows: 95% were accepted by the prescriber, 5% were not accepted

Figure 1: Distribution of pharmaceutical interventions according to drug categories.
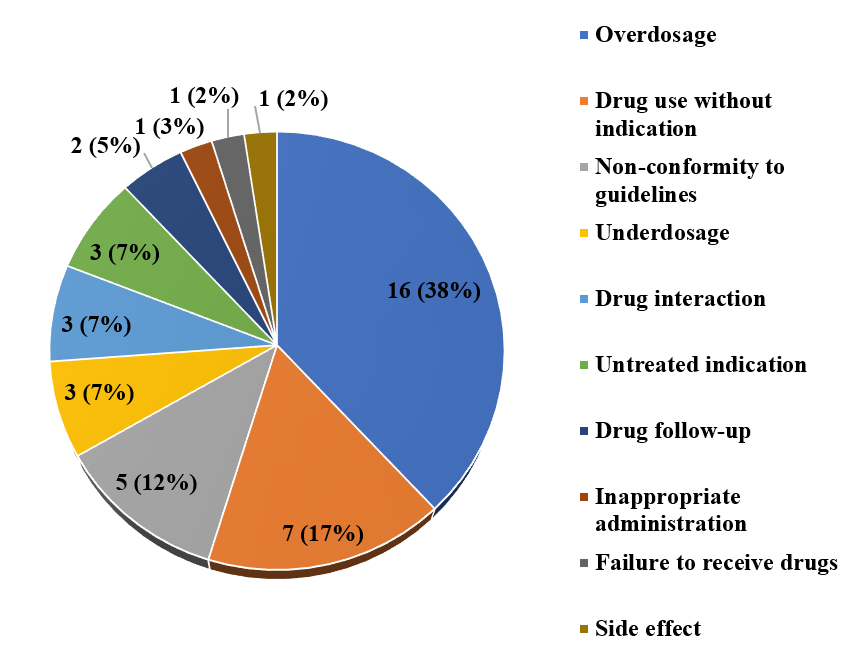
Figure 2: Types of drug-related problems identified.
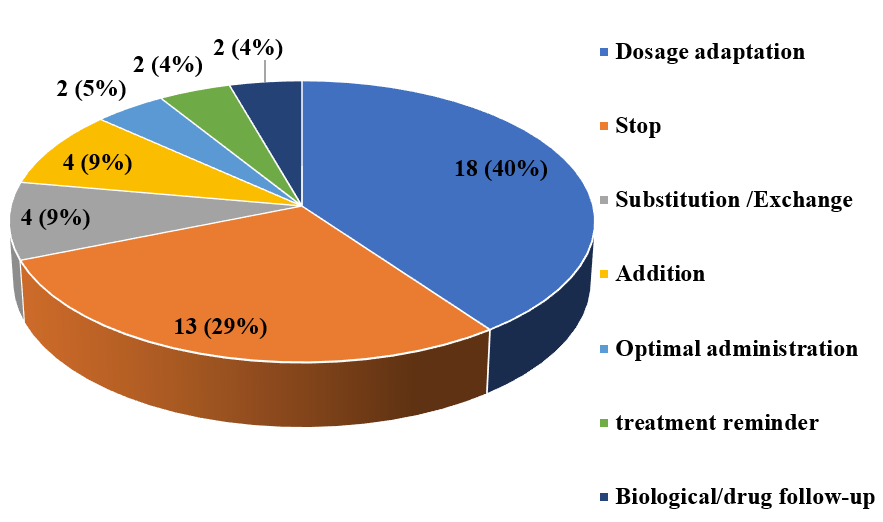
Figure 3 : Distribution of pharmaceutical interventions.
Discussion
Many studies have been conducted on the essential services provided by pharmacists during the COVID-19 pandemic. Pharmacists were likely to play an important role during this pandemic in multiple aspects, such as providing drug information to health care providers [11,12], patient counseling [13] and alternative suggestion therapy [14]. This study highlights the experience of integrating clinical pharmacy within a COVID unit at the university hospital center.
The clinical pharmacist is presented as a complementary health professional in the management of these patients, allowing them to secure their care pathways. They played an extremely important role in ensuring patient safety during the coronavirus pandemic, according to Li et al [15].
In our institution, the clinical pharmacy team has been reorganized to provide drug management for patients infected or suspected of having COVID-19.
The first step in securing the management of patients with COVID-19 was the elaboration of charts by a multi-professional therapeutic group. This was implemented during the first wave. It is composed of (infectiologists, pharmacists, etc.) who develop practical help charts that constitute a reliable database for the management of COVID patients [9].
In our study, the patients were mainly elderly women with hypertension and diabetes, the results of our study are in line with the American study by Richardson et al, which included 5700 American patients, which identified the same comorbidities with higher rates (56.6%, 33.8% for hypertension and diabetes respectively) [4].
During this period, the main drug-related problems were related to general anti-infective agents for systemic use (ATC J) which accounted for 25% (16/63). These problems were also identified in a French study by Mabille C. et al [16]. This therapeutic category was the cornerstone of the treatment of suspected or confirmed respiratory co-infections. Indeed, antibiotics are often prescribed in overdose, especially in patients with impaired kidney function.
For ATC P “Antiparasitics, insecticides and repellents”, clinical pharmacists mainly participated in optimizing the appropriate use of hydroxychloroquine, particularly in cases of interaction with drugs that prolong the QT interval.
Due to the many drug interactions of hydroxychloroquine with certain drugs that cause QT prolongation, it was essential to control their use, especially since they were very often prescribed by doctors who were not accustomed to their use.
This appropriate use has been framed as follows:
- An electrocardiogram (ECG) is done systematically before the initiation of treatment and during treatment.
- Development of a prescribing aid in cases of kidney or liver failure.
- Vigilance when hydroxychloroquine is associated with other drugs that prolong QT interval (the association must be discussed with a senior)
- Management of adverse reactions to hydroxychloroquine
During this study period, problems related to antithrombotic agents (ATC B) represent 17% (11/63). These PIs were important because of the high risk of thromboembolic complications in patients with COVID-19. In the majority of cases, a dosing error was observed and the PIs led to a change in treatment [17].
Thus, some pharmaceutical interventions have focused on the switch from oral anticoagulants (direct oral anticoagulants (DOACs) or vitamin K antagonists (VKAs)) to curative heparin therapy: low molecular weight heparin (LMWH), unfractionated heparin (UFH) to avoid the risk instability and drug interactions with the different COVID-19 treatments, especially with hydroxychloroquine.
The panel states that continuation of prophylactic anticoagulation with a DOAC after hospital discharge should be proposed in COVID-19 patients with two or more risk factors for venous thromboembolic disease (VTE) [18].
Prescribing a DOAC instead of injectable LMWH avoids the need for nurses to travel to patients who are unable to self-inject [18].
In more than a quarter of cases, a dosing error in case of relay with a DOAC was observed at hospital discharge. The PIs led to a change in treatment dose.
In the same context of treatment with an anticoagulant, cases of adaptation of the dosage to the kidney function in case of overdose have been reported in patients with kidney failure.
For the ATC H (Systemic hormonal drugs), the clinical pharmacist was mainly involved in the optimization of the appropriate use of corticosteroids. The High Council of Public Health (HCSP) recommends the application of the RECOVERY protocol, i.e., 6 mg/day of dexamethasone for 10 days, and failing that, the use of methylprednisolone at a dose of 32 mg/day, or prednisone at a dose of 40 mg/day, or finally, as a last resort, hydrocortisone at a dose of 160 mg/day for 10 days, with a gradual decrease in dose over three or four days [19]
Indeed, corticosteroids are often over-prescribed or over-dosed, and most PIs have been recommended by reducing the dosage or adapting the frequency of intake, as is the case of dexamethasone which was prescribed at a dosage of 6 mg/day but in three doses instead of one, and also the duration of treatment.
Medical acceptance rate:
According to Renaudin et al [20], a PI is considered accepted when the prescriber changes the prescription in accordance with the PI within a period not exceeding 48 hours. In our study, this percentage was significant and amounted to 95%. This reflects the familiarity of the physicians in the unit with clinical pharmacy as they were working in medical units that already had a clinical pharmacist integrated into the care team.
Conclusion
The presence of clinical pharmacists has led to a significant reduction in prescription problems related to drugs, particularly for general anti-infective drugs and antithrombotic drugs. This feedback shows us that their presence is all the more important in this context of health emergency to secure the management of COVID patients.
Conflicts of Interest: Authors declares no conflicts of interest
Grant Information: The author(s) received no specific funding for this work.
References
- Bragazzi NL, Mansour M, Bonsignore A, Ciliberti R. The role of hospital and community pharmacists in the management of COVID-19: towards an expanded definition of the roles, responsibilities, and duties of the pPharmacy, 2020; 8(3): 140.
- Ministère de la santé Maroc. Épidémie de COVID- 19 au Maroc. Site internet : https://www.sante.gov.ma. [accès en date du 22/05/2021].
- Adhikari SP, Meng S, Wu YJ, et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infectious Diseases of Poverty, 2020; 9(1): 1-12.
- Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. Jama, 2020; 323(20): 2052-2059.
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The lancet, 2020; 395(10229): 1054-1062.
- Simonnet A, Chetboun M, Poissy J, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation.Obesity, 2020; 28(7): 1195-1199.
- Chaibi A, Mrani Alaoui A, Lasri FZ, Abouqal R, Madani N. New strategies proposed by clinical pharmacists in the management of the COVID-19 pandemic in a developing country [Nouvelles stratégies proposées par les pharmaciens hospitaliers dans la gestion de la pandémie de Covid-19 dans un pays en développement]. Le pharmacien hospitalier & clinicien, 2021; 56(2): 133-136.
- Saqrane S, El Mhammedi MA. Review on the global epidemiological situation and the efficacy of chloroquine and hydroxychloroquine for the treatment of COVID-19. New microbes and new infections, 2020; 35:
- Chaibi A, Mrani Alaoui A, Fatima-zahra L ,Redouane A, Naoufel M. Clinical Pharmacists role in the therapeutic management of COVID-19 patients in Morocco. Medical Research Archives, 2020; 8(10).
- Juste M. Recommandation de bonne pratique en pharmacie clinique. Analyse d’ordonnance et niveaux d’analyse pharmaceutique. Le pharmacien hospitalier et clinicien, 2012; 47(4): 293–29
- Dhahri AA, Arain SY, Memon AM, et al.The psychological impact of COVID-19 on medical education of final year students in Pakistan: A cross-sectional study. Annals of medicine and surgery, 2020; 60: 445-450.
- Louiselle K, Elson EC, Oschman A, Duehlmeyer S. Impact of COVID-19 pandemic on pharmacy learners and preceptors. American journal of health- system pharmacy, 2020; 77(14): 1097-1099.
- Meng L, Qiu F, Sun S. Providing pharmacy services at cabin hospitals at the coronavirus epicenter in China. International journal of clinical pharmacy, 2020; 42(2): 305-308.
- Ong SW, Tan YK, Chia PY, et al. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. Jama 2020;323(16):1610-1612.
- Li H, Zheng S, Liu F, Liu W, Zhao R. Fighting against COVID-19: Innovative strategies for clinical pharmacists. Research in social and administrative pharmcy, 2021; 17(1): 1813-1818.
- Mabille C, Joseph C, Schmit J, Belhout M, Terrier-Lenglet A. Analyse pharmaceutique des prescriptions de patients COVID. Médecine et Maladies Infectieuses, 2020; 50(6):
- Perez M, Masse M, Deldicque A, et al. Analysis of clinical pharmacist interventions in the COVID-19 units of a French university hospital. European Journal of Hospital Pharmacy, 2022; 29(e1): e30-e35.
- Stephan D, Cordeanu M, Mirea C, et al. Maladie veineuse thromboembolique et COVID-19 [Venous thromboembolic disease and COVID-19].La Presse Médicale Formation, 2021; 2(1): 33-38.
- Haut Conseil de la santé publique Covid-19 : conditions d’utilisation de la dexaméthasone ou d’autres corticoïdes de substitution chez les patients hospitalisés, 2022.
- Renaudin P, Esteve MA, Berbis J, Delorme J, Pisano P, Honore S. Les interventions pharmaceutiques dans un centre hospitalier universitaire : influence du mode de transmission sur leur acceptation par le prescripteur.Le Pharmacien Hospitalier et Clinicien, 2016; 51(1): 2–8.

