Optimal Mean Airway Pressure in High Frequency Oscillatory Ventilation Guided by Electrical Impedance Tomography in Children with Acute Respiratory Distress Syndrome
Natchaya Thaiyanon1 and Rujipat Samransamruajkit2,*
1Division of Pediatric Pulmonology and Critical Care, Department of Pediatrics, King Chulalongkorn Memorial Hospital, Faculty of Medicine, Chulalongkorn University, Thailand
2Department of Pediatrics, King Chulalongkorn Memorial Hospital, Faculty of Medicine, Chulalongkorn University, Thailand
Received Date: 21/12/2022; Published Date: 11/01/2023
*Corresponding author: Rujipat Samransamruajkit, Department of Pediatrics, King Chulalongkorn Memorial Hospital, Faculty of Medicine, Chulalongkorn University, Division of Pediatric Pulmonology and Critical Care, 1873, Rama 4 Road, Pathumwan Sub District, Pathumwan District, Bangkok, Thailand, 10330
Abstract
Objective: To assess the clinical feasibility and safety of electrical impedance tomography (EIT) to identify the optimal Mean Airway Pressure (MAP) in high frequency oscillatory ventilation (HFOV) in children with Acute Respiratory Distress Syndrome (ARDS).
Design and setting: Prospective, observational descriptive study in the pediatric intensive care unit (PICU) at King Chulalongkorn Memorial Hospital (KCMH) (super tertiary level)
Patients: Patients aged 1 month – 15 years admitted to PICU with moderate to severe ARDS using HFOV from October 2020 to February 2022.
Interventions: To determine the optimal MAP in HFOV, we used our previous protocols which identified the optimal MAP in HFOV by decremental MAP titration after lung recruitment maneuvers (conventional approaches) combined with EIT analysis. After decremental MAP titration, the treating physicians set the appropriate MAP for each patient, referred to as the practical MAP.
Measurements and result: There were six participants diagnosed moderate to severe ARDS. All had their optimal MAP identified in HFOV following the protocol combined with EIT analyses for 13 times. One analyses could not be compared due to technical errors. The optimal MAP values in HFOV obtained using EIT were lower significantly from the practical MAP (median 21.25 (18.5-28) and 23.00 (17-26) cmH2O respectively, P = 0.025). After the lung recruitment maneuver, most patients had a reduction in MAP and oxygen index (OI) except for patients No. 2 and No. 5 who had increased OI. OI decreased by median value of 1.1 (0.2-2.45). No significant changes were found in the hemodynamics and lung volume from chest x-rays after the lung recruitment maneuver was performed. There was no complication related to EIT in this study.
Conclusions: The use of EIT in children with moderate to severe ARDS is appeared to be safe and feasible. The optimal MAP on HFOV obtained using the EIT was lower significantly from the practical MAP set by the treating physicians depending on the individual patient.
Keywords: Pediatric; Acute respiratory distress syndrome; High frequency oscillatory ventilation; Electrical impedance tomography; Optimal mean airway pressure; Lung recruitment
Introduction
Acute Respiratory Distress Syndrome (ARDS) is a leading cause of morbidity and mortality in intensive care units. Among pediatric patients, the incidence of ARDS was 3.5 cases per 100,000 people per year and its mortality rate was 33.7% [1]. There are alternative treatment modalities for severe ARDS including high frequency oscillatory ventilation (HFOV) [2]. However, a recent study of adult ARDS patients found that HFOV did not reduce mortality [3], but rather increases the number of vasoactive drugs, sedatives and muscle relaxants used. It may also show increased mortality [4,5]. While some pediatric studies found that using HFOV in severe ARDS resulted in superior oxygenation to conventional ventilation[6], the outcomes of HFOV are based on multifactorial. One is of the key success in using HFOV is to optimize lung volume by initial Mean Airway Pressure (MAP).
Currently, we have a safe lung volume monitoring device called an electrical impedance tomograph (EIT), which has the capability to assess lung volume at the bedside. Inany HS et al., found that EIT images were well associated with a patient's lung pathology and there was no complication associated with using EIT [7]. It has been used with conventional mechanical ventilation (CMV) in patients with ARDS for estimation of recruitable alveolar collapse and hyperdistension [8], finding personalized optimal positive end-expiratory pressure (PEEP) [9]. Additionally, there is limited study of the application of EIT with HFOV in pediatric ARDS.
Methods
Study design & setting
This prospective, observational descriptive study was approved by the Institutional Review Board of the Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand (153/64). All patients were admitted to the Pediatric Intensive Care Unit (PICU) at King Chulalongkorn Memorial Hospital (KCMH), (super tertiary level) with moderate to severe ARDS using HFOV, from October 2020 to February 2022. Informed consent was obtained.
Study population
Pediatric patients aged 1 month – 15 years with moderate to severe ARDS (oxygen index (OI) ≥ 12) according to the Pediatric Acute Lung Injury Consensus Conference (PALICC) definition using HFOV were included. Those with cyanotic heart disease, severe obstructive airway disease, unstable spines or did not consent were excluded.
Data collection
To determine the optimal MAP in HFOV (sensor medic, USA) we used the protocols of a previous study (ISRCTN 19924570) (Appendix A), which identified the optimal MAP in HFOV by decremental MAP titration after lung recruitment maneuvers. The conventional protocol will start MAP at 30 cmH2O, continuous distending pressure was sustained for 30 second. Then, the piston started and gradually weaned down MAP by 2 cmH2O every 5 minutes and observe SpO2. Record the MAP at which the SpO2 starts to decrease by more than 2 percentage points (lung DE recruitment). MAP at this point is the optimal MAP in conventional protocol.
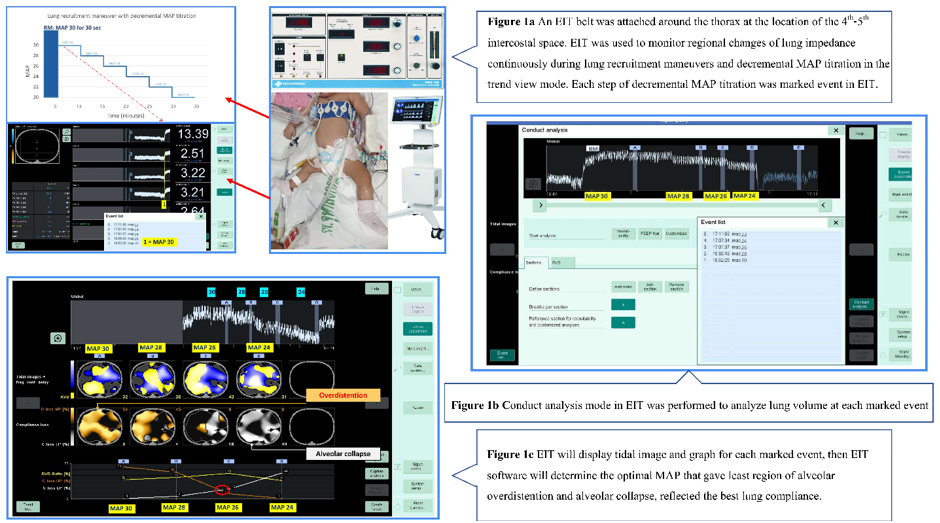
A Dräger PulmoVista® 500 (EIT analysis unit with software version 1.2 (latest version 2020), Germany) was used in the study. An EIT belt was attached around the thorax at the location of the 4th-5th intercostal space with a reference electrode at the abdomen used to perform the analysis at a rate of 50 frames per second.
EIT was used to monitor regional changes of lung impedance which correlated with lung volume continuously during lung recruitment maneuvers and decremental MAP titration in the trend view mode. Starting, MAP at 30 cmH2O was sustained for 30 seconds then decrease MAP by 2 cmH2O every 5 minutes and stop when the oxygen saturation (SpO2) starts to decrease by more than 2 percentage points (lung de-recruitment point) (Figure 1a).
Each step of decremental MAP titration was marked event in EIT. After decremental MAP titration was done, conduct analysis mode in EIT was performed to analyze lung volume at each marked event. (Figure 1b). EIT will display tidal image and graph for each marked event which included region of alveolar overdistention and region of alveolar collapse, then EIT software will determine the optimal MAP that gave least region of alveolar overdistention and alveolar collapse, reflected the best lung compliance (Figure 1c).
After decremental MAP titration, the appropriate MAP was set by the treating physicians for each patient according to clinical, SpO2, hemodynamics, blood gas and chest x-ray, referred to the practical MAP. The practical MAP may be lower than the optimal MAP obtained using EIT, if the treating physicians concern of overdistention of alveoli from clinical, blood gas and chest x-ray, or may be higher if the clinical, blood gas and chest x-ray suggested collapsed alveoli.
Statistical Analysis
Continuous variables were expressed as mean (standard deviation: SD), median (interquartile range: IQR) and percentage for categorical variables. IBM SPSS Statistics 22 software was used for analysis.
Results
Baseline clinical characteristics
There were six participants in the study. All participants had moderate to severe ARDS ( Table 1).
Table 1: Baseline clinical characteristics.
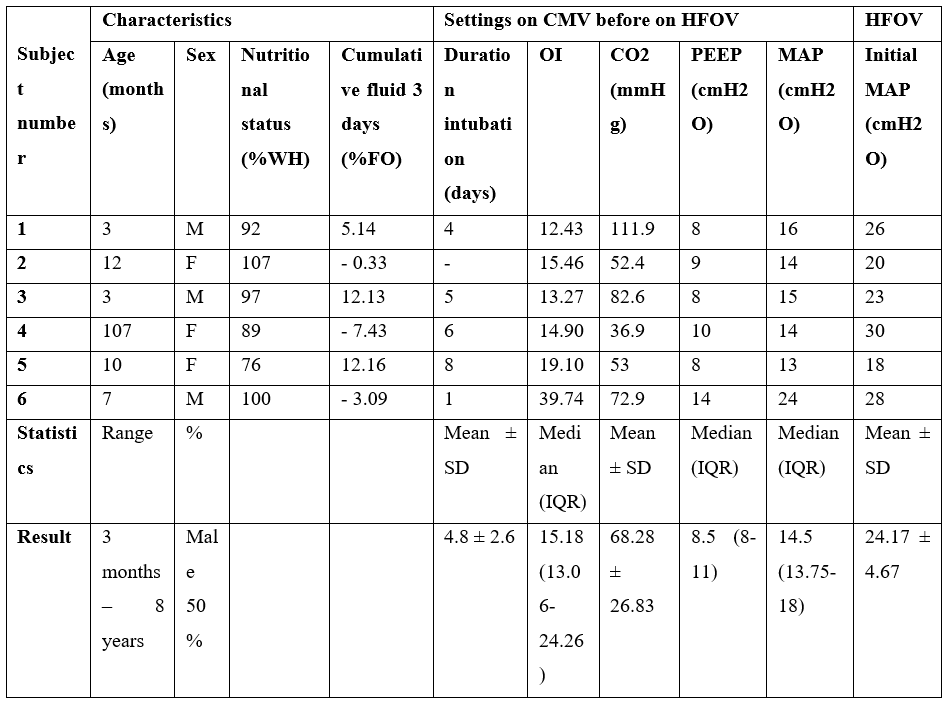
*Diagnosis of subject:
Number 1. Respiratory syncytial virus pneumonia, bronchopulmonary dysplasia.
Number 2. Ventilator-associated pneumonia, bronchopulmonary dysplasia with congenital diaphragmatic hernia, patent ductus arteriosus with ventricular septal defect with pulmonary hypertension.
Number 3. Ventilator-associated pneumonia, preterm with hiatal hernia with upper gastrointestinal bleeding.
Number 4. Pulmonary hemorrhage, microscopic polyangiitis with rapidly progressive glomerulonephritis.
Number 5. Ventilator-associated pneumonia, Down's syndrome with atrioventricular canal defect with pulmonary hypertension.
Number 6. Hospital-acquired pneumonia with pulmonary hemorrhage, Alagille syndrome with congenital anomalies of kidney and urinary tract, Gut obstruction with small bowel resection.
Optimal mean airway pressure guide by electrical impedance tomography and practical MAP
The total number of analysis times was 13. One analysis could not be compared due to technical errors. The optimal MAP values in HFOV obtained using EIT were lower significantly from the practical MAP set by the treating physicians (median 21.25 (18.5-28) and 23.00 (17-26) cmH2O respectively, P = 0.025). No significant changes were found in the hemodynamics and lung volume from chest x-rays between Optimal MAP obtained using EIT and practical MAP. (Table 2). There were no complications related to EIT in this study. The survival rate was 50% (Figure 2).
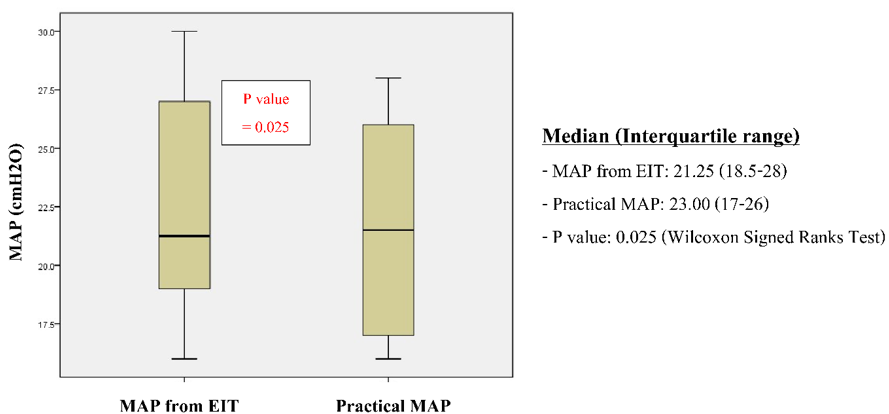
Figure 2: Optimal MAP guide by electrical impedance tomography and practical MAP.
Table 2: Hemodynamics and chest x-rays between optimal MAP obtained using EIT and practical MAP.

Effect of lungs recruitment maneuvers
Almost all participants had their optimal MAP in HFOV identified following the protocol except in the first titration for the initial settings of patient No. 6. There was no indication for a recruitment maneuver due to a low HFOV setting with appropriate oxygenation.
After the lung recruitment maneuver, most patients had a reduction in their MAP and OI except patient No. 2 and No. 5 who had an increased OI. The OI decreased by a median value of 1.1 (0.2-2.45).
No significant changes were found in the hemodynamics and lung volume from chest x-rays after the lung recruitment maneuver was performed.
All six participants received ventilation with HFOV for an average of 8.3 days (SD 4.63 days), received intubation from 17 - 52 days. Of them, 67% were admitted to the PICU for more than 28 days. The results for the intubation day were not included for patient No. 2 because that patient was a preterm infant received intubation since birth.
Discussion
From our study, in children with moderate to severe ARDS, EIT-guided the optimal MAP in HFOV is feasible and safe. There was no complication related to EIT. The optimal MAP values in HFOV obtained using EIT were lower significantly from the practical MAP. Similar to the study by Rosemeier et al., found that EIT guided PEEP titration protocol in children with mild to moderate ARDS is feasible and led to higher PEEP levels than the PEEP levels set by the treating physician [9].
The study by Liu S et al., in which they used EIT to adjust the optimal MAP in HFOV and compared these results to the optimal MAP obtained via a conventional approach. They used a SpO2 response to the change of MAP in an experimental model of ARDS. The difference of MAP values ranged from 0-6 cmH2O [10]. In the study of Miedema M et al., it was used to examine changes in lung volume and ventilation in preterm infants with RDS using HFOV. They found oxygenation-guided recruitment maneuvers alone cannot effectively represent the lung volume when compared with EIT [11].
A key factor in successfully using HFOV is to set an initial optimum MAP. The optimal MAP setting in HFOV will give the best recruitable lung units, provide adequate gas exchange, and thereby maintain good SpO2 as well as hemodynamics. Therefore, SpO2 is often used as indicator of proper lung volume, especially in recruitment maneuvers.4, 5 Nevertheless, the level of SpO2 also depends on other factors, such as tissue perfusion, hemodynamics, cardiac output, and especially pulmonary blood flow [12]. This finding demonstrates the limitation of the current protocol to find the best MAP-recruitable lung units in HFOV. Using a safe device that can assess lung volume at the bedside while using HFOV to determine the optimal MAP to achieve the best recruitable lung unit would be an ideal approach in severe ARDS.
After identifying the optimal MAP in HFOV using a protocol. The OI decreased by a median value of 1.1 (IQR 1.3) except patient No. 2 and No. 5 who had an increased OI. This could be partly explained by their underlying diseases (congenital heart disease and pulmonary hypertension) in which the change in MAP may quickly affect lung volume, pulmonary blood flow, shunting of blood flow that will affect PaO2 and SpO2.
Additionally, there was no significant change of lung volume observed from CXR. Thus, CXR may be an inaccurate method to assess the optimal lung volume during the use of HFOV. There were no significant changes in the hemodynamics after a lung recruitment maneuver was performed, which agrees with the results of Samransamruajkit R et al [6].
Our study is the first study to obtain the optimal MAP in HFOV guided by EIT in children with moderate to severe ARDS. Although, there are limitations in this study due to the small number of enrolled subjects. Thus, it may be insufficient to make solid conclusions about the efficacy of using EIT and HFOV in severe pediatric ARDS. However, the study demonstrates potential clinical benefits without causing adverse effects on patients. A subsequent larger clinical study to confirm the effectiveness of EIT-guided MAP settings in children with severe ARDS on HFOV is certainly warranted.
Conclusion
The use of EIT in children with moderate to severe ARDS is appeared to be safe and feasible. It may be used to assess the dynamic changes of pulmonary pathology at the bedside and find individualized optimal MAP values in HFOV to provide the best recruitable lung units with the least effect on hemodynamics.
Conflict of Interest: The authors declare that they have no conflicts of interest.
Financial support and sponsorship: The study received no financial support.
References
- Schouten LR, Veltkamp F, Bos AP, van Woensel JB, Serpa Neto A, Schultz MJ, et al. Incidence and Mortality of Acute Respiratory Distress Syndrome in Children: A Systematic Review and Meta-Analysis. Crit Care Med, 2016; 44: 819-829.
- Pediatric acute respiratory distress syndrome: consensus recommendations from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med, 2015; 16: 428-439.
- Sud S, Sud M, Friedrich JO, Meade MO, Ferguson ND, Wunsch H, et al. High frequency oscillation in patients with acute lung injury and acute respiratory distress syndrome (ARDS): systematic review and meta-analysis. BMJ, 2010; 340: c2327.
- Young D, Lamb SE, Shah S, MacKenzie I, Tunnicliffe W, Lall R, et al. High-frequency oscillation for acute respiratory distress syndrome. N Engl J Med, 2013; 368: 806-813.
- Ferguson ND, Cook DJ, Guyatt GH, Mehta S, Hand L, Austin P, et al. High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med, 2013; 368: 795-805.
- Samransamruajkit R, Rassameehirun C, Pongsanon K, Huntrakul S, Deerojanawong J, Sritippayawan S, et al. A comparison of clinical efficacy between high frequency oscillatory ventilation and conventional ventilation with lung volume recruitment in pediatric acute respiratory distress syndrome: A randomized controlled trial. Indian J Crit Care Med, 2016; 20: 72-77.
- Inany HS, Rettig JS, Smallwood CD, Arnold JH, Walsh BK. Distribution of Ventilation Measured by Electrical Impedance Tomography in Critically Ill Children. Respir Care, 2020; 65: 590-595.
- Costa EL, Borges JB, Melo A, Suarez-Sipmann F, Toufen C, Jr., Bohm SH, et al. Bedside estimation of recruitable alveolar collapse and hyperdistension by electrical impedance tomography. Intensive Care Med, 2009; 35: 1132-1137.
- Rosemeier I, Reiter K, Obermeier V, Wolf GK. Mechanical Ventilation Guided by Electrical Impedance Tomography in Children With Acute Lung Injury. Crit Care Explor, 2019; 1: e0020.
- Liu S, Zhao Z, Tan L, Wang L, Möller K, Frerichs I, et al. Optimal mean airway pressure during high-frequency oscillatory ventilation in an experimental model of acute respiratory distress syndrome: EIT-based method. Ann Intensive Care, 2020; 10: 31.
- Miedema M, de Jongh FH, Frerichs I, van Veenendaal MB, van Kaam AH. Changes in lung volume and ventilation during lung recruitment in high-frequency ventilated preterm infants with respiratory distress syndrome. J Pediatr, 2011; 159: 199-205.e2.
- David F, Butler, Kenneth A. Schenkman: Noninvasive respiratory monitoring and assessment of gas exchange. In: Fuhrman and Zimmerman's Pediatric Critical Care. Zimmerman JJ, Rotta AT (Eds). Sixth Edition. Philadelphia, Elsevier, 2021; pp 483-491.

