Cyclosporin A Differentially Regulates Matrix Metalloproteinases in Gingival Fibroblasts
Colonya C Calhoun* and Robert Chiu
Division of Oral & Maxillofacial Surgery and Dentistry, Oral and Maxillofacial Surgery, Harbor – UCLA Medical Center Lecturer, UCLA School of Dentistry, USA
Received Date: 10/02/2023; Published Date: 10/04/2023
*Corresponding author: Colonya C Calhoun, Program Director, Division of Oral & Maxillofacial Surgery and Dentistry, Oral and Maxillofacial Surgery, Harbor – UCLA Medical Center Lecturer, UCLA School of Dentistry, USA
Abstract
A disturbance in the metabolism of the extracellular matrix (ECM) has been implicated in the pathogenesis of Cyclosporin A (CsA) induced gingival overgrowth. The family of matrix metalloproteinases (MMPs) such as collagenases (MMP-1, -13), gelatinases A (MMP-2) and B (MMP-9) which are secreted in many cell types, play an essential role in the degradation of the ECM. The aim of this study is to determine whether the immunosuppressant drug, CsA, suppresses collagenases and gelatinases A and B expression in primary cultured human gingival fibroblasts (HGFs) upon exposure to the drug. Conditioned media from HGFs used in this study were isolated either from healthy individuals or a renal transplant patient who previously displayed gingival overgrowth during past CsA treatment. Zymography showed a decrease in the activity of 42 (consistent with MMP-1 and MMP-13), 62 (MMP-2) and 82 kDa (MMP-9) molecular weight molecules. The decrease in MMP-1 expression by CsA was due to destabilization of MMP-1 mRNA while the decrease in MMP-2 was found to be limited to the secreted protein expression suggesting that CsA’s affects are limited to the secretion of MMP-2. CsA also appeared to inhibit NFkappaB which is known to transcriptionally activate MMP-9 expression. These results suggest that MMP-1, -2, and -9 all may contribute to the pathogenesis of CsA induced gingival overgrowth by different mechanisms.
Introduction
Cyclosporin A (CsA) is a widely used immunosuppressant, which can be considered a “wonder drug” because of its variety of uses. It is mainly used for prevention of organ transplant rejection, as well as a multitude of diseases such as systemic lupus erythematous, insulin-dependent diabetes mellitus, psoriasis, rheumatoid arthritis, multiple sclerosis, AIDS, myasthenia gravis, erosive lichen planus, primary biliary cirrhosis, and schistmiasis [1]. CsA is produced from the fungus, Tolypocladium inflatum, and its action is directed to the inhibition of T cell proliferation via inhibiting the production of IL-2 [2]. CsA has many side effects including nephrotoxicity, hepatotoxicity, neurotoxicity, lymphoproliferative neoplasms, and hirutism [3]. The most important adverse effect in the oral cavity is gingival overgrowth or enlargement. This adverse tissue response is a multifactoral phenomenon that occurs in approximately 20-40% of adult patients [4,5]. Patients who exhibit CsA-induced gingival overgrowth have been termed responders. Those who do not have CsA-induced gingival overgrowth are termed nonresponders [1]. CsA-induced overgrowth also appears to be isolated mainly in the canine-to-canine region [3]. Despite periodontal therapy including gingivectomy, 27% of patients with severe gingival overgrowth had recurrence [6].
Historically, gingival enlargement was referred as hyperplasia but many histological studied failed to demonstrate an increase in the density of fibroblasts in the connective tissue stroma [1,2,7,8]. Previous studies examined changes in various extracellular matrix macromolecules (type I and type IV collagens) in patients exhibiting drug induced gingival overgrowth [8,9]. Using immunohistological study, excess accumulation of type I and type IV collagens was observed, however the number of fibroblasts remain unchanged. Other studies examined the role of prostaglandins and glycosaminoglycans in drug induced gingival enlargement but the results have been controversial [10,11]. Regardless of which ECM component involved, this side effect of CsA reflects a metabolic disturbance in the homeostasis of the ECM [1]. ECM metabolism is orchestrated by groups of neutral proteases called matrix metalloproteinases (MMPs) and a family of inhibitory proteins called tissue inhibitors of metalloproteinases (TIMPs). There are more than 20 MMPs that have been classified into four groups based on their substrate: 1) collagenases (MMP-1, -8, -13) which cleave at a specific site in the triple helical collagen fibrils causing fragmentation and subsequent denaturing into gelatin; 2) gelatinases A and B (MMP-2, -9) which degrade the denatured collagens produced by collagenases; 3) stromelysins (MMP-3, -10, -11, -12) whose major substrate is proteoglycans and 4) membrane type MMPs (MMP-14, -15, -16, -17) which can activate other MMP’s and degrade collagens. In general, MMPs can degrade all ECM proteins. Specifically, MMP-1, -8 and -13 degrade type I, II and III collagen, MMP-2 and 9 degrade type IV, V, VII and X as well as elastin, fibronectin and denatured collagen type I [12]. In this study, we provide evidence that CsA treatment decreases the expression of MMP-1 MMP-2 and MMP-9 in normal human gingival fibroblasts (NHGFs) obtained from healthy patients as well as the renal transplant patient who displayed gingival overgrowth secondary to CsA use (CHGFs). We also show that the suppression of MMP-1 is due to destabilization of the message while suppression of MMP-2 may be due to inhibition of the secretion of MMP-2. We also demonstrate that CsA suppressed NFkappaB binding which may result in -transcriptional inhibition of MMP-9 expression.
Materials and Methods
Tissue origin, cell culture and reagents - Gingival fibroblasts were isolated from 5 healthy individuals designated as NHGFs and one patient taking CsA designated as CHGFs. This renal patient previously took 200 mg of CsA twice a day for approximately 3 years, displayed gingival overgrowth, but stopped taking CsA more than 2 months prior to surgery and had significant resolution of overgrowth at the time of surgery/tissue collection. The isolated fibroblasts were cultured in complete media (10% fetal bovine serum, 100 U/ml penicillin, and 100 µg/ml streptomycin). Second to fifth passages of HGFs were exposed to 500 ng/ml of CsA or remained untreated in complete media for 48 hours at 37ºC prior to harvest for all experiments.
Informed consent was obtained in compliance with UCLA office for the protection of research subjects internal review board. Fibroblasts from both sources were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (Gibco) and 100 units/ml penicillin, 50 g/ml streptomycin (Gibco) in a humidified atmosphere of 5% CO2 at 37ºC. Cyclosporin A, and Actinomycin D were obtained from Sigma. Recombinant human TNFα was obtained from US Biological. Monoclonal antibodies for MMP-1, -2, TIMP-2, MT1-MMP, phosphorylated and unphosphorylated IκBα were purchased from Calbiochem. Secondary antibody alkaline phosphatase-conjugated rabbit anti-mouse IgG was purchased from Promega.
Collagen and Gelatin Zymography- Zymographic analysis was carried out as described by [13]. Conditioned medium was harvested and protein concentration was measured using the Bradford method. 100μg of protein were loaded onto a 10% polyacrylamide gel (10 cm height, 0.75 mm thickness) containing 500 mg/ml of rat-tail collagen (BD Biosciences, Bedford, MA) or 1mg/ml of gelatin (Sigma St. Louis MO) dispersed in buffered solution consisting of 2.5 ml of 30% acrylamide/BIS, 1.88 ml of 1.5 M Tris▪ Cl containing 0.4% SDS, 3.13 ml bidistilled water, pH 8.8. Stacking gel contained 4% polyacrylamide in 0.5 M Tris▪ Cl containing 0.4% SDS, pH 6.8. Gels were polymerized by adding 50 ml of 10% ammonium persulfate and 10 ml of 0.1% TEMED. Gels were run under Laemmli conditions (24 mM Tris, 192 mM glycine, 3.47 mM SDS; 40 mA, 1 hr). Gels were washed twice in 200 ml of 2.5% Triton X-100 for 30 minutes, then incubated in activation buffer containing 50mM Tris-Cl, 150mM NaCl and 5mM CaCl2 for 32-48 hours at 37°C. Gels were stained with Coomassie stain Solution (BioRad Lab., Hercules CA, USA) to visualize the relative collagenolytic and gelatinolytic activities as clear bands in a blue background.
Methods
Northern Blot - MMP-1 and -2 cDNA probes were purchased from ATCC (Manassas, VA). The 5.1 kb MMP-1 and the 4.8 kb MMP-2 probes were digested with SmaI/HindIII or BamHI/SmaI restriction enzymes (Invitrogen, Carlsbad, California), resulting in a 2.0 kb fragment corresponding to MMP-1 or a 3.0 kb fragment corresponding to MMP-2. The probes were purified using Geneclean II Kit (Bio 101, La Jolla, CA) and labeled with [32P]-adCTP using Random Primed DNA Labeling Kit (Boehringer Mannheim). Total RNA was isolated using Total RNA Isolation Reagent for Liquid Samples (TRIzol®LS Reagent) following manufacturers instructions and denatured @ 95ºC for 5 minutes in 50% formamide, 2.2-M formaldehyde, 20-mM morpholinepropane sulfonic acid, 5 mM NaOAc, and 1 mM EDTA. The RNA was separated by electrophoresis through a 1% agarose gel containing 2.2 M formaldehyde, followed by transfer to a nylon membrane in 20X SSC (1X SSC = 0.15 M NaCl and 0.015 M sodium citrate) and immobilized by ultraviolet crosslinking (Stratalinker; Stratagene, Inc., San Diego, CA) Probes specific for MMP-1 and 2 were hybridized to the membrane using 50% formamide, 5X SSC, 50 mM sodium phosphate (pH 6.8), 0.1mg/ml of sonicated salmon sperm DNA and 2X Denhardt’s (1X Denhardt’s solution: 0.02% bovine serum albumin, 0.02% Ficoll, and 0.02% polyvinylpyrrolidone) solution, hybridization was done at 42º C overnight. Klenow polymerase was used to generate [32P]-labeled hybridization probes. Filters were washed in 0.1X SSC, 0.1% SDS at 55˚C and exposed to Kodak XAR-5 film at –80 C with intensifying screens for varying duration of time. Densitometric scan was used for analysis of autoradiograms on a Pharmacia laser densitometer (Ultra Scan XL).
Isolation of cell lysates - Conditioned media was removed and culture plates rinsed with PBS at room temperature. 600 µl of RIPA buffer (with freshly added inhibitors) was added to a 100 mm cell culture plate. Cells were harvested using a cell scraper and passed through the 21-gauge needle to shear the DNA. 10 µl of 10 mg/ml PMSF stock was added and incubated for 30-60 minutes on ice. Cell lysates were centrifuged at 10,000xg for 10 minutes at 4° C. The supernatant fluid is the total cell lysate. (RIPA buffer: 1x PBS (137 mM NaCl, 2.7 mM KCl, 4.3 mM sodium phosphate dibasic, 1.4 mM potassium phosphate monobasic), 1% Nonidet P-40 (Amaresco) or Igepal CA-630 (Sigma Chemicals), 0.1% SDS.
Nuclear Extraction - HGFs were trypsinzed centrifuged for 10 min at 1200 rpm. HGFs will then be washed with 1ml of ice-cold PBS, centrifuged for 5 min at 1200 rpm at 4°C and resuspended in buffer A (10mM HEPES, 10mM KCL, 2mM MgCl2, 1mM DTT, 0.1mM EDTA, 0.1mM PMSF, 5 mg/ml antipain and 5 mg/ml leupeptin). After incubation on ice for 15 min, 25 ml of 10% Nonidet P-40 was added, and the suspension vortexed for 15s, then centrifuged for 30s at 14000 rpm. The resultant pelleted nuclei were resuspended in 50ml of buffer C (50mM HEPES, 50mM KCL, 300mM NaCl, 0.1 mM EDTA, 1mM DTT, 0.1 mM PMSF, 10% (v/v) glycerol) mixed for 20 min at room temperature and centrifuged for 5 min at 14000rpm at 4°C. The supernatant, containing nuclear proteins, were analyzed using western blot analysis.
Gel shift - Nuclear proteins (4mg) from CsA treated and untreated HGF were combined with binding buffer (50mM NaCl, 1 mM EDTA, 0.1mM DTT, 10% (v/v) glycerol and 10mM Tris-HCL pH 7.5), 2 mg of poly [dI-dC] and 40,000 cpm of 32P-labled nucleotide probe to a final volume of 20ml. After incubation for 25 min at room temperature, the mixture was resolved on 5% page using 0.25xTBE buffer (50mM Tris-Cl, 45 mM boric acid, 0.5mM EDTA, pH 8.4) and expose to x-ray film overnight at -70˚C.
Western Blot Analysis - Conditioned medium was harvested and protein concentration was measured using the Bradford method. 100μg of protein was loaded onto a 10% SDS-Page gel. The gel will then be transferred to a nitrocellulose membrane after being incubated in 5% nonfat dry milk/ 1x PBS/ 0.1% Tween-20 for 3 hours. Then nitrocellulose membrane was incubated with the primary antibody overnight at 4˚C. The membrane was washed with 1X TBS and then incubated with the secondary antibody at room temperature for 1 hour, and developed using SuperSignal West Pico Chemiluminescent Substrate from (Piece, Rockford, Il). Developed membranes were exposed to Kodak XAR-5 film.
Isolation of Membrane Proteins - Using, Mem-PER® Eukaryotic Membrane Protein Extraction Reagent Kit (Promega), cells were lysed with a proprietary detergent and then a second proprietary detergent is added to solubilize the membrane proteins. The cocktail was incubated at 37°C to separate the hydrophobic proteins from the hydrophilic proteins through phase partitioning.
Statistics - Paired T-test was used to compare control versus CsA exposed group. A one tailed p value less than 0.05 was considered to be significant.
Results
Human gingival fibroblasts exposed to cyclosporin differentially regulate various cellular proteins - To determine CsA’s effect on protein expression, 2-D gel electrophoresis was applied to CsA stimulated and unstimulated CHGFs (represents gingival fibroblast from a patient with a previous history of CsA induced gingival overgrowth) in order to give us a key to protein regulations that are linked to the structural changes of the CsA induced gingival overgrowth. Whole cell lysates extracted from treated and untreated human gingival fibroblasts were analyzed by 2D gel electrophoresis as described by (O’Farrell, et al. 1975). 50 ng of tropomycin was added to each sample for standardization. Tropomycin migrates as a doublet with lower polypeptide spot of MW 33,000 and pI 5.2; a black arrow on the stained gels marks its position.
Several proteins were either up regulated (blue arrows) or down regulated (green arrow) after treatment with CsA (Figure 1). This data helps us to form the basis for the understanding of the mechanism of CsA induced gingival overgrowth on a protein level.
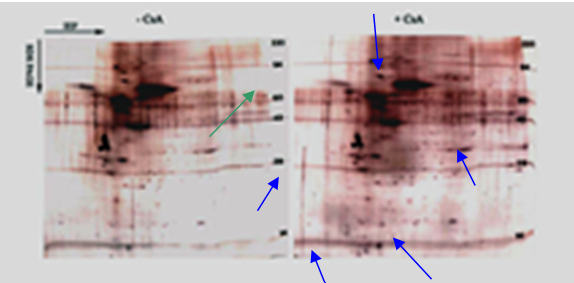
Figure 1: Silver-stained two-dimensional reducing gel of whole cell lysate from untreated and treated gingival fibroblasts. Molecular weight standards appear as horizontal lines across the silver stained (Oakley et al. 1980) gel. The blue arrows represent proteins that showed increased expression, while the green arrow shows a protein that displayed decreased expression.
Cyclosporin inhibits matrix metalloproteinase (MMP) activity and expression in human gingival fibroblasts - MMPs are categorized into four groups based on their substrate [12]. MMP-1, MMP- 8 and 13 belong to the collagenases, which primarily degrade collagen type 1. Type 1 collagen is the most abundant collagen in gingival tissues and MMP-1 is widely expressed in normal remodeling tissue including human gingival fibroblasts (HGF) (Murawaki et al, 1998 [14,15]. We used collagen zymography to demonstrate that CsA inhibits activity of 42-kDa enzyme corresponding with MMP-1 as well as MMP-13 (Figure 2A). Using Western blot analysis with specific monoclonal antibodies our results indicate that CsA inhibits MMP-1 protein expression (Figures 2B). CsA’s inhibitory effect on MMP-1 is also demonstrated at the RNA level (Figure 2C).
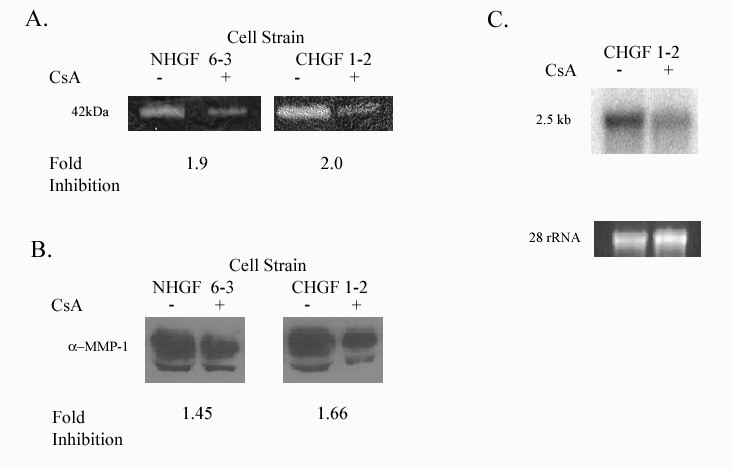
Figure 2: Expression of MMP-1. NGF 6-3 represents gingival fibroblast from a healthy patient and CHGF 1-2 represents a patient with a previous history of CsA induced gingival overgrowth.
2A: Collagenolytic activity of the culture medium from human gingival fibroblasts exposed to CsA versus untreated. Collagen zymography was performed as described in Methods.
2B: MMP-1 protein secreted from human gingival fibroblast treated with CsA versus untreated.
2C: Northern blot analysis of total RNA obtained from gingival fibroblasts previously showing decreased activity and protein expression with exposure to CsA. Decreased mRNA in gingival fibroblast exposed to 500ng/ml of CsA after 48 hrs. Lower panel shows 28s rRNA as a control for equal RNA loading.
To determine whether the decrease in collagenase activity and protein expression after CsA exposure shows statistical significance, we analyzed a minimum of 3 cell strains. We then scanned the lytic and protein bands for relative intensity. We used student’s t-test to calculate the statistical significance of the average mean difference. Figure 3 show histograms showing the relative intensity between controls and treatments groups. Figure 3A demonstrates the results from all cell strains examined by collagen zymography. The average mean difference (one tailed t-test) between all treated and untreated cell strains was statistically significant with a p value less than 0.005. All cell strains examined by Western blot analysis are shown in figure 3B. The p value for the average mean difference was less than 0.05.

Figure 3: Analysis of collagenase activity and expression of all cell strains examined. 3A. Analysis of collagenolytic activity of the culture media from all human gingival fibroblasts exposed to CsA versus untreated. Collagenase activity was measured as relative intensity of the lytic bands. 3B. Analysis of MMP-1 protein expression of the culture media from all human gingival fibroblasts exposed to CsA versus untreated.
MMP-2 (gelatinase A) is expressed in normal remodeling tissue and has been implicated in the spread of carcinomas Borhane Annabi et al. 2002 [16,17]. We examined the effect of CsA treatment on MMP-2 activity, using gelatin zymography and showed that CsA inhibited the activity of a 62-kDa MW enzyme (consistent with MMP-2) in gingival fibroblasts (Figure 4A). Secreted MMP-2 protein expression was also inhibited by CsA exposure (Figure 4B). Unlike MMP-1, CsA effect on MMP-2 was not evident at the level of transcription (Figure 4C).
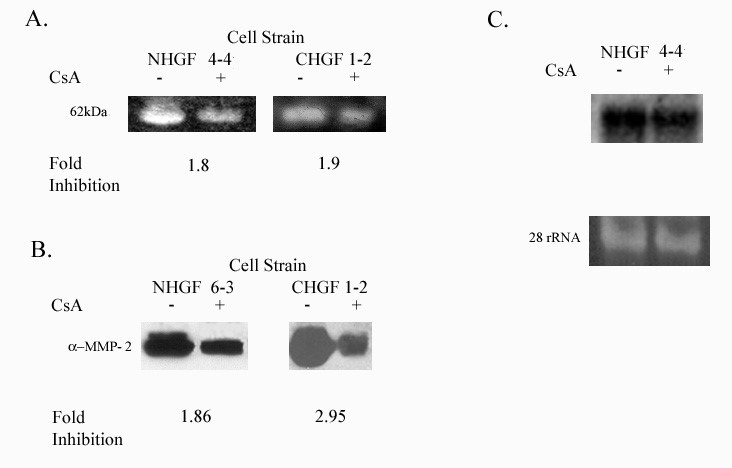
Figure 4: Expression of MMP-2 in HGF exposed to 500 ng/ml CsA for 48 hours. NHGF 4-4 and NHGF 6-3 represents gingival fibroblast from a healthy patient and CHGF 1-2 represents a patient with a previous history of CsA induced gingival overgrowth.
4A: Gelatinolytic activity of MMP-2 in the culture media from human gingival fibroblasts exposed to CsA versus untreated. Gelatin zymogram was performed as described in the Methods section of this proposal.
4B: MMP-2 protein secreted from human gingival fibroblasts treated with CsA versus untreated.
4C: Northern blot analysis of total RNA obtained from gingival fibroblasts previously showing decreased activity and protein expression with exposure to CsA. Decreased mRNA in gingival fibroblast exposed to 500ng/ml of CsA after 48 hrs. Lower panel shows 28s rRNA as a control for equal RNA loading.
To determine whether the inhibition of MMP-2 by CsA is statistically significant, we analyzed a minimum of 3 cell strains for MMP-2 activity and protein expression before and after CsA exposure [18,19]. We scanned the lytic and protein expression bands for relative intensity. Figure 5 show histograms of all the cell strains studied. CsA significantly inhibits the activity of gelatinase A (MMP-2) in both cell stains with a p value less than 0.05 (Figure 5A). Secreted protein expression was significantly reduced after CsA exposure with a p value less than 0.01 (Figure 5B).
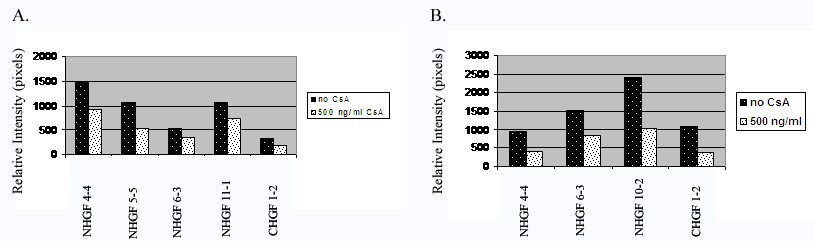
Figure 5: Histograms of gelatinase activity and expression of all cell strains examined. 5A. Analysis of gelatinolytic activity of the culture media from all human gingival fibroblasts exposed to CsA versus untreated. Gelatinase activity was measured as relative intensity of the lytic bands. 5B. Analysis of MMP-2 protein expression of the culture media from all human gingival fibroblasts exposed to CsA versus untreated.
MMP-9 (gelatinase B) is enzymatically digesting the basement membrane along with MMP-2 and has been implicated in tumor invasion (Jimenez et al. 2000) [20-22]. We examined the effect of CsA treatment on MMP-9 activity, using gelatin zymography and showed that CsA inhibited the activity of a 92-kDa MW enzyme (consistent with MMP-9) in gingival fibroblasts (Figure 6A). Secreted MMP-9 protein expression was also inhibited by CsA exposure (Figure 6B). CsA’s inhibitory effect on MMP-9 is also demonstrated at the RNA level, although minimal (Figure 6C).

Figure 6: Expression of MMP-9. NHGF 9-3 represents gingival fibroblast from a healthy patient and CHGF 1-2 represents a patient with a previous history of CsA induced gingival overgrowth.
6A: Collagenolytic activity of the culture medium from human gingival fibroblasts exposed to CsA versus untreated. Collagen zymography was performed as described in Methods.
6B: MMP-9 protein secreted from human gingival fibroblast treated with CsA versus untreated.
6C: Northern blot analysis of total RNA obtained from gingival fibroblasts previously showing decreased activity and protein expression with exposure to CsA. Decreased mRNA in gingival fibroblast exposed to 500ng/ml of CsA after 48 hrs. Lower panel shows 28s rRNA as a control for equal RNA loading.
Using 3 cell strains, we determined whether the inhibition of MMP-9 activity by CsA is statistically significant. To determine the significance of the suppression of secreted protein expression of MMP-9, we used 7 cell lines before and after CsA exposure. We scanned the lytic and protein expression bands for relative intensity. Figure 7 show histograms of all the cell strains studied [23-25]. CsA significantly inhibits the activity of gelatinase B (MMP-9) in both cell stains with a p value less than 0.05 (Figure 7A). Secreted protein expression was significantly reduced after CsA exposure with a p value less than 0.01 (Figure 7B).
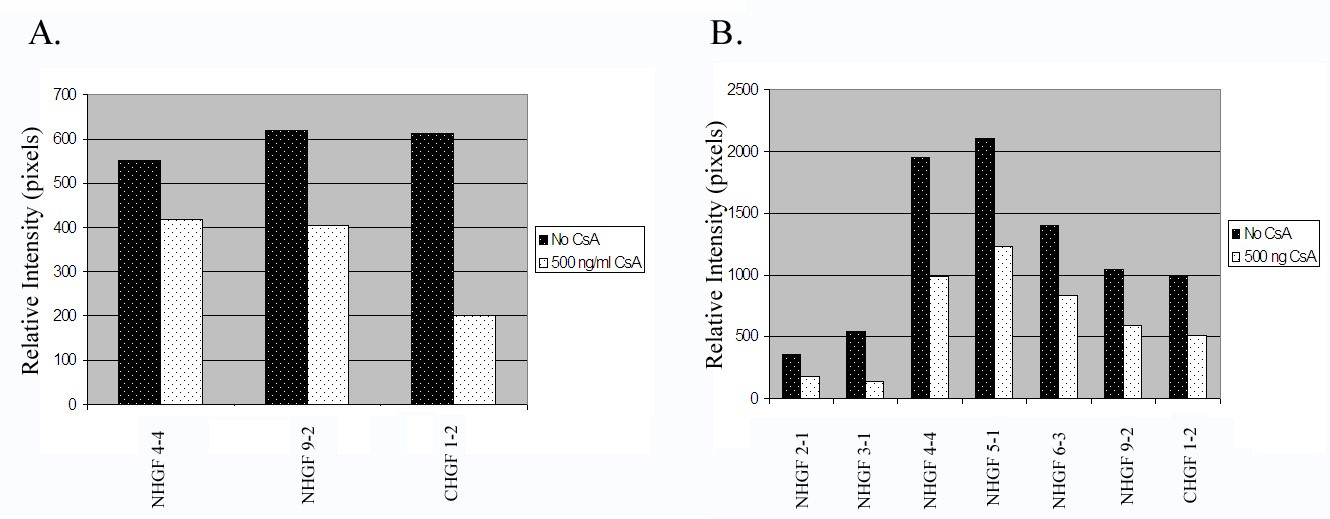
Figure 7: Histograms of gelatinase activity and expression of all cell strains examined.
7A. Analysis of gelatinolytic activity of the culture media from all human gingival fibroblasts exposed to CsA versus untreated. Gelatinase activity was measured as relative intensity of the lytic bands.
7B. Analysis of MMP-9 protein expression of the culture media from all human gingival fibroblasts exposed to CsA versus untreated.
Cyclosporin inhibits MMP-1 posttranscriptionally - To determine whether CsA regulates MMP-1 transcriptionally regulates MMP-1 expression we examined whether CsA requires de novo protein synthesis to inhibit MMP-1 expression [26,27]. Since transcription is enhanced or repressed by transcriptional factors, we blocked translation using a protein synthesis inhibitor, cycloheximide to determine whether CsA requires de novo protein synthesis to regulate MMP-1 expression. As shown in Figure 8A and 8B, cycloheximide does not affect the extent of CsA-induced down-regulation of MMP-1. It appears that CsA doesn’t regulate MMP-1 via transcription; therefore, we will determine whether CsA’s inhibition of MMP-1 is due to decreased mRNA stability by inhibition of new mRNA synthesis using actinomycin D. Figure 9 shows that with the inhibition of new mRNA, CsA destabilizes MMP-1 mRNA at a steady and increasing rate as compared to the control. With this data, we can surmise that CsA posttranslationally regulates MMP-1 expression [28,29].

Figure 8: Effect of cycloheximide (CHX) on MMP-1 expression in untreated and CsA-treated gingival fibroblasts cells. A. Cells incubated with 10 g/ml CHX were exposed to 500 ng/CsA or vehicle for 48 h in complete medium. Cells were then harvested for Northern blotting. B. Histogram of relative density of mRNA.
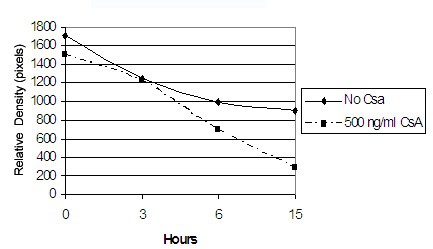
Figure 9: Effect of CsA on MMP-1 mRNA half-life in gingival fibroblasts. Gingival fibroblasts were exposed to vehicle or 500ng/ml for 24 h before the addition of actinomycin D (10g/mL). Total RNA was extracted from the cells at the indicated times after actinomycin D treatment. Semiquantitative RT–PCR was performed.
Cyclosporin inhibits MMP-2 secretion – Since CsA inhibits both MMP-2 activity and secreted protein levels, but not the mRNA levels, we sought to determine whether the intracellular and/ or membrane bound MMP-2 was affected by CsA treatment which would implicate that CsA regulates MMP-2 secretory mechanism.
CsA inhibits MMP-9 expression via inhibition of NFkB binding - To determine CsA’s effects on transcription factors known regulate MMP-9 gene expression we exposed HGFs to CsA and/or TNF-α for 24 hours. Nuclear extraction was performed on treated and untreated HGFs [30]. 4 ug of nuclear proteins were combined with binding buffer (50mM NaCl, 1 mM EDTA, 0.1mM DTT, 10% (v/v) glycerol and 10mM Tris-HCL pH 7.5), 2 mg of poly [dI-dC] and 40,000 cpm of 32P-labled nucleotide probe and incubated 25 min @ RT. This mixture was resolved on 5% page using 0.25xTBE buffer (50mM Tris-Cl, 45 mM boric acid, 0.5mM EDTA, pH 8.4) and exposed to x-ray film overnight. Figure 6 shows that CsA inhibits TNF-α induced NFκB binding.

Figure 10: Nuclear translocation of NF-κB is decreased by CsA and increased by TNF-α in CHGF 8 treated with 500ng/ml of CsA, 10ng/ml of TNF-α, both or untreated for 3 hours. Confluent HGF were stimulated either with 500ng/ml of CsA, 10ng/ml of TNF-α, both or untreated for 3 hours. Nuclear extracts were prepared from the stimulated fibroblasts and EMSA was performed (lanes B-E). To determine its specificity, binding was also performed in the presence of 5-fold excess non-radiolabeled NF-κB probe (lane F). Lane A shows radiolabeled probe without nuclear proteins.
Discussion
Cyclosporin and MMP-1
Our study demonstrated that the decrease in MMP-1 expression by CsA was due to destabilization of MMP-1 mRNA. This finding is consistent with the results of Vahabi et. al. (2014) who found that CsA inhibited expression of MMP-1 by fibroblasts from adults. In contrast, Nazemisalman et. al. (2019) demonstrated that MMP-1 level increased with the treatment of CsA. This difference may be due to the lower dose of CsA when treated with (50 and 150 ng/mL) whereas we used 500ng/ml of CsA. Their study also was limited to fibroblast from one subject.
Cyclosporin and MMP-2
We show that MMP-2 secreted protein expression was decreased when treated with CsA’s suggesting that the effects are limited to the secretion of MMP-2. CsA inhibited the expressions of MMP-2 in another study (Fu et. al. 2015) however there was increased MMP-2 when treated with CsA (Nazemisalman et. al. 2019).
CsA, MMP-9 and NFkB
CsA also appeared to inhibit NFkappaB which is known to transcriptionally activate MMP-9 expression. CsA treatment reduced MMP- 9 secretion (Johanson et. al. 2012), CsA-induced attenuation expressions of MMP-9 in the gingival tissues of Sprague-Dawley rats (Fu et. al. 2015) [31,32]. With these studies it can be considered that CsA may inhibits MMP-9 expression via NFkappaB but this is something that we must confirmed in future studies.
Conclusion
These results suggest that MMP-1, -2, and -9 all may contribute to the pathogenesis of CsA induced gingival overgrowth by different mechanisms.
Acknowledgments
This work was supported by the Oral and Maxillofacial Surgery Foundation Fellowship and the NIH Training Grant # T32 DE07296 awarded to Colonya C. Calhoun. The authors would like to acknowledge Joseph McQuirter D.D.S., Richard D. Leathers D.D.S., Edward Black D.D.S., Reginald Gowans D.D.S.,
Richard D. Meekins D.D.S., Hai Duong and Jun Song Ph.D.
References
- Boltchi FE, Rees TD, Iacopino AM. Cyclosporine A-induced gingival overgrowth: A comprehensive review. Quintessence Int, 1999; 30: 775-783.
- Williamson MS, Miller EK, Plemons J, Rees T, Iacopino AM. Cyclosporine A upregulates interleukin-6 gene expression in human gingiva: Possible mechanism for gingival overgrowth. In: Journal of Periodontology, 1994; 65(10): 895-903.
- Daley TD, Wysocki GP. Cyclosporine therapy. Its significance to the periodontists. Journal of Periodontology, 1984; 55: 708-712.
- McGraw WT, Porter H. Cyclosporine-Induced Gingival Overgrowth an Ultrastructural Stereologic Study Oral Surgery Oral Medicine Oral Pathology, 1988; 65(2): 186-190.
- Seymour RA, Smith DG. The effect of a plaque control program on the incidence and severity of cyclosporin-induced gingival changes. Journal of Clinical Periodontology, 1991; 18(2): 107-110.
- Ilgenli T, Atilla G, Baylas H. Effectiveness of periodontal therapy in patients with drug-induced gingival overgrowth. Long-term results. In: J Periodontol, 1999; 70 (9): 967-972.
- Myrillas TT, Linden GJ, Marley JJ, Irwin CR. Cyclosporin a regulates interleukin-1beta and interleukin-6 expression in gingiva: Implications for gingival overgrowth. In: Journal of Periodontology March, 1999; 70(3): 294-300.
- Kataoka M, Shimizu Y, Kunikiyo K, Asahara Y, Yamashita K, Ninomiya M, et al. Cyclosporin A decreases the degradation of type I collagen in rat gingival overgrowth. In: Journal of Cellular Physiology, 2000; 182(3): 351-358.
- Bonnaure-Mallet M, Tricot-Doleux S, Godeau GJ. Changes in extracellular matrix macromolecules in human gingiva after treatment with drugs inducing gingival overgrowth. In: Archives of Oral Biology, 1995; 40(5): 393-400.
- Wondimu B, Modeer T. Cyclosporin A upregulates prostaglandin E-2 production in human gingival fibroblasts challenged with tumor necrosis factor alpha in vitro. In: Journal of Oral Pathology & Medicine, 1997; 26(1): 11-16.
- Rocha LAG, Martins RCL, Werneck CC, Feres-Filho EJ, Silva LCF. Human gingival glycosaminoglycans in cyclosporin-induced overgrowth. In: Journal of Periodontal Research, 2000; 35(3): 158-164.
- Woessner JF, Nagase H. Matrix Metalloproteinases and TIMPs. New York, NY, Oxford Press, 2000.
- Gogly B, Groult N, Hornebeck W, Godeau G, Pellat B. Collagen zymography as a sensitive and specific technique for the determination of subpicogram levels of interstitial collagenase. In: Analytical Biochemistry, 1998; 255(2): 211-216.
- Thomason JM, Sloan P, Seymour RA. Immunolocalization of Collagenase (MMP-1) and stromelysin (MMP-3) in the gingival tissues of organ transplant patients medicated with cyclosporin. Journal of Clinical Periodontology, 1998; 25: 554-560.
- Bolzani G, Coletta RD, Júnior HM, Almeida OP, Graner E. Cyclosporin A inhibits production and activity of matrix metalloproteinases by gingival fibroblasts. Journal of Periodontal Research, 2000; 35: 51-58.
- Taraboletti G, D’Ascenzo S, Borsotti P, Giavazzi R, Pavan A, Dolo V. Shedding of the Matrix Metalloproteinases MMP-2, MMP-9, and MT1-MMP as Membrane Vesicle-Associated Components by Endothelial Cells. American Journal of Pathology, 2002; 160(2).
- Yamada H, Nishimura F, Naruishi K, Chou HH, Takashiba S, Albright G, et al. Phenytoin and cyclosporin A suppress the expression of MMP-1, TIMP-1 and cathepsin L, but no cathepsin B in cultured fibroblasts. Journal of Periodontology, 2000; 71: 955-960.
- Mariani G, Calastrini C, Carinci F, Marzola R, Calura G. Ultrastructural features of cyclosporine A-induced gingival hyperplasia. In: Journal of Periodontology, 1993; 64(11): 1092-1097.
- Ueki K, Dongari-Bagtzoglou AI. Regulation of gingival fibroblast interleukin-6 secretion by cyclosporine A. In: Journal of Periodontology, 1999; 70(12): 1464-1471.
- Murphy G, Knauper V. Relating matrix metalloproteinase structure to function: why the "hemopexin" domain? Matrix Biol, 1997; 15: 511–518.
- Miyamori H, Takino T, Seiki M, Sato H. Human membrane type-2 matrix metalloproteinase is defective in cell-associated activation of progelatinase A. Biochem Biophys Res Commun, 2000; 267: 796–800.
- Ueki K, Tabeta K, Yoshie H, Yamazaki K. Self-heat shock protein 60 induces tumour necrosis factor-alpha in monocyte-derived macrophage: Possible role in chronic inflammatory periodontal disease. In: Clinical and Experimental Immunology January, 2002; 127(1): 72-77.
- Bond M, Baker AH, Newby AC. Biochemical and Biophysical Research Communications, 1999; 264: 561–567.
- Bondeson J, Foxwell B, Brennan F, Feldmann M. Natl. Acad. Sci. USA. Mmunology, 1999; 96: pp. 5668–5673.
- Benbow U, Brinckerhoff CE. The AP-1 site and MMP gene regulation: What is all the fuss about? In: Matrix Biology, 1997; 15(8-9): 519-526.
- Annabi B, Lachambre M, Bousquet-Gagnon N, Page M, Gingras D, Beliveau R. Green tea polyphenol (3)-epigallocatechin 3-gallate inhibits MMP-2 secretion and MT1-MMP-driven migration in glioblastoma cells. Biochimica et Biophysica Acta, 2002; 1542: 209-220.
- Spinale Francis G. Matrix metalloproteinases: Regulation and dysregulation in the failing heart. In: Circulation Research, 2002; 90(5): 520-530.
- Han Yuan-Ping, Tuan Tai-Lan, Wu Huayang, Hughes M, Garner TNF-a stimulates activation of pro-MMP2 in humanskin through NF-kB mediated induction of MT1-MMP. Journal of Cell Science 114: 131-139.
- Bruno de Almeida J, Doherty J, Ausiello DA, Stow JL. Binding of the cytosolic p200 protein to Golgi membranes is regulated by heterotrimeric G proteins. Journal of Cell Science, 1993; 106: 1239-1248.
- Riley GP, Curry V, DeGroot J, van El B, Verzijl N, Hazleman BL, et al. Matrix metalloproteinase activities and their relationship with collagen remodelling in tendon pathology. Matrix Biol, 2002; 21(2): 185-195.
- Bondeson J, Brennan F, Foxwell B, Feldman M. Effective adenoviral transfer of IkBa into human fibroblasts and chondrosarcoma cells reveals that the induction of matrix metalloproteinases and proinactivators flammatory cytokines is nuclear factor-kB dependent. J Rheumatol, 2000; 27: 2078–2089.
- Galis ZS, Muszynski M, Sukhova GK, et al. Cytokine-stimulated human vascular smooth muscle cells synthesize a complement of enzymes required for extracellular matrix digestion. Circulation Res, 1994; 75: 181–189.

