Seroprevalence of Human Cytomegalovirus Infection among Pregnant Women Attending Ante-Natal Clinic at Federal Teaching Hospital Ado-Ekiti, Nigeria
Richard Yomi Akele1, Oreoluwa Oluwayemisi Bakare2, Janet Funmilayo Akinseye2, Bernard Oluwapelumi Oluboyo2, Seyi Samson Enitan3,*, Ayuba Sunday Buru2 and Michael Olugbamila Dada3
1Department of Biomedical Science, School of Applied Science, University of Brighton, London, United Kingdom
2Department of Medical Laboratory Science, Afe Babalola University, Ado-Ekiti, Nigeria
3Department of Medical Laboratory Science, Babcock University, Ilishan-Remo, Ogun State, Nigeria
Received Date: 10/02/2023; Published Date: 30/03/2023
*Corresponding author: Sey Samson Enitan, Department of Medical Laboratory Science, Babcock University, Ilishan-Remo, Nigeria
Abstract
Background: Human Cytomegalovirus (HCMV) infection in pregnancy, especially in the first trimester often lead to congenital abnormalities and is associated with serious complications, such as microcephaly, mental retardation, spastic paralysis, hepatosplenomegaly, anaemia, thrombocytopenia, deafness, and optic nerve atrophy leading to blindness in infants. These conditions are threats to accomplishing the sustainable developmental goal three (To ensure healthy lives and promote well-being for all at all ages) as there are not deliberate measures put in place to track and manage the virus in Ido-Ekiti and environs. This present study evaluated the seroprevalence of the virus among pregnant women attending antenatal clinic at Federal Teaching Hospital Ido-Ekiti, Nigeria.
Method: A total of one hundred and sixteen (116) pregnant women were examined in this study. The subjects were administered a structured questionnaire to obtain demographic and risk data. Five millimeters (5 ml) of blood samples were collected from consenting subjects to obtain sera which were analyzed for the presence of CMV IgG and IgM antibodies using Enzyme linked immunosorbent assay (ELISA) technique. Avidity testing was also carried out using ELISA.
Results: Results obtained from this study showed prevalence of 87.9% and 7.8% for IgG and IgM respectively. Samples that tested positive for both IgG and IgM were 6.0%. These samples were examined for avidity to evaluate strength of the IgG antibodies. Those with avidity index >35% were 28.6% showing IgG antibody predominance, while those with avidity index < or equal to 35% were 71.4% indicating that IgM was the predominant antibody. A significant (p<0.05) number of the pregnant women recorded the presence of IgG and IgM antibody to the virus. There was no statistical significance (p>0.05) difference between the prevalence of CMV IgG antibody across age ranges, number of pregnancies, miscarriages, marital status, trimester, still birth and transfusion.
Conclusion: With the information provided in this study, there is need for the adoption of CMV screening into the antenatal profile tests, more public awareness of the virus to educate the general public especially women on how it is acquired and contributing factors like personal and community hygiene as preventive approaches to avert future consequences.
Keywords: Cytomegalovirus; Immunoglobulin; Pregnancy; Prevalence; Women
Introduction
Human Cytomegalovirus (HCMV) is a beta human herpes virus type 5 [1]. It is an enveloped DNA virus that is a member of the herpes family and belongs to a group of vertically transmitted infections known as TORCH (Toxoplasmosis, Rubella, Cytomegalovirus, and Herpes simplex, [2]). Human Cytomegalovirus has been confirmed as the major cause of viral infection in pregnancy [3].
The virus is ubiquitous and infection is often asymptomatic [4]. It’s mode of transmission is usually either horizontal, through infected secretions like saliva, semen, blood etc or vertical via genital tract, breast milk etc. The most significant mode of post-natal spread is through blood transfusion [5]. Once someone has been infected with CMV, he/she has it for life due to the ability of the virus to establish latency [6]. HCMV is one of the major causes of birth defects and can be transmitted from mother to fetus during gestation period. It is mainly detrimental when the mother experiences a primary infection during the pregnancy [7].
HCMV is a leading cause of disability in children and a common cause of congenital viral infection that results in conditions such as mental retardation, neurological impairment, and permanent hearing and vision loss. HCMV infection of pregnant women, especially in the first trimester may lead to congenital abnormalities and is often associated with serious complications, such as microcephaly, mental retardation, spastic paralysis, hepatosplenomegaly, anaemia, thrombocytopenia, deafness, and optic nerve atrophy leading to blindness in infants [2]. HCMV infections can have serious consequences in pregnant women and in immunocompromised patients, and may even cause a teratogenic danger during pregnancy [2].
The characteristic cytomegalic cells of HCMV disease were first noted by Hugo Ribbert in sections of the kidney of a luetic stillborn and in the parotic glands of a syphilitic neonate, and confirmed with the report he saw by Jesionek and Kiolemenoglou who described similar cells as protozoan like cells in the lungs, kidneys and liver of an 8-month luetic fetus, but for a long time the disease was thought to be of protozoan nature [8]. The term ''salivary gland virus'' was coined as a result of the prominence of these cells in salivary glands and a guinea pig model of salivary gland virus disease confirmed the viral agent of this disease as transmissible through saliva. As knowledge advanced, a neonatal illness with petechiae, hepatosplenomegaly, brain calcifications were characterized and correlated with the presence of cytomegalic cells and was generalized as cytomegalic inclusion disease [8]. Fetterman having heard of the presence of the viral inclusions in kidney tubules cells began using urine as a sample for diagnosis in infants. Thomas Huckle Weller together with Smith and Rowe independently isolated the virus giving credence to earlier reports [8]. Weller proposed the term "cytomegalovirus" for this viral agent, from two Greek words cyto meaning cell and megalo, meaning large. The first draft of human cytomegalovirus genome was published in 1990 and was the biggest contagious genome at that time [9].
Cytomegalovirus infection in pregnancy can either be acute or chronic [10]. Infection transmitted in acute infections during pregnancy has an estimated incidence of 25-75% with chronic condition having an estimation of 0.2-2% [11]. Congenital infection in the fetus usually occurs if the mother has a primary infection or a recurrent infection during pregnancy. Maternal infection during the first trimester seems to be associated with adverse effect in the fetus [12].
Human Cytomegalovirus screening is not among the routine antenatal screening tests [13]. It is only done upon request despite the fatal implication to the health development of infants [14]. This negligence is part of the contributing factors to high intrauterine transmission leading to high recorded cases of irreversible sequel and mental disorders in foetuses [15].
The transmission of the virus to the baby may occur through the placenta during pregnancy [13] and seroprevalence of CMV among women of childbearing age has been reported to range from 35% to 95% in different countries, a range which increases with age and may also depend on sexual activity, occupation - particularly occupations involving close contacts with children in a community setting [16]. Once a person becomes infected, the virus remains alive, but usually dormant within that person’s body for life [17]. However, if a person's immune system is seriously weakened, the virus can become active and cause chronic CMV disease.
Materials and Methods
Study location
The study was carried out at the Federal Teaching Hospital (FTH), Ido-Ekiti, Ekiti State, located in South west Nigeria and lies on latitude 7°35 and 7°38 North of the equator and on longitude 5°10 and 5°15 east of the Greenwich meridian [18]. The FTH-Ido-Ekiti is a tertiary health institution, the foremost in Ekiti State. The hospital serves as a referral center for all other health institutions (general hospital, specialist hospitals, and comprehensive health centers) in Ekiti state. The FTH has 24 fully functional departments comprising of 18 clinical department and 5 supportive departments. People from various parts of the State come to access medical care in FTH, Ido-Ekiti. Samples collected in this hospital for the present study were transported to the Department of Medical Laboratory Science, Afe Babalola University, for analysis.
Study population
The study targeted pregnant women (age range, 15 to 45 years) attending ante-natal clinic at Federal Teaching Hospital, Ido-Ekiti, Ekiti State.
Sample size
A total of 113 consenting pregnant women were recruited for the study.
Inclusion criterion
The study included pregnant women aged between15-45 years attending the ante natal clinic of FTH, Ido-Ekiti who consented to the study.
Exclusion criterion
The study excluded non-pregnant women, women below 15 years or above 45 years, pregnant women whose ages fell within the acceptable age group but did not consent to the study.
Study area
Samples were collected from a cross-section of pregnant women attending the ante-natal clinic of Federal teaching hospital Ido-Ekiti, Ekiti State, Nigeria.
Ethical consideration
Ethical approval was obtained from the research ethics review committee of Federal Teaching Hospital, Ido-Ekiti, Ekiti State. Result was kept strictly confidential. Privacy was ensured as the name of the participants or any form of identity was not required in the questionnaire. No result is traceable to the participants as only laboratory identifiers were used to number the samples collected. The nature and purpose of research was explained to each participant using an informed consent form for literate participants and verbal explanation for illiterate participants. The right to self determination was followed by providing the participants with the rights to voluntarily consents or decline to participate and to withdraw at any time without penalty. Participants did not suffer any hazard in this study. Samples were collected aseptically.
Sample collection
A well-structured questionnaire was used to obtain information from the volunteers. Five milliliter (5 ml) of venous blood was collected from each consented pregnant woman. The blood was transferred into a sterile anticoagulant free bottle and labeled properly with patient's identification number. The blood sample was centrifuged at 3,000 revolutions per minute for 10 minutes. Using Pasteur pipette, the serum was transferred into clean screw-capped containers, and stored at −20 °C, until ready for analysis [19].
Sample analysis
The serum samples were analyzed using ELISA assay for the detection of Human Cytomegalovirus IgG and IgM antibodies.
Test principle
The kit uses capture ELISA principle to detect CMV IgG and IgM. Purified CMV antigen is pre-coated on the strips; the enzyme-labeled antigen CMV complex will combined with IgG and IgM in human serum/plasma. Into the wells, samples, positive control, negative control and HRP conjugated protrin were added and incubated. After incubation, it is washed to remove the uncombined enzyme, after which chromogen A and B mixture is added changing the colour of the liquid to blue. At the effect of the acid, the colour finally becomes yellow. The colour change is measured spectrophotometrically at a wavelength of 450nm. The presence or absence of CMV IgG and IgM in the samples is determined by comparing the O.D of the samples to the CUT-OFF.
Test procedure
The manufacturer’s procedure was strictly followed. The reagents provided were allowed to reach room temperature for 15 minutes before use. The wash buffer was diluted with distilled water using ratio 1:40 dilution before use. The microtiter plate was set up with 1 well as blank, 2 wells as negative control and 1well as positive control. 100 µl of sample dilution fluid was dispensed into the corresponding wells except the blank well, negative control well and the positive control well. 10 µl of samples was added to corresponding wells and mixed thoroughly using the pipette. 100 µl of negative control and positive control were added to the negative and positive control wells. Microtiter plate was shaken gently by tapping to mix for 30 seconds. The microtiter plate was covered with a sealing paper and was incubated in a microtiter plate incubator (SI505, Avantor, Inc. Radnor, Pennsylvania, U.S.) at 37°C for 20 minutes. After incubation, the microtiter plate was washed five times using wash buffer. 50 µl of conjugate was added to the wells except the blank, the microtiter plate was covered with a sealing paper and was incubated in the microplate incubator at 37 °C for 20 minutes. After the incubation, the microtiter plate was washed five times with the diluted wash buffer in an automatic microplate plate washer (Erba Lisa, Texas, U.S) 50 µl of substrate solution A and B mixture was added to each well respectively and was mixed; the plate was covered and incubated at 37°C for 15 minutes. 50 µl of stop solution was added to each well and was mixed by shaking gently. The absorbance was read in an ELISA reader machine (KC-100 Caretium microplate reader, Germany) at wavelength of 450 nm.
Interpretation of results
If mean negative control O.D < 0.1 and the mean positive O.D > 0.8, the test is valid.
CUT OFF = the mean O.D value of the negative control × 2.1
POSITIVE RESULTS: Sample O.D > Cut-off O.D
NEGATIVE RESULT: Sample O.D < Cut-off O.D
Avidity testing
The samples that tested positive to both CMV IgM and CMV IgG were evaluated for IgG avidity. Avidity test was carried out to evaluate the strength of IgG and IgM antibodies in order to classify the infection as acute and chronic. The CMV specific IgG optical density was divided by the CMV specific IgG optical density of the previous results then multiplied by a hundred and this provided the Avidity index. If the index is < 35 % it was considered low. Avidity index ≥ 35 % was considered high [2]. Low IgG avidity levels strongly suggest an infection contracted less than three months before, whereas a high avidity tends to exclude this [20].
Statistical analysis
Data collected were subjected to non-parametric statistical analysis using Statistical package for social sciences (SPSS) version 23. Chi square analysis was performed with significance at P˂0.05.
Result
Of the 116 study subjects, 102/116 (87.9%) tested positive for CMV IgG antibodies while 14/116 (12.1%) tested negative. For CMV IgM antibodies, 9/116 (7.8%) were IgM positive while 107/116 (92.2%) tested negative. All study subjects were between age ranges 15- 45 years, 24years was the mode age. They were grouped into three age groups; 15-24 years, 25-34 years and 35-44 years. Subjects within age group 15-24 years had the highest prevalence IgG CMV antibodies 27/29(93.1%), while women in age group 25-34 years had the lowest prevalence 58/68(85.3%). Subjects within age group 35-44 years had the highest prevalence 2/19(10.5%) of IgM antibodies to CMV. Seven subjects (6.8%) who tested positive for CMV IgG also tested positive for CMV IgM. The prevalence of IgG and likewise IgM antibodies was not significantly different (p = 0.544 and p = 0.883 respectively) across age ranges (Table 1).
Table 1: Prevalence of CMV IgG and IgM antibodies according to the age ranges of pregnant women.

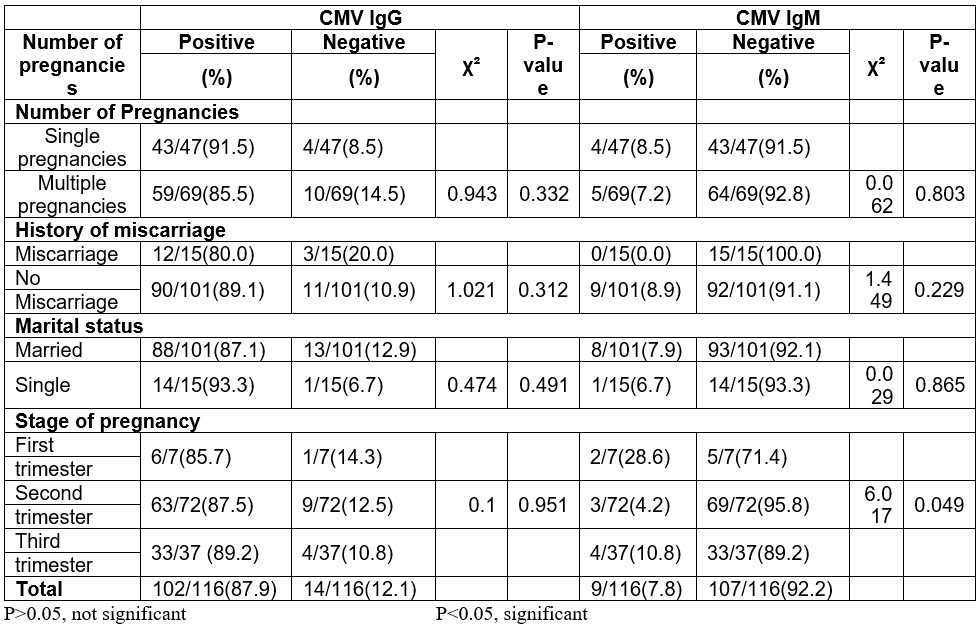
Considering number of pregnancies, 85.5% (59/69) of subjects who have had multiple pregnancy tested positive for CMV IgG antibodies while 91.5% (43/47) of those who were pregnant for the first time tested positive for CMV IgG antibodies. For CMV IgM, 7.2% (5/69) of subjects that have had multiple pregnancies tested positive for CMV IgM antibodies while 8.5% (4/47) of those pregnant for the first time tested positive for CMV IgM antibodies. There was no statistically significant difference in distribution of prevalence of CMV IgG or CMV IgG (p = 0.332 and p = 0.803 respectively) among those who have had multiple and single pregnancy (Table 2).
A higher percentage of the mothers that were not married tested positive to CMV IgG than the married mothers (93.3%). The opposite was the case for CMV IgM (7.9%). The highest incidence of CMV IgM was observed among women in the first trimester of pregnancy. The prevalence of CMV IgM (p = 0.049) based on stages of pregnancy was statistically significant (Table 2).
Table 2: Prevalence of CMV IgG and IgM antibodies among pregnant women in relations to marital status, history of miscarriages, number and stage of pregnancy.
Cytomegalovirus antibody avidity index
The 6.8 % of study population that tested positive for both IgG and IgM were evaluated for avidity of IgG antibodies. Two study subjects (28.6 %) had avidity index > 35 % showing IgG antibody predominance which suggest a chronic infection while 5 (71.4 %) had an avidity index < 35 % indicating that IgM was the predominant antibody suggesting an acute infection (Table 3).
Table 3: Avidity index among pregnant women with both IgM and IgG antibodies.

Discussion
The findings in this study indicate high prevalence of HCMV IgG antibodies (87.9%) among pregnant women attending the ante-natal clinic at Federal Teaching Hospital Ido-Ekiti. It suggests that most of the study subjects have previously been infected with CMV, while only few had primary CMV infection. This result is consistent with reports from other parts of the world including Nigeria [21] in which a high seroprevalence of CMV IgG antibodies and low CMV IgM antibodies (indicating recent CMV infection) in pregnant women was reported [22]. The result from this study agrees with previous investigations of the seroprevalence of HCMV in Nigeria with 84.2% in Bida, Niger State [13] and 88% in Sokoto [15]. The percentage is slightly lower than 91.1% in Kano [23], 93.3% in Benue [3], 94.8% in Kaduna and 97.2% in Lagos [24]. Higher seroprevalence of anti-CMV IgG antibodies have been reported in Turkey 98.5% [25], 100% in Thailand, 93% in Iranian women of childbearing age [26] and 98.7 in western Yemen [27]. However, results from this study were higher in relation to those obtained in developed countries: 56.9% in Australia and 46.8% in France [28]. The low rates recorded in developed countries are probably due to inclusion of routine HCMV screening among the pregnant women attending the antenatal clinics [29] and observation of improved hygienic standards [30].
The higher seroprevalence may probably be associated with lack of proper hygienic practices and lack of adequate awareness on prevention measures. All these play a major role to the high HCMV transmission and infection rates [31].
There was no significant relationship (p > 0.05) across ages of pregnant women. Infection can occur at any age if exposed to the virus. However, age ranges of 15-24 recorded the highest prevalence (93.1%). This is in tandem with work of [15] from Sokoto and [13] which reported no significant association between CMV and age. There was no predictable pattern between seroprevalence and age, though the youngest and oldest ages gave the highest prevalence. The highest seroprevalence among the youngest age group could be due probable increase sexual activities of this age group as the virus can be transmitted sexually and the high prevalence among the older group could be a cumulative effect longer duration of exposure to the virus.
The prevalence of CMV IgG antibody among pregnant women that had multiple pregnancies is not significant (p > 0.05) in relation to women who are just getting pregnant for the first time. The prevalence of CMV IgG in women pregnant for the first time was higher than those with multiple pregnancies. This result is in accord with a study among pregnant women in London which reported high (88.2%) seroprevalence in first pregnancies among women [23]. The prevalence is however at variance with the work done in Khorramabad, Iran where the CMV infection frequency had a significant relationship with the number of pregnancies [32]. This is probably due to the lacking of healthier behaviors in the time of delivery which may have led to increased contamination risk (I do not understand this sentence). This study did not also agree with reports in Sokoto State [15]. The reason for the disagreement could be as a result of the disproportion in the size of women who were pregnant for the first time to women with multiple pregnancies.
The prevalence of CMV IgG antibody among women who have had miscarriages was not statistically significant (p>0.05) in relation to women who had no miscarriages in the past. In this study, the percentage of women without history of miscarriage (89.1%) was higher than those with history of miscarriage (80.0%). This report is in accord with the work done in Benue [3] in which history of miscarriage showed no significant relationship with the seroprevalence of CMV IgG antibodies.
The prevalence of CMV IgG antibody among married women was not statistically significant (p>0.05) in relation to women who were single. 87.1% of married women were positive for CMV IgG and 93.3% of single women were positive for CMV IgG antibodies. The record on the prevalence of the virus in relation to marital status was scanty. Reports in Benue [3] showed that seroprevalence of CMV IgG was not significantly associated with marital status of the pregnant women in accordance to this work.
The prevalence of CMV IgG antibody among women in relation to trimester is not statistically significant. Hence, this is not associated with the risk of CMV positivity. Seroprevalence increased with increase in trimester. The prevalence was least in those who were in the first trimester of pregnancy and highest in the third trimester. This report is in accordance with the prevalence reported in Benue State [3]. This report disagreed with the work done in Kaduna State in which the CMV IgG was highest in the second trimester and this could be due to the fact that most pregnant women report for ante natal in their second trimester than in the first.
The low prevalence of IgM antibodies (7.8%) observed in this study is possibly due to the fact that majority of the women would have recovered from previous infections and had become immunized. The prevalence of primary infection among subjects was in the present study is of great concern due to the risk of congenital CMV infection which may ensue if mother contracts a primary infection during pregnancy especially in the first trimester [33].
Furthermore, CMV specific IgM may reappear during reactivation of CMV infection. IgG avidity is preferable as the presence of low IgG avidity has been shown to be a more unique and reliable serologic indicator of primary CMV infection [34] and using IgG avidity testing which can help to distinguish primary CMV infection from reactivation [35-37]. In this study, the pregnant women with avidity index >35% were 2 (28.6 %) showing IgG antibody predominance while those with avidity index ≤ 35 % were 5 (71.4 %) indicating that IgM was a predominant antibody. This report is in discordance with work done in Kenya in which avidity index > 35 % were 79.63 % showing IgG antibody predominancy while those with avidity index ≤ 35 % were 20.37 %.
In pursuance of the sustainable developmental goal number 3, it is important to take advantage of the high prevalence rate of CMV IgG in the population studied which suggest a high herds immunity to work towards a possible elimination of CMV from the community. This is necessary because presence of CMV IgM suggest that there is still an ongoing infection and reinfection among the population. The spread can however be easily halted by testing and vaccinating the susceptible member of the community thereby contributing to the effort towards the achievement of the sustainable developmental goals.
Conclusion
This study shows that HCMV infection is widespread among women of child bearing age in Ido-Ekiti and environs with the prevalence of 87.9% CMV IgG antibody. There is also a 7.8% CMV IgM incident rate of the infection among the study population. Actual incident rate (acute infection) as confirmed by avidity index testing was 4.3% (5/116). The prevalence of CMV IgG and CMV IgM antibodies were independent of age, marital status, and number of pregnancy and history of miscarriage.
Recommendation
Based on the findings of the present study we recommend that relevant authorities ensure that CMV routine screening be enlisted among antenatal screening tests. Vaccination of susceptible mothers should be encouraged so as to achieve total eradication of CMV in the population. The infected individuals serve as a reservoir through whom infection can spread and mutation can possibly occur thus generating a variant strain that could result in a new epidemic.
Limitation to study: A number of potential subjects were conservative with giving consent to be enrolled in the studies and some were not ready to provide necessary data as required by the structured questionnaire. Such potential participants had to be removed from the study.
Competing Interests: The authors have declared that no competing interests exist.
References
- Swanson EC, Gillis P, Hernandez-Alvarado N, Fernández-Alarcón C, Schmit M, Zabeli JC, et al. Comparison of Monovalent Glycoprotein B with Bivalent Gb/Pp65(Gp83) Vaccine for Congenital Cytomegalovirus Infection in a Guinea Pig Model: Inclusion of Gp83 Reduces Gb Antibody Response but Both Vaccine Approaches Provide Equivalent Protection against Pup Mortality. Vaccine, 2015; 33(32): 4013-
- Maingi Z, Nyamache AK. Seroprevalence of Cytomegalo Virus (CMV) among pregnant women in Thika, Kenya. BMC Res Notes, 2014; 7: 794-796.
- Umeh EU, Onoja TO, Aguoru CU, Umeh JC. Seroprevalence of Cytomegalovirus Antibodies in Pregnant Women, Benue State, Nigeria. J Infect Dis Ther., 2015; 3: 242-247.
- Mocarski ES, Shenk T, Pass RF. Cytomegalovirus. In: Fields BN, Knipe DM, Howley PM, et al., Virology. (5. Ed.). Philadelphia: Lippincott Williams and Wilkins, 2007; pp. 2701–2772.
- Steininger C. Clinical Relevance of Cytomegalovirus Infection in Patients with Disorders of the Immune System.Clinical Microbiology and Infection, 2007; 13(10): 953-
- Boppana SB, Rivera LB, Fowler KB, Mach M, Britt WJ. Intrauterine transmission of cytomegalovirus to infants of women with preconceptional immunity.New England Journal of Medicine, 2001; 344:1366–1371.
- Nigro G, Adler SP. Cytomegalovirus Infections During Pregnancy. Current Opinion in Obstetrics and Gynecology, 23(2): 123-
- Ho M. The History of Cytomegalovirus and its diseases.Med Micro Immunol. 2008; 197: 65-73.
- Martí CJ, Maes P. Human cytomegalovirus genomics and transcriptomics through the lens of next-generation sequencing: revision and future challenges. Virus Genes, 2019; 55(2):138-164.
- Spano LC, Gatti J, Nascimento JP. Prevalence of human cytomegalovirus infection in pregnant and non-pregnant women.Journal of infectious Diseases, 2004; 48(3): 213-220.
- Murray PR, Rosenthal KS, Kobayashi GS, pfaller MA. Human Herpesviruses. (14. Ed.). Medical Microbiology.Elsevier: Philadelphia, 2022; pp 475-498
- Hamdan HZ, Abdelbagi IE, Nasser NM, Adam I.Seroprevalence of cytomegalovirus and rubella among pregnant women in western Sudan.Virol J., 2011; 8: 217-220.
- Okwori A, Olabode A, Emumwen E, Echeonwu G, Lugos M, Okpe E, et al. Sero-Epidemiological Survey of Human Cytomegalo Virus Infection Among Expectant Mothers In Bida, Nigeria. The Internet Journal of Infectious Diseases, 2008; 7(1): 1-9.
- Sheevani N, Jindal AA. A pilot seroepidemiological study of cytomegalovirus infection in women of child bearing age.Indian Journal of Medical Microbiology, 2005; 23(1): 34-36.
- Ahmad RM, Kawo AH, Udeani TC, Manga SB, Ibrahim ML. Seroprevalence of cytomegalovirus antibodies in pregnant women attending two selected hospitals in Sokoto State, Northwestern Nigeria.Journal of Pure and Applied Science, 2010; 3: 298-303.
- Liesnard C, Donner C, Barancart F, Gosselin F, Delforge ML, Rodesh F. Prenatal diagnosis of congential cytomegalovirus infection: prospective study of 237 pregnancies at risk. Obstetrics Gynecology Journal, 2000; 95(6): 881-888.
- Daiminger A, Bader U, Enders G. Pre and periconceptional primary cytomegalovirus infection: risk of vertical transmission and congenital diseases. Journal OfGynaecology, 2005; 122: 166-172.
- Ogundele JA, Jegede AO. Environmental Influences of Flooding on Urban Growth and Development of Ado-Ekiti, Nigeria.Studies in Sociology of Science, 2011; 2(2): 89-93.
- Redwan N, Ahmed M, Awfi MA. Prevalence Study of Cytomegalovirus (Cmv) Infection among Foreign Manpower in Jeddah Saudi Arabia. African Journal of Microbiology Research, 2011; 5(17): 2539-2549.
- Shimada K, Toriyabe K, Kitamura A, Morikawa F, Minematsu T, Ikejiri M, et al. Primary cytomegalovirus infection during pregnancy and congenital infection: a population-based, mother–child, prospective cohort study. J Perinatol., 2021; 41: 2474-2481.
- Akinbami AA, Rabiu KA, Adewunmi AA, Wright KO, Dosunmu AO, Adeyemo TA, et al. Seroprevalence of cytomegalovirus antibodies amongst normal pregnant women in Nigeria. J. Womens Health, 2011; 3: 423–428.
- Leila B, Hossein M, Narges S, Mohammad G. Seroprevalence of cytomegalovirus infection among pregnant women in Eastern Iran.The brazilian journal of infectious diseases, 2012; 16(4): 402–403.
- Hamid KM, Onoja AB, Tofa UA, Garba KN. Seroprevalence of cytomegalovirus among pregnant women attending Murtala Mohammed Specialist Hospital Kano, Nigeria.African Health Sciences, 2014; 14(3): 125-130.
- Dollard SC, Grosse SD, Ross DS. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev Med Virol.,2007; 17(5): 355–363.
- Ali S, Askin G, Hakan O, Inanç M. CMV seroconversion in pregnancy and the incidence of congenital CMV infection. Turk J Pedriatr.,2007; 49: 30–36.
- Arabpour M, Kariyanee K, Jankhah A, Yaghobi R. Human cytomegalovirus in women of child bearing age throughout Fars’s province-Iran: A population-based cohort study. Malaysian Journal of Microbiology, 2007; 3(2): 23–28.
- Alghalibi SMS, Abdullah QYM, Al-Arnoot S, Al-Thobhani A. Seroprevalence of Cytomegalovirus among Pregnant Women in Hodeidah city, Yemen. J Hum VirolRetrovirol, 2016; 3(5): 00106.
- Picone O, Vauloup FC, Cordier AG. A 2-year study on cytomegalovirus infection during pregnancy in a French hospital.Journal of Gynaecology, 2009; 116: 818-821.
- Robert F, Pass. Cytomegalovirus Infection.Pediatrics in Review, 2000; 23: 163-169.
- Dowd JB, Aiello AE, Alley DE. Socioeconomic disparities in the seroprevalence of cytomegalovirus infection in the US population.Epidemiology of Infection, 2009; 137: 58-65.
- Colugnati FA, Staras SA, Dollard SC, Cannon MJ. Incidence of cytomegalovirus infection among the general population and pregnant women in the United States.Bio Med Central Infectious Diseases, 2007; 71: 1471-2334.
- Delfan BM, Sheikhian A, Birjandi M, Fazeli M. Seroprevalence of Cytomegalovirus Infection in Pregnant Women Referred to Health Care Center of Khorramabad. Iranian Journal of Virology, 2011; 5(4): 11-16.
- Griffiths PD, Mclean A, Emery VC. Encouraging prospects for immunization against primary cytomegalovirusVaccine, 2001; 19: 1356-1362.
- Emovon EO, Oduyebo O, Lofor PV, Onakewhor JU, Elikwu CJ. Seroprevalence and risk factors for cytomegalovirus infection among pregnant women in southern Nigeria.J Microbiol Infect Dis., 2013; 3(3): 123-127.
- Cahill AG, Odibo AO, Stamilio DM, Macones GA. Screening and testing for primary cytomegalovirus in pregnancy: where do we stand? A decision-analytic and economic analysis.Am J ObstGynecol, 2009; 201(466): 1-7.
- Singh AS, Masuku MB. Sampling techniques and Determination of sample size in Applied Statistics Research: An Overview.International Journal of Economics, Commerce and Management, 2014; 2(11): 1-22.
- Zhang S, Hu L, Chen J, Xu B, Zhou YH, et al. Cytomegalovirus seroprevalence in pregnant women and association with adverse pregnancy/neonatal outcomes in Jiangsu Province, China. PLoS One, 2014; 9: e107645.

